Abstract
Biological invasions are responsive to changing wildfire regimes related to human activities that are altering biological communities. Our objective was to investigate how fire, rodent activity, and competition among plant species modify plant community structure, invasion patterns, and vulnerability to altered fire regimes. We imposed experimental fires, and reduced rodent density using fencing in a full factorial design and quantified competitive interactions among plant species in the northeast Mojave Desert that has experienced dramatic increases in plant invasion and fire in recent years. Vegetation surveys were conducted in the experimental plots to determine plant density, cover, and biomass of herbaceous plants over a 5-year period. Rodent exclusion increased the density, cover, and biomass of Bromus rubens, an invasive annual grass, and density of forb species. In contrast, rodent exclusion decreased the density, cover, and biomass of Schismus spp. another dominant annual invader. Fire increased Schismus spp. and forb species density, cover, and biomass but decreased B. rubens density. Negative spatial correlation between B. rubens and Schismus spp., and forbs indicated interspecific competition among the dominant plant species. Fire reduced rodent community diversity (Shannon’s) 2.5-fold, which was correlated with increases in B. rubens cover and biomass, and native forb diversity. Fire, high rodent diversity, and competition from the other plant species may decrease fire potential in our study system by reducing the density and biomass of B. rubens, which because of its taller growth form tends to ignite and carry fire better than Schismus spp. and forbs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction and spread of exotic species is among the most widespread and problematic human impacts on Earth’s ecosystems (Pimentel et al. 2005; Vilà et al. 2010; Vitousek et al. 1997). Plant invasions can trigger state changes in vegetation that result in reductions of biodiversity and loss of ecosystem services (Pimentel et al. 2005). Patterns of plant invasions are influenced by biotic interactions between plant invaders and native organisms via competition and herbivory, which can both enhance and reduce biotic resistance to invasion (Levine et al. 2004; Pearson et al. 2012). Disturbance can increase the invasibility of an ecosystem by impacting native community composition and structure, leading to reduced biotic resistance to invaders (Davis et al. 2000). For example, disturbance-driven decreases in native plant cover increases space, light, and soil resource availability, which can increase the establishment success of plant invaders (Esque et al. 2010a; Steers and Allen 2012). Disturbance can also modify the composition and abundance of native consumer communities that often exert top-down controls on plant invaders (St. Clair et al. 2016).
Human activities are altering wildfire regimes at a global scale with broad implications for the invasibility of ecosystems (Brooks 1999a; Germino et al. 2016 and references therein; Vitousek et al. 1997). In dryland ecosystems, which cover as much as 40% of the Earth’s terrestrial surface (Safriel et al. 2005), there is a close association among disturbance, increased exotic annual grass presence, and increased potential for fire ignition and spread (Gelbard and Belnap 2003; Mack 1981). In deserts of North America, wildfires have occurred historically on century time scales; however, with the introduction of exotic annual grasses in the late 1800s sub-decadal fire return intervals are now being observed (Bowman et al. 2011; Germino et al. 2016 and sources therein; Whisenant 1992). Wildfires promote invasion success in deserts of North America by decreasing biotic resistance through removal of native vegetation (St. Clair et al. 2016; Steers and Allen 2012). Removal of intact native plant species by fire provides resource opportunities and competitive release that promotes invasive annual grass establishment (Germino et al. 2016; Horn et al. 2015; Levine et al. 2004). Dominance of invasive grasses after fire can decrease the establishment success of native plant species that alters plant community composition (Brooks et al. 2004; Germino et al. 2016). Fire can also indirectly affect the plant community by modifying the behavior and composition of consumer communities that regulate plant community assembly (Elton 2000; Horn et al. 2012; Levine et al. 2004). Shifts in plant community composition and structure in response to these interactions can alter the fuel characteristics of the plant community that influence the probability and spread of wildfires that promote invasive grass–fire cycles (Brooks et al. 2004; D’Antonio and Vitousek 1992; St. Clair and Bishop 2019).
Rodent herbivory creates top-down control on plant community assembly (Beatley 1976; Inouye et al. 1980; Pearson et al. 2014) and biotic resistance against the establishment of invasive species through seed predation and seedling herbivory (Pearson et al. 2014; St. Clair et al. 2016). Rodents structure plant communities through seed consumption and seed dispersal (Beatley 1969; Price and Joyner 1997). Rodents may therefore facilitate invasion by increasing dispersal of invasive seeds or preferentially consuming native seeds (Beatley 1976; Horn et al. 2017; Price and Joyner 1997). However, post-dispersal establishment of plant invaders may be suppressed by rodent activity (Pearson et al. 2012; St. Clair et al. 2016). Because rodent abundance, richness, and diversity can decrease in response to wildfire (Horn et al. 2012; Ostoja and Schupp 2009), post-fire conditions may create windows of opportunity for plant invaders to be released from rodent suppression (Allington et al. 2013).
Competition is a key driver of plant community structure and biotic resistance against plant invasions (Levine et al. 2004). Intact native plant communities have been shown to reduce the establishment and spread of exotic plant species (Pearson et al. 2012; St. Clair et al. 2016). However, competition within plant communities typically decreases following fire resulting in increased availability of soil resources (Allen et al. 2011; Horn et al. 2017; Shea and Chesson 2002). In arid and semi-arid environments, inter-shrub spaces typically have lower native annual plant abundance, and those that do establish have shorter stature than those that grow under shrub canopies (Brooks and Chambers 2011; Filazzola et al. 2018) creating resource opportunities in the open spaces between shrubs (Brooks and Chambers 2011; Schafer et al. 2012). This niche opportunity (niche opportunity hypothesis; (Davis et al. 2000)) could allow an exotic plant to move away from a direct competitor and exploit a neighboring but relatively uninhabited area by native plants, filling in the inter-shrub spaces (Shea and Chesson 2002). Plant density and composition in inter-shrub spaces influence the spread and size of wildfires in deserts (St. Clair and Bishop 2019). Since grasses and forbs (exotic and native) have different fuel properties, niche exploitation based on competitive interactions can strongly influence the size and frequency of wildfires in desert ecosystems (D’Antonio and Vitousek 1992; Shea and Chesson 2002).
The Mojave Desert was chosen as a study system to examine the interplay of disturbance, herbivory, and competition on ecosystem invasibility because fires are becoming more frequent (Brooks and Matchett 2006), rodents are abundant as a primary consumer, and there are multiple plant invaders influencing fire ecology in the system (Brooks et al. 2016; Brooks and Chambers 2011). The two dominant plant invaders in the Mojave Desert are Bromus rubens L. and Schismus spp. (Schismus barbatus or Schismus arabicus), and several studies have examined their role in and response to fire (Brooks and Matchett 2003, 2006; DeFalco et al. 2007; reviewed in Germino et al. 2016). Fewer studies have examined the competitive effects between invasive and native plants in the Mojave Desert in response to fire and little research exists examining the influence of consumers on invasibility of the Mojave Desert through experimental treatments (but see Brooks 1995). In high precipitation years, B. rubens and Schismus spp. can fill in inter-shrub spaces that typically are left void of enough vegetation to carry fire (Brooks and Matchett 2003). However, when both are present on the landscape, B. rubens is found more commonly under shrub canopies, particularly the fire-susceptible shrub Coleogyne ramosissima Torr. (Beatley 1966) and dominant shrub Larrea tridentata, while Schismus spp. fills the inter-shrub spaces (Allen et al. 2011; Brooks and Matchett 2003). However, there is a lack of understanding of how these spatial relationships may be modified by fire or rodent activity and impact competitive relationships and fire potential.
The objective of this study was to experimentally investigate the effects of fire and rodent activity on plant community structure and invasion outcomes in the Mojave Desert. We asked the following questions: (1) What are the effects of fire and rodent exclusion on the establishment and growth of invasive annual grasses and herbaceous forbs, including exotic and native species? (2) Are changes in rodent diversity, richness, and abundance correlated with post-fire plant community characteristics? (3) Is there evidence of competitive interactions between invasive grass species or between exotic grasses and forb species and are they modified by fire or rodent exclusion across space?
Methods
Study site
The study was conducted at Lytle Research Preserve, Washington Co., UT, USA, in the northeast region of the Mojave Desert (37°08′54″N, 114°00′51″W). No known fires have occurred at the study site in recent decades based on a well-developed perennial shrub community along with personal communication with the preserve manager (Heriberto Madrigal, personal communication). Cattle grazing has not occurred at the site since 1985. The study site is a semi-desert shallow hardpan (blackbrush) with soil classified as a gravelly sandy loam (Soil Survey Staff 2015). It is located at 915 m elevation and mean annual precipitation is 272 mm and mean annual temperature is 16 °C (Western Regional Climate Center 2000). Annual precipitation during the study period was as follows: 228 mm (2012–13), 253 mm (2013–14), 314 mm (2014–15), and 315 mm (2015–16). Precipitation data were collected from the Badger Springs, Ivins Remote Automated Weather Station (RAWS) (Western Regional Climate Center 2000). Vegetation at the study site is dominated by Larrea tridentata (DC.) Coville, Coleogyne ramosissima Torr., Ambrosia dumosa (A. Gray) Payne, and Yucca brevifolia Engelm. Common native herbaceous (perennial and annual) plants included Sphaeralcea ambigua A. Gray spp. monticola Kearney, Baileya multiradiata Harv. & A. Gray, Astragalus nuttaliianus DC., Plantago spp. (P. patagonica and P. ovata), and Descurainia pinnata (Walkter) Britton. At the beginning of the study in 2011, shrub interspaces were dominated by non-native annuals, Bromus rubens and Erodium cicutarium, while Schismus spp. was generally absent.
Plot design
The study tests the main effects of fire and rodent exclusion in a full factorial block design replicated five times (St. Clair et al. 2016). Each 60 m × 60 m experimental block was split into four randomly assigned 30 m × 30 m treatment sub-plots with the following treatment combinations: burned–rodents present, burned–rodents excluded, unburned–rodents present, unburned–rodent excluded. Wire mesh fencing was installed around the perimeter of each treatment plot (both rodents present and excluded) with 30 cm buried below ground and 70 cm above ground. Rodent exclusion plots had a 20 cm strip of aluminum flashing placed at the top to keep rodents from climbing over the fences. Rodents present treatment was achieved by cutting 12 cm × 10 cm opening every 4 m around the fence perimeter to allow rodent entry. The rodent exclusion fences reduced the number of unique individuals trapping session 4.4-fold over the study period. Mean rodent abundance of unique individuals in rodents present plots was 2.76 ± 0.36 individuals per 3-day trapping session, whereas the mean unique individuals in rodent exclusion plots was 0.63 ± 0.15 individuals (P < 0.001). The study area was enclosed by barbed wire fencing to exclude livestock, but had an 80 cm gap at the bottom to allow the entry of mammals and reptiles in and out of the study area. The burn treatments in each block were conducted in June 2011 using a drip torch as an ignition source. The study site was already invaded with B. rubens when the study began with average densities of 1319 stems m−2 in the inter-shrub spaces, which carried fire across the plots. The experimental fire decreased native shrub cover by > 80% indicating that burn severity was moderate to high.
Vegetation surveys
Plant density surveys were conducted annually in April–May for the years 2013–2016. Density counts were done using four parallel randomized transect lines, spaced at least 2 m apart with a modified Daubenmire frame (25 cm × 50 cm) placed every 2 m for a total of 12 quadrats per transect line. Transect lines were placed in randomized locations, as well as alternating orientation each year (N–S, E–W). Frame placement on each transect line started at least 2 m from the fence to avoid edge effects. All herbaceous plants rooted within the frame were counted. Because they were so abundant, invasive annual grasses B. rubens and Schismus spp. were counted in a subframe of 10 cm × 25 cm within the modified Daubenmire frame. Native grasses were largely absent and were not included in the statistical analyses.
Plant canopy cover measurements were done in 2016 using the line-point intercept method (Herrick et al. 2006) along the same four transect lines used for density measurements. A pin was dropped starting at least 2 m from the fence, every 0.5 m for a total of 48 pin drops per transect line. For each pin drop, the topmost plant intersecting the pin was recorded as a canopy layer.
Herbaceous plant biomass was collected in April 2016. All living aboveground herbaceous biomass rooted within the 25 cm × 50 cm modified Daubenmire frame used for vegetation density counts was removed by clipping to ground surface. Biomass was collected along the same four randomized transects used for vegetation density measurements starting at the 2 m mark and sampled every 4 m for a total of six sampling frames per transect. All biomass was taken back to the laboratory and dried at 80 °C for 48 h, then weighed to the nearest gram.
Rodent surveys
Rodent surveys were conducted every spring, summer, and fall period for three consecutive nights per trapping session. Eight large Sherman live traps were placed in a circle away from the fence edge in each plot with two control trap circles for each block located at randomized locations outside the treatment plots. Each morning, rodents were collected and assessed for species, gender, age, reproductive status, and weight (to the closest 0.5 g). New individuals were given an ear tag with a unique identifier to track for subsequent nights and trapping sessions. Rodent abundance was calculated as the number of unique individuals per species trapped within a 3-day trapping session. Rodent species in order of abundance were Dipodomys merriami, Peromyscus crinitus, Chaetodipus formosus, Neotoma lepida, Ammospermophilus leucurus, Peromyscus boylii, Peromyscus maniculatus, Peromyscus truei, and Onychomys torridus. The Brigham Young University Animal Care and Use Committee approved the small mammal survey protocols (IACUC#120,202).
Statistical analysis
Plant density, cover, biomass, and plant community richness, and Shannon’s diversity index were modeled using linear mixed effects models using the nlme package in R (Pinheiro et al. 2017) with main and interactive fixed effects of fire, rodent, and year with experimental blocks designated as the random effect. To meet homogeneity of variance assumptions a varIdent covariance structure for the fixed effects (fire, rodent, and/or year) was used when needed. \(R_{\text{c}}^{2}\) values are presented to help interpret how much of the model explains the variation in the data with both fixed and random effects included in the model. A simple linear regression was used to analyze the effects of rodent diversity, abundance, and richness on native and total plant diversity and density averaged across treatment blocks.
Piecewise structural equation modeling (pSEM, also known as confirmatory path analysis) (Shipley 2009) was used to model the type (positive or negative) and strength (statistical significance and critical values) that treatments had on plant density between plant species using ‘psem’ package in R (Lefcheck 2016). We chose pSEM because of the flexibility to have multiple structural equations with different covariance structures as required to meet the assumptions for the linear mixed effects models (Shipley 2009). However, one limitation to using piecewise structural equation models is that they cannot test reciprocal relationships as with traditional SEM. To better understand possible reciprocal relationships between plant species, different pSEM analyses were used with a particular plant species as a predictor vs a response.
To analyze the competitive spatial correlation a simulated permutation Spearman’s correlation test (n = 2000) was used to test the strength of the correlation between B. rubens and Schismus spp. as measured by densities in each quadrat used for density sampling. This was done for all years combined as well as each year individually across all treatment possibilities and combinations. Each year was included in the analysis because of the unique opportunity for comparing the strength of the competitive interference over time as Schismus spp. was not present at the onset of the experiment.
Results
Plant community responses to rodent exclusion
Rodent exclusion increased B. rubens density (216 m−2–294 m−2) (P = 0.001 Fig. 1, Table 1), cover (11%–17%), and biomass (19 g m−2–28 g m−2) (P = 0.029 and P = 0.067, respectively, Table 2) when averaged across years. Rodent exclusion increased forb density 7% from 156 to 214 m−2 (P = 0.0001, Fig. 1, Table 1), but did not significantly affect forb cover or biomass compared to rodent access plots (P = 0.95 and P = 0.52 respectively, Table 2). In contrast, rodent exclusion reduced Schismus spp. density (993 m−2–751 m−2) (P < 0.0001, Fig. 1, Table 1), cover (30%–22%), and biomass (25 gm−2–14 gm−2) (P = 0.0001 and P = 0.015, respectively, Table 2) 1.2-, 1.5-, and 1.7-fold compared to rodent present plots. Excluding rodents also slightly decreased total herbaceous density (P = 0.068, Fig. 1, Table 1), but did not affect cover or biomass (P = 0.55 and P = 0.75, respectively, Table 2). There was a significant interaction between fire and rodent exclusion on forb density in which the effects of fire were greater in rodent exclusion plots (P = 0.017, Fig. 1, Table 1). Exotic forbs were 2.5-fold more dense than native forbs, no matter the rodent treatment. Rodent exclusion increased diversity of the plant community 1.4-fold (P = 0.017, Fig. 2), but had no significant effect on richness of the plant community (P = 0.16, Fig. 2).
Effects of fire and rodent exclusion on (a) Bromus rubens, (b) Schismus spp., (c) forb species, and (d) total herbaceous plant density (mean ± SE) over time for the entire study period from 2013 to 2016. F-statistics and levels of significance (P value α = 0.05) are given in Table 1
Effects of rodent exclusion and fire on Shannon’s diversity index (left axis) and species richness (right axis) for the entire herbaceous plant community in 2016. Mean values presented with ± SE. Levels of significance (P value α = 0.05) for each treatment and treatment interaction indicated for diversity and richness on the top left of the figure
Plant community responses to fire
Forbs and invasive grass species had varying responses to fire in this study. Fire decreased B. rubens densities by 9% compared to unburned plots when averaged across the 4-year study period (P = 0.0107, Fig. 1, Table 1), while fire had no significant effect on B. rubens cover and biomass (P = 0.18 and P = 0.66, respectively, Table 2). In contrast, Schismus spp. densities (P < 0.0001, Fig. 1, Table 1), cover, and biomass doubled (P < 0.0001 and P < 0.0001, respectively, Table 2) in burned plots compared to unburned plots when averaged across the study period. Fire nearly doubled forb density (P < 0.0001, Fig. 1, Table 1) and cover (P = 0.006, Table 2) compared to unburned plots. Exotic forbs were threefold more dense than native forbs in burned plots, but only 1.5-fold denser in unburned plots. Similarly, forb biomass increased 2.3-fold in burned plots compared to unburned plots in 2016 (P = 0.002, Table 2). Fire increased total herbaceous density (P < 0.0001, Fig. 1, Table 1), cover (P < 0.0001, Table 2), and biomass (2016 only) 1.5-fold or higher (P = 0.0001, Table 2). Plant community diversity decreased 1.4-fold in response to fire (P = 0.014), while fire had no significant effect on plant species richness (P = 0.51, Fig. 2). Except where stated above, there were no other significant fire and rodent interactions for plant density, cover, biomass, richness, or diversity (P > 0.1 for all measurements (Tables 1 and 2, Figs. 1 and 2).
Rodent community responses to fire and rodent exclusion
Burned plots had a 2.5-fold reduction in rodent diversity (Shannon’s diversity index, P < 0.0001) and a slight decrease in rodent richness (P = 0.064) compared to unburned plots averaged across the study period (Fig. 3). Fire did not alter rodent abundance (P = 0.16) in comparison to unburned plots when averaged across time (Fig. 3).
Effects of fire on (a) rodent abundance (left axis), (b) species richness (primary right axis), and (c) Shannon’s diversity index (secondary right axis) for the study period (2013–2016). Rodent diversity was the only rodent community measurement with significant differences (P < 0.001) denoted with an asterisk (*). Mean values presented with ± SE
Plant–rodent correlative relationships
Rodent Shannon’s diversity was negatively correlated with forb Shannon’s index of diversity (R2 = 0.68, P < 0.01) (Fig. 4) and B. rubens cover and biomass (R2 = 0.52, P < 0.05 for cover; R2 = 0.59, P < 0.01 for biomass). Rodent Shannon’s diversity and abundance were not correlated with B. rubens density, Schismus spp. density, cover, or biomass (R2 < 0.1).
Plant competition
There was evidence of competitive interactions between B. rubens, Schismus spp., and forbs (Figs. 5 and 6). The strength of the negative correlation between the two invasive grasses doubled from 2013 (rho = − 0.24, P < 0.0001), when Schismus spp. first started to appear, to 2016 when Schismus spp. attained higher densities and percent cover than B. rubens (rho = − 0.43, P < 0.0001) (Figs. 1 and 5, Table 1). All treatment combinations had significant negative correlation between the two exotic grasses (Fig. 5).
Correlation of B. rubens and Schismus spp. density over time in each experimental treatment plot. Spearman’s rho presented in middle right from the simulated permutation correlation tests (n = 2000) for all possible treatment combinations averaged over the study period. Asterisks (***) denote P < 0.0001
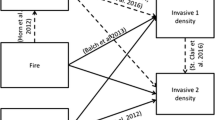
Direct and indirect effects of rodent exclusion, fire, and plant species on density of Bromus rubens (BRRU), Schismus spp. (SCSP), and forb plant species. Black solid lines indicate positive significant (P < 0.05) relationships, red short-dashed lines indicate negative significant relationships, gray long-dashed lines indicate non-significant (0.15 < P > 0.05) negative relationship. Line widths indicate the strength of the relationship as determined by the critical value. \(R_{\text{c}}^{2}\) values are given for each unidirectional response for each model. Color version of this figure is available online
The results from the piecewise structural equation model (pSEM) are displayed in a graphical synthesis of how the experimental treatments affected plant species and potential competitive interactions between plant species (Fig. 6, Table 3). Rodent exclusion positively affected B. rubens and forb densities (P < 0.001; P = 0.02), but negatively affected Schismus spp. (P < 0.01). The pSEM showed that fire benefitted Schismus spp. and forb densities (P < 0.0001), but negatively affected B. rubens (P = 0.02). Schismus spp. and forbs increased with time (P < 0.001; P = 0.04) and B. rubens decreased (P = 0.02). Because pSEM is unidirectional with no testing of reciprocal relationships, B. rubens, Schismus spp., and forbs were each run in a model as the main predictor totaling in three pSEM models. In the first pSEM, B. rubens negatively affected Schismus spp. (P = 0.08) and both B. rubens and Schismus spp. negatively impacted forb densities (P = 0.1; P < 0.001) (Fig. 6). The second pSEM model showed Schismus spp. negatively affected B. rubens (P = 0.05) (Fig. 6; Table 3). The third pSEM model forbs negatively impacted B. rubens and Schismus spp. (P = 0.12, P = 0.08) (Fig. 6 and Table 3).
Discussion
This study provides evidence that top-down effects of consumers on plants, competition among plants, and their response to fire (Chambers et al. 2007; Melgoza et al. 1990; St. Clair et al. 2016) influence patterns of invasion and susceptibility to invasive grass–fire cycles in arid ecosystems (Brooks et al. 2004; Gill et al. 2018). Plants, particularly invasive species, often respond positively to post-fire conditions (Brooks et al. 2004; St. Clair and Bishop 2019), which is consistent with the twofold increase in Schismus spp. and forb species (which include exotic annuals) in this study (Figs. 1 and 6, Table 2). Rodents have been shown to have both positive and negative effects on plant establishment (Maron et al. 2012; Orrock et al. 2008), which was consistent in our data as the two dominant invasive grasses had opposite responses to rodent treatments (Fig. 6). This is likely due to variation in their functional traits and competitive interactions (Bowman et al. 2017; Cubera et al. 2009; Steers and Allen 2012). Shifts in the rodent communities altered plant community structure and invasibility of the study system (Figs. 4 and 5), which is consistent with a comparable study in the Great Basin Desert (St. Clair et al. 2016). Our results also suggest that invasive grasses had strong competitive interactions in our study system (Figs. 5 and 6), along with forbs, that may dictate fine fuel composition under shrub canopies and in inter-shrub spaces (Brooks 1999a; Shea and Chesson 2002). While fine fuel composition thresholds in areas such as the Mojave and Sonoran deserts have not been strongly defined (Rao and Allen 2010 and sources therein), understanding how fine fuel composition may change due to competition and consumer activity may dictate future state changes within arid ecosystems experiencing more frequent wildfires.
Effects of rodents on invasive and herbaceous plant communities
Rodent exclusion positively affected both B. rubens and forb species in our study (Figs. 1 and 6, Table 1 and 2) (St. Clair et al. 2016). Seed predation is a primary mechanism by which rodents influence plant community assembly (Brown and Heske 1990). Rodent consumers have been shown to prefer larger seeds over small seeds (Brooks 1999b; Maron et al. 2012). The positive effect of rodent exclusion on B. rubens may be due it having a larger seed than most of the other species. The increase in forbs in rodent exclusion plots is consistent with other studies that suggest most native forbs, albeit small seeded species in our study, are most likely preferred by small mammals over exotic plant species (Bowman et al. 2017; Maron et al. 2012). Seedling herbivory is another mechanism by which rodents exert top-down control on plant community assembly (Bowman et al. 2017). Forb seedlings are typically preferred by rodents due to their increased forage quality compared to grasses (Bowman et al. 2017; Cubera et al. 2009), but seedling size is also a contributing factor (Pérez-Harguindeguy et al. 2003). Given that B. rubens seedlings may be larger or more abundant due to earlier germination than most natives, rodents may exert higher pressure on B. rubens seedlings at certain times of the year (Beatley 1969; Veech 2001). Excluding rodents increased total plant Shannon’s diversity (Fig. 2) (Keane and Crawley 2002), but did not change plant species richness, suggesting that the rodent community may have more of a generalist strategy rather than targeting specific native plants (Keane and Crawley 2002).
Rodents had positive impacts on Schismus spp. (Figure 1, Table 1 and 2). Schismus spp. has miniscule seeds that have been documented to fall into small soil cracks thereby avoiding seed predation by rodents (Gutterman 1994). The positive effect of rodents on Schismus spp. are also likely indirectly driven by reduced competitive pressure by B. rubens and forbs, as they experience greater top-down control by rodents (Figs. 1 and 6) potentially due to greater seed predation (Orrock et al. 2008; Shea and Chesson 2002). Rodent consumers have been shown to influence invasions indirectly by reducing competition from native species (Allington et al. 2013; Orrock et al. 2008; Shea and Chesson 2002; Veech 2001).
Effects of fire on invasion and the herbaceous plant community
Fire decreased B. rubens density with little to no effect on cover and biomass (Fig. 1, Table 2). Shortly after fire, B. rubens has been known to decrease in abundance most likely due to high seed mortality because of the lethal temperatures from the fire (Brooks 2002; Esque et al. 2010b). Fire increased the density, cover, and biomass of Schismus spp. and forbs over time (Fig. 1, Table 2), perhaps due to reduced competition and increasing soil resource availability (Figs. 1 and 6) (Chambers et al. 2007; Melgoza et al. 1990; Shea and Chesson 2002). Increased soil nutrients in post-fire environments (Allen et al. 2011; Horn et al. 2017) can lead to increased density, biomass, and cover in annual plants which is consistent with the responses of Schismus spp. and forbs in our study (Figs. 1 and 6; Table 2) (Allen et al. 2011; Steers and Allen 2012). Schismus spp.’s positive response to fire (Fig. 1) appears to have reduced the abundance (Fig. 6) and Shannon’s diversity of forbs (Brooks 2000; Steers and Allen 2012). Due to its distinctive phenology, early season invaders such as Schismus spp. may be superior competitors in a burned environment by capitalizing on the post-fire nitrogen pulse and water earlier than most native plants (Fig. 6) (Brooks et al. 2004; Esque et al. 2010a; Melgoza et al. 1990). This may explain why Shannon’s diversity of herbaceous plants was lowest in burned plots (Fig. 2), where Schismus densities were highest (Fig. 1) along with observed increases in Erodium cicutarium (exotic forb) in burned plots.
The effects of fire on plant and rodent community diversity
Fire decreased Shannon’s diversity of the herbaceous plant community, but not plant species richness in our study (Fig. 2). Disturbance initially can decrease diversity by removing individual plants and altering herbivore behavior (Grime 1973; Horn et al. 2012). But as succession continues, diversity can increase until competitive effects are manifested (Catford et al. 2012; Shea et al. 2004). Native plant diversity has shown positive responses after fire in the Mojave, particularly with long disturbance-free time periods (Vamstad and Rotenberry 2010). However, increasing the frequency of disturbance likely favors the fast, early successional invasive grass species where niche pre-emption or environment transformation can decrease local diversity long term (Catford et al. 2012). Species such as Schismus spp. or B. rubens have faster growth and higher propagule production, which means that they can pre-empt native species inhabiting microsites and niche space (niche pre-emption) (Catford et al. 2012). Also, transforming native shrubland by decreasing shrub cover (> 80% in this study) and density into invasive grassland will promote fire and establish an alternate transient state (Brooks et al. 2004; Fukami and Nakajima 2011), preventing the community from attaining greater biodiversity in the future (Catford et al. 2012).
Fire decreased rodent Shannon’s diversity as also observed by Horn et al. (2012), but not species richness or abundance (Fig. 3). This was likely because Dipodomys merriami, a rodent species that spends more time in open spaces, compensated for the losses of quadrupedal species which prefer shrub cover and emigrated from burned areas (Horn et al. 2012; Ostoja and Schupp 2009). The loss of shrub cover (> 80%) due to the experimental fires increased open canopy space and increased inter-shrub space (Bishop, unpublished data) perhaps providing residual soil nutrients for plant growth (Horn et al. 2017). Exotic grasses such as Schismus spp. and B. rubens re-establish plant cover after fire (Brooks et al. 2004; St. Clair et al. 2016), but do not provide the same cover structure as native shrubs (Freeman et al. 2014; St. Clair et al. 2016). This interpretation is supported by our data showing rodent Shannon’s diversity was negatively correlated with forb Shannon’s diversity and B. rubens growth (Fig. 4).
The role of competition in structuring an invasive annual grass community
Competitive exclusion by exotic plant species can reduce native plant establishment in both unburned and burned environments (Brooks 2000). Bromus rubens and Schismus spp. negatively affected the establishment of forbs (Fig. 6) (Brooks 2000; DeFalco et al. 2007). In one study, however, higher precipitation increased cover and biomass of a native forb even in the presence of invasive grasses, which may indicate some native species have competitive abilities toward exotic grasses dependent on temporal availability of resources (Rao and Allen 2010). There was evidence of strong competition between Schismus spp. and B. rubens in our study (Figs. 5 and 6). One mechanism by which two strong competitors can maintain co-existence on the same landscape is through spatial niche differentiation (Davis et al. 2000; Shea and Chesson 2002). Open shrub interspaces are a common characteristic of vegetation patterns in hyper-arid deserts. At the beginning of our experiment, B. rubens was commonly found throughout the plots and was one of the few species inhabiting this open space. When Schismus spp. appeared, possible direct plant–plant competition may have led to a reduction of B. rubens over time (Fig. 1). An indication that Schismus spp. is driving niche differentiation is seen in the strengthening of the negative spatial correlation as Schismus spp. enters and increases in the experimental plots over time (Fig. 5), with B. rubens increasing under native shrubs (Horn et al. 2017; Price and Joyner 1997), and Schismus spp. becoming dominant in the inter-shrub space (Pucheta et al. 2011) (Bishop, personal observation).
Conclusion
The activity of rodent communities may be a critical mitigating factor in establishment of the invasive grass–fire cycle in the Mojave Desert (Fig. 6). The loss of diversity in the rodent community due to fires (Fig. 3) may provide a window of opportunity for B. rubens to increase propagule pressure (Fig. 4) and overcome predation control from rodents and facilitate invasion. This positive feedback between rodent herbivory in post-fire environments and B. rubens growth may increase the size and frequency of wildfires in the Mojave Desert by providing more flammable and continuous fine fuels (Fig. 4) (Brooks and Matchett 2006; St. Clair and Bishop 2019; Steers and Allen 2012). In the Great Basin, St. Clair and Bishop (2019) showed that reduction in rodent activity in a post-fire environment created areas with high Bromus tectorum (cheatgrass) propagule pressure that led to more severe secondary fires. However, our data also suggest that if rodent consumer activity is high, the differential effects of rodent herbivory between B. rubens and Schismus spp. could cause Schismus spp. to become a dominant fuel in the inter-shrub space. Schismus spp. has shorter stature and does not carry fire as well as B. rubens (Brooks 1999a); therefore, fire ignition and spread may be less severe when Schismus spp. abundance increases. How invasive grasses compete with native and exotic forbs is also important in understanding the ecological underpinnings of invasive grass–fire cycles and state changes in arid ecosystems. As forbs have less favorable fuel properties for spreading fire, years with higher precipitation or low herbivory that favor forbs may lead to a forb-dominated community with lower fire risk (Brooks 2000; Schutzenhofer and Valone 2006). In summary, our data indicate that shifts in plant community composition in response to fire, rodent activity, and competitive interactions among plant species can influence the fuel structure of the vegetation that determines vulnerability to invasive grass–fire cycles.
References
Allen EB, Steers RJ, Dickens SJ (2011) Impacts of fire and invasive species on desert soil ecology. Rangel Ecol Manag 64:450–462. https://doi.org/10.2111/rem-d-09-00159.1
Allington GR, Koons DN, Morgan Ernest S, Schutzenhofer MR, Valone TJ (2013) Niche opportunities and invasion dynamics in a desert annual community. Ecol Lett 16:158–166
Beatley JC (1966) Ecological status of introduced brome grasses (Bromus spp) in desert vegetation of southern Nevada. Ecology 47:548–554. https://doi.org/10.2307/1933931
Beatley JC (1969) Dependence of desert rodents on winter annuals and precipitation. Ecology 50:721–724. https://doi.org/10.2307/1936267
Beatley JC (1976) Environments of kangaroo rats (Dipodomys) and effects of environmental change on populations in southern Nevada. J Mammal 57:67–93. https://doi.org/10.2307/1379513
Bowman DMJS et al (2011) The human dimension of fire regimes on Earth. J Biogeogr 38:2223–2236. https://doi.org/10.1111/j.1365-2699.2011.02595.x
Bowman TRS, McMillan BR, St Clair SB (2017) Rodent herbivory and fire differentially affect plant species recruitment based on variability in life history traits. Ecosphere 8:10. https://doi.org/10.1002/ecs2.2016
Brooks ML (1995) Benefits of protective fencing to plant and rodent communities of the western Mojave desert, California. Environ Manag 19:65–74. https://doi.org/10.1007/bf02472004
Brooks ML (1999a) Alien annual grasses and fire in the Mojave desert. Madroño 46:13–19
Brooks ML (1999b) Effects of protective fencing on birds, lizards, and black-tailed hares in the western Mojave desert. Environ Manag 23:387–400. https://doi.org/10.1007/s002679900194
Brooks ML (2000) Competition between alien annual grasses and native annual plants in the Mojave Desert. Am Midl Nat 144:92–108. https://doi.org/10.1674/0003-0031(2000)144%5b0092:cbaaga%5d2.0.co;2
Brooks ML (2002) Peak fire temperatures and effects on annual plants in the Mojave Desert. Ecol Appl 12:1088–1102. https://doi.org/10.1890/1051-0761(2002)012%5b1088:pftaeo%5d2.0.co;2
Brooks ML, Chambers JC (2011) Resistance to invasion and resilience to fire in desert shrublands of North America. Rangel Ecol Manag 64(5):431–438
Brown JH, Heske EJ (1990) Control of a desert-grassland transition by a keystone rodent guild. Science 250:1705–1707
Brooks ML, Matchett JR (2003) Plant community patterns in unburned and burned blackbrush (Coleogne ramosissima Torr.) shrublands in the Mojave Desert. W North Am Nat 63(3):2
Brooks ML, Matchett JR (2006) Spatial and temporal patterns of wildfires in the Mojave Desert, 1980–2004. J Arid Environ 67:148–164
Brooks ML, D’Antonio CM, Richardson DM, Grace JB, Keeley JE, DiTomaso JM, Hobbs RJ, Pellant M, Pyke D (2004) Effects of invasive alien plants on fire regimes. BioScience 54(7):677–688
Brooks ML, Brown CS, Chambers JC, D’Antonio CM, Keeley JE, Belnap J (2016) Exotic annual Bromus invasions: comparisons among species and ecoregions in the Western United States. In: Germino MJ, Chambers JC, Brown CS (eds) Exotic Brome-Grasses in arid and semiarid ecosystems of the Western US: causes, consequences, and management implications. Springer International Publishing, Cham, pp 11–60
Catford JA et al (2012) The intermediate disturbance hypothesis and plant invasions: implications for species richness and management. Perspect Plant Ecol Evol Syst 14:231–241. https://doi.org/10.1016/j.ppees.2011.12.002
Chambers JC, Roundy BA, Blank RR, Meyer SE, Whittaker A (2007) What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecol Monogr 77:117–145. https://doi.org/10.1890/05-1991
Cubera E, Nunes JM, Madeira M, Gazarini L (2009) Influence of Quercus ilex trees on herbaceous production and nutrient concentrations in southern Portugal. J Plant Nutr Soil Sci 172:565–571
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Ann Rev Ecol Syst 23:63–87
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534. https://doi.org/10.1046/j.1365-2745.2000.00473.x
DeFalco LA, Fernandez GCJ, Nowak RS (2007) Variation in the establishment of a non-native annual grass influences competitive interactions with Mojave Desert perennials. Biol Invasions 9:293–307. https://doi.org/10.1007/s10530-006-9033-5
Elton CS (2000) The ecology of invasions by animals and plants. University of Chicago Press, Chicago. ISBN-10: 0226206386, ISBN-13: 978-0226206387
Esque TC, Kaye JP, Eckert SE, DeFalco LA, Tracy CR (2010a) Short-term soil inorganic N pulse after experimental fire alters invasive and native annual plant production in a Mojave Desert shrubland. Oecologia 164:253–263. https://doi.org/10.1007/s00442-010-1617-1
Esque TC, Young JA, Tracy CR (2010b) Short-term effects of experimental fires on a Mojave Desert seed bank. J Arid Environ 74:1302–1308. https://doi.org/10.1016/j.jaridenv.2010.04.011
Filazzola A, Sotomayor DA, Lortie CJ (2018) Modelling the niche space of desert annuals needs to include positive interactions. Oikos 127:264–273. https://doi.org/10.1111/oik.04688
Freeman ED, Sharp TR, Larsen RT, Knight RN, Slater SJ, McMillan BR (2014) Negative effects of an exotic grass invasion on small-mammal communities. PLoS One 9:7. https://doi.org/10.1371/journal.pone.0108843
Fukami T, Nakajima M (2011) Community assembly: alternative stable states or alternative transient states? Ecol Lett 14:973–984
Gelbard JL, Belnap J (2003) Roads as conduits for exotic plant invasions in a semiarid landscape. Conserv Biol 17:420–432. https://doi.org/10.1046/j.1523-1739.2003.01408.x
Germino MJ, Belnap J, Stark JM, Allen EB, Rau BM (2016) Ecosystem impacts of exotic annual invaders in the genus Bromus. In: Germino M, Chambers J, Brown C (eds) Exotic brome-grasses in arid and semiarid ecosystems of the Western US. Springer series on environmental management. Springer, Cham
Gill RA, O’Connor RC, Rhodes A, Bishop TB, Laughlin DC, St. Clair SB (2018) Niche opportunities for invasive annual plants in dryland ecosystems are controlled by disturbance, trophic interactions, and rainfall. Oecologia 187:1–11. https://doi.org/10.1007/s00442-018-4137-z
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344
Gutterman Y (1994) Strategies of seed dispersal and germination in plants inhabiting deserts. Bot Rev 60:373–425
Herrick JE, Schuman GE, Rango A (2006) Monitoring ecological processes for restoration projects. J Nat Conserv 14(3–4):161–171. https://doi.org/10.1016/j.jnc.2006.05.001
Horn KJ, McMillan BR, St Clair SB (2012) Expansive fire in Mojave Desert shrubland reduces abundance and species diversity of small mammals. J Arid Environ 77:54–58. https://doi.org/10.1016/j.jaridenv.2011.10.003
Horn KJ, Wilkinson J, White S, St Clair SB (2015) Desert wildfire impacts on plant community function. Plant Ecol 216:1623–1634. https://doi.org/10.1007/s11258-015-0546-9
Horn KJ, Bishop TBB, St. Clair SB (2017) Precipitation timing and soil heterogeneity regulate the growth and seed production of the invasive grass red brome. Biol Invasions 19:1339–1350. https://doi.org/10.1007/s10530-016-1348-2
Inouye RS, Byers GS, Brown JH (1980) Effects of predation and competition on survivorship, fecundity, and community structure of desert annuals. Ecology 61:1344–1351
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. https://doi.org/10.1016/s0169-5347(02)02499-0
Lefcheck JS (2016) piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol 7:573–579
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989. https://doi.org/10.1111/j.1461-0248.2004.00657.x
Mack RN (1981) Invasion of Bromus tectorum L. into western North America—an ecological chronicle. Agro-Ecosyst 7:145–165. https://doi.org/10.1016/0304-3746(81)90027-5
Maron JL, Pearson DE, Potter T, Ortega YK (2012) Seed size and provenance mediate the joint effects of disturbance and seed predation on community assembly. J Ecol 100:1492–1500. https://doi.org/10.1111/j.1365-2745.2012.02027.x
Melgoza G, Nowak RS, Tausch RJ (1990) Soil water exploitation after fire: competition between Bromus tectorum (cheatgrass) and two native species. Oecologia 83:7–13. https://doi.org/10.1007/BF00324626
Orrock JL, Witter MS, Reichman O (2008) Apparent competition with an exotic plant reduces native plant establishment. Ecology 89:1168–1174
Ostoja SM, Schupp EW (2009) Conversion of sagebrush shrublands to exotic annual grasslands negatively impacts small mammal communities. Divers Distrib 15:863–870. https://doi.org/10.1111/j.1472-4642.2009.00593.x
Pearson DE, Potter T, Maron JL (2012) Biotic resistance: exclusion of native rodent consumers releases populations of a weak invader. J Ecol 100:1383–1390. https://doi.org/10.1111/j.1365-2745.2012.02025.x
Pearson DE, Hierro JL, Chiuffo M, Villarreal D (2014) Rodent seed predation as a biotic filter influencing exotic plant abundance and distribution. Biol Invasions 16:1185–1196. https://doi.org/10.1007/s10530-013-0573-1
Pérez-Harguindeguy N, Díaz S, Vendramini F, Cornelissen JH, Gurvich DE, Cabido M (2003) Leaf traits and herbivore selection in the field and in cafeteria experiments. Aust Ecol 28:642–650
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Pinheiro J et al. (2017) Package ‘nlme’. Linear and nonlinear mixed effects models, version:3-1
Price MV, Joyner JW (1997) What resources are available to desert granivores: seed rain or soil seed bank? Ecology 78:764–773
Pucheta E, Garcia-Muro VJ, Rolhauser AG, Quevedo-Robledo L (2011) Invasive potential of the winter grass Schismus barbatus during the winter season of a predominantly summer-rainfall desert in Central-Northern Monte. J Arid Environ 75:390–393. https://doi.org/10.1016/j.jaridenv.2010.11.010
Rao LE, Allen EB (2010) Combined effects of precipitation and nitrogen deposition on native and invasive winter annual production in California deserts. Oecologia 162:1035–1046. https://doi.org/10.1007/s00442-009-1516-5
Safriel U, Adeel Z, Niemeijer D, Puigdefabregas J, White R, Lal R, Winslow M, Ziedler J, Prince S, Archer E, King C, Shapiro B, Wessels K, Nielsen T, Portnov B, Reshef I, Thonell J, Lachman E, McNab D (2005) Millenium ecosystem assessment. In: El-Kassas M, Ezcurra E (eds) Current state and trends, Chapter 22, Dryland systems. A report of the millennium ecosystem assessment. World Resource Institute. http://www.millenniumassessment.org/documents/documents
Schafer JL, Mudrak EL, Haines CE, Parag HA, Moloney MA, Holzapfel C (2012) The association of native and non-native annual plants with Larrea tridentata (creosote bush) in the Mojave and Sonoran Deserts. J Arid Environ 87:129–135. https://doi.org/10.1016/j.jaridenv.2012.07.013
Schutzenhofer MR, Valone TJ (2006) Positive and negative effects of exotic Erodium cicutarium on an arid ecosystem. Biol Cons 132:376–381
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176. https://doi.org/10.1016/S0169-5347(02)02495-3
Shea K, Roxburgh SH, Rauschert ES (2004) Moving from pattern to process: coexistence mechanisms under intermediate disturbance regimes. Ecol Lett 7:491–508
Shipley B (2009) Confirmatory path analysis in a generalized multilevel context. Ecology 90:363–368. https://doi.org/10.1890/08-1034.1
Soil Survey Staff (2015) Web soil survey. natural resources conservation service, United States Department of Agriculture
St. Clair SB, Bishop TBBB (2019) Loss of biotic resistance and high propagule pressure promot invasive grass–fire cycles. J Ecol. https://doi.org/10.1111/1365-2745.13156
St. Clair SB, O’Connor R, Gill R, McMillan B (2016) Biotic resistance and disturbance: rodent consumers regulate post-fire plant invasions and increase plant community diversity. Ecology 97:1700–1711. https://doi.org/10.1002/ecy.1391
Steers RJ, Allen EB (2012) Impact of recurrent fire on annual plants: a case study from the western edge of the Colorado Desert. Madrono 59:14–24
Vamstad MS, Rotenberry JT (2010) Effects of fire on vegetation and small mammal communities in a Mojave Desert Joshua tree woodland. J Arid Environ 74:1309–1318. https://doi.org/10.1016/j.jaridenv.2010.04.002
Veech JA (2001) The foraging behavior of granivorous rodents and short-term apparent competition among seeds. Behav Ecol 12:467–474. https://doi.org/10.1093/beheco/12.4.467
Vilà M et al (2010) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front Ecol Environ 8:135–144
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499. https://doi.org/10.1126/science.277.5325.494
Western Regional Climate Center (2000) Western regional climate center. Tuweep, Arizona Station Report, Reno, Nevada, USA
Whisenant SG (1992) Changing fire frequencies on Idaho’s Snake River plains: ecological and management implications: General Technical Report — US Department of Agriculture, Forest Service, INT-276, 1990, pp 4–10. Biol Conserv 59(2):276. https://doi.org/10.1016/0006-3207(92)90659-B
Acknowledgements
We sincerely appreciate the support of the St. George Bureau of Land Management office and the Lytle Ranch Preserve for logistical support of this study and Adam Heyder and his fire crew for burning the experimental plots. We express gratitude for field work conducted by Rory O’Connor, Justin Taylor, Tiffanny Sharp-Bowman, and Samantha Phillips. We also express appreciation to Dr. Matthew Heaton for his advice and guidance on statistical analysis and Michael Bishop for graphic design and editing on figures.
Funding
This research was funded with generous support by Brigham Young University, Sant Educational Endowment for a Sustainable Environment, the Redd Center, the United States Department of Interior BLM, and United States Department of Agriculture NIFA award number 2010-04092.
Author information
Authors and Affiliations
Contributions
SBS and BRM conceived the study design and SBS, BRM, and RAG set up the experiment, TBBB and BRM collected the data, TBBB analyzed the data and produced figures, and TBBB led the writing of the manuscript with the assistance of SBS and feedback from BRM and RAG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human and/or animal participants
This article does not contain any studies with human participants. Small mammal survey protocols were approved by the Brigham Young University Animal Care and Use Committee (IACUC#120202).
Additional information
Communicated by Edith B. Allen.
Rights and permissions
About this article
Cite this article
Bishop, T.B.B., Gill, R.A., McMillan, B.R. et al. Fire, rodent herbivory, and plant competition: implications for invasion and altered fire regimes in the Mojave Desert. Oecologia 192, 155–167 (2020). https://doi.org/10.1007/s00442-019-04562-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04562-2