Abstract
Recent studies have reported positive net diversity effects on aboveground tree growth. However, whether similar effects occur belowground through root investment, and whether such effects are related to evergreenness of tree communities, is less clear. Here we studied vertical distribution of standing fine root biomass of twelve North American temperate tree species planted in a common garden tree diversity experiment of varying species richness and evergreenness to test whether belowground niche complementarity of trees could explain positive diversity effects reported aboveground. We tested two alternative hypotheses: trees in mixtures increase uptake of soil resources (1) by increasing vertical root stratification and/or producing a greater fine root density (mg cm−3) or (2) by producing similar or fewer fine roots that are potentially more efficient. Additionally, we hypothesized that proportional allocation to belowground biomass increases with evergreenness of tree communities. Fine roots were sampled in six layers of 5–10 cm, from 0 to 40 cm depth in single-, two- and four-species mixtures. We did not observe an effect of species richness on rooting depth or root density, refuting the hypothesis that aboveground overyielding in tree mixtures is linked to fine root overyielding. Rather, we observed a significant negative diversity effect (− 7.6%) on total fine root density, suggesting overall less investment to fine roots with increasing diversity. The strong positive effect of evergreeness on proportional allocation to fine roots over aboveground parts suggests that deciduous tree roots may be generally more efficient at absorbing soil resources, at least in the early years after tree establishment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Awareness about the high rate of species extinction has resulted in increasing research efforts to understand the consequences of biodiversity loss on ecosystem functioning (Cardinale et al. 2012; Hooper et al. 2012). Among the many positive effects of biodiversity on ecosystem functioning, the general increase in aboveground biomass yield (i.e. positive diversity effects) found in many terrestrial ecosystems, including forests, is of particular interest (Nadrowski et al. 2010; Paquette and Messier 2011; Zhang et al. 2012). However, the underlying biological mechanisms for these positive biodiversity effects on tree productivity remain poorly understood, and a better mechanistic understanding is generally recognized as a priority for biodiversity–ecosystem function research (Cardinale et al. 2012; Loreau et al. 2012; Tobner et al. 2016). One promising approach is to improve our understanding of belowground investment to tree roots, given that belowground biomass in forest ecosystems represents between 43 and 60% of total annual net primary production and tree roots store large amounts of carbon and nutrients, playing an important role in the dynamics of these elements (Cairns et al. 1997).
Despite their importance, how tree roots directly interact belowground and compete for limiting soil resources, i.e. if they partition in space or time to reduce interspecific competition, is still an active field of research (Bardgett et al. 2014; Jones 2015; Iversen et al. 2017). The few existing biodiversity studies on tree root biomass show mixed results, making generalizations difficult, in part due to effects that seem to vary with stand age (Ma and Chen 2016, 2017). In herbaceous communities, several studies reported that root biomass was positively correlated with diversity (Cardinale et al. 2007; Mueller et al. 2013; Ravenek et al. 2014). However, in studies on tree and forest ecosystems, negative (McKay and Malcolm 1988, Bolte and Villanueva 2006), neutral (Bauhus et al. 2000; Meinen et al. 2009a, b; Jacob et al. 2013; Domisch et al. 2015; Finér et al. 2017), and positive (Lei et al. 2012a; Brassard et al. 2011, 2013; Sun et al. 2017) diversity effects on fine root biomass have been reported.
Such positive or negative net biodiversity effects, when observed, can be partitioned into selection and complementarity effects (Loreau and Hector 2001; Loreau et al. 2012), which are understood to be associated with the diversity of functional traits in communities (Cadotte 2011; Handa et al. 2014). The selection effect follows from the mass ratio hypothesis (Grime 1998) and states that ecosystem functioning is driven by the local dominance of species with particular traits (Loreau et al. 2001). The complementarity effect, on the other hand, results from interspecific differences and species interactions, and combines both niche partitioning and facilitation among species (Loreau and Hector 2001), both resulting in reduced competition. For example, in plants, niche complementarity might include a better capture (and possibly use) of light (aerial) (Yachi and Loreau 1999) and soil resources (water, nutrients) (Fargione et al. 2007). However, concrete tests of these complementarity hypotheses remain scarce (Sapijanskas et al. 2014; Tobner et al. 2013b).
When applied to tree root systems, contrasting complementarity hypotheses might explain the aboveground diversity effect often observed in mixed tree communities (Potvin and Gotelli 2008; Tobner et al. 2016). First, niche partitioning could lead to a higher total root biomass in mixed forests compared to their component monocultures, increasing soil resource uptake. For example, roots of some tree species could penetrate different depths to explore additional volume or use different patches of soils more intensively for resources (niche differentiation, e.g. organic vs. mineral layers) (Ewel and Mazzarino 2008; Loreau 1998; Pate and Bell 1999). Indeed, numerous studies have documented species occupying different rooting depths (Jackson et al. 1996; Mamolos et al. 1995; Mamolos and Veresoglou 2000), which could lead to a more stable coexistence (Fargione and Tilman 2005). However, other studies did not find such differentiation (Meinen et al. 2009b), and species-specific rooting depths are notoriously difficult to tease apart from site effects in natural settings, where species tend to grow on different soil types and environmental conditions (Brassard et al. 2011; Tobner et al. 2013a). Alternatively, rather than spatial segregation, a contrasting hypothesis is that trees growing in mixed communities show positive aboveground diversity effects due to a reduction in belowground competition by increased efficiency and, therefore, reduced allocation to roots. For example, trees showing stimulated aboveground growth on richer and moister sites have been documented to show no difference or even a reduction in belowground growth (Comeau and Kimmins 1989; Lehtonen et al. 2016).
Another factor in interpreting tree community responses may be linked to contrasting life strategies of evergreen and deciduous trees, evident in different leaf functional traits such as leaf longevity (Kikuzawa et al. 2013; Reich 2014), which may contribute to explaining variation in allocation to roots (Finér et al. 2017). Evergreen trees typically invest more in leaf construction costs, which reflects a more conservative life strategy and likely has repercussions at the whole plant scale, including towards root investment (Reich 2014). In a continued effort to understand allocation in trees, attempts have been made to determine if evergreen trees allocate proportionally more or less belowground than deciduous trees, but results to date are conflicting (e.g. Cairns et al. 1997; Jackson et al. 1996). One problem with such general assessments is that often, studied evergreen and deciduous trees species did not grow on similar soil and climatic conditions, making comparison difficult (e.g. Finér et al. 2007). When both evergreen and deciduous tree species were planted together, results tended to indicate a greater allocation belowground in evergreen tree species, particularly in younger stands (Chen et al. 2016a), in poorer soil (Domisch et al. 2015) or in soil with more organic matter (Finér et al. 2017).
Our study aimed specifically at explaining aboveground positive net diversity effects previously reported on tree biomass yield at our international tree diversity experimental site (Tobner et al. 2016) and elsewhere (Nadrowski et al. 2010; Paquette and Messier 2011; Zhang et al. 2012) by testing whether belowground niche complementarity of fine roots occurs. To do so, we compared the root growth patterns (rooting depth, root density and fine root productivity) of twelve North American temperate tree species (six deciduous and six evergreen tree species) planted in a common garden tree diversity experiment of one, two and four tree species richness. We first tested the hypothesis that soil under increasing tree diversity is more intensively prospected through increasing rooting depth and/or rooting density, which could explain aboveground overyielding. An alternative hypothesis tested was that aboveground overyielding can be achieved with similar, or even less belowground fine root biomass, presumably due to more efficient extraction of soil resources. While testing evergreenness was not an initial consideration in the tree diversity experimental design, evergreen life strategy emerged as a factor of increasing interest as we began to explore tree community responses in fine root investment. Consequently, we tested the additional hypothesis that the proportional allocation to fine roots (over aboveground biomass) and fine root productivity (1 year), increase with the proportion of evergreen tree species present in the plot.
Materials and methods
Site description
The tree diversity experiment was established in 2009 on a former agricultural field that was intensively managed for several decades, and is located in Ste-Anne-de-Bellevue (Lat: 45.4247 Long: − 073.939, alt. 39 m), near Montreal, Québec, Canada (Tobner et al. 2014). The site has an area of 0.6 ha and contains 9472 trees of 12 species native to North American temperate forests that are characteristic to the region (the experiment includes more plots and species not used here; see Tobner et al. 2014 for further details). The species pool, covering a wide range of life-history strategies, includes five broadleaved angiosperm tree species: Acer saccharum, Acer rubrum, Betula alleghaniensis, Betula papyrifera and Quercus rubra, one deciduous gymnosperm, Larix laricina, as well as six evergreen gymnosperm tree species: Abies balsamea, Pinus strobus, Pinus resinosa, Picea glauca, Picea rubens and Thuja occidentalis. Mean annual precipitation is 963 mm with a mean annual temperature of 6.2 °C (climate.weatheroffice.gc.ca). The soil consists of a 20–70-cm sandy layer (91% sand) over a heavier clay layer (soil texture of the 30–40-cm layer was obtained by the Bouyoucos hydrometer method at the plot level). The area is relatively flat and precise elevation was also measured (microtopography) at the plot level using standard surveying equipment (cm; total station theodolite) to account for minor depressions. A fence to protect against herbivory surrounded the experiment and all plots were regularly weeded manually to keep them free of herbaceous competition during the first 3 years (after which all plots had complete canopy closure). Corridors (~ 1.25 m wide) between plots allowed for movement of personnel and equipment, and minimized interplot interactions. Roots were also sliced vertically 30 cm deep in the centre of the corridors in the third and fourth growing seasons (2011 and 2012). Aboveground stem biomass was estimated for 2012 in a previous study (Tobner et al. 2016), as stem volume (stem diameter at 5 cm from ground × tree height) multiplied by species-specific wood density.
The experimental design was described previously and is part of International Diversity Experiment Network with Trees (IDENT) that includes several sites in North America and Europe (Tobner et al. 2014, 2016). Briefly, treatments consist of experimental square plots where trees were planted (8 × 8) at 50-cm spacing, to favour early interactions. The species richness gradient includes monocultures of all 12 species, 14 combinations of two-species mixtures and ten combinations of four-species mixtures. Each community was replicated four times (block design) for a total of 144 plots for the present study. Within plots, trees in mixtures were planted at random with restrictions (at least two of the eight neighbours had to be different species, and the outside rows, used as buffer, had the same species relative abundances as the inner core). Planting patterns within plots were repeated and randomly distributed within blocks.
Rooting depth and root density
We sampled roots from October 2–18, 2012, to capture the peak fine root production that occurs between early spring and late summer in temperate deciduous forests (Burke and Raynal 1994; McCormack et al. 2014). Given that trees do not shed their fine roots in the fall but do so continuously during the year (Hendrick and Pregitzer 1993, 1996) and that peak fine root production periods vary across tree species and even years (McCormack et al. 2014), our choice to sample at the end of the temperate growing season, when leaf senescence was occurring, provided the possibility to capture this potential interspecific variability across treatment plots. Five and three soil cores were sampled per plot in mixed communities and monocultures, respectively, to a depth of 40 cm. In mixed communities, the first core was taken in the centre of the plots and four more were taken in a cross shape 100 cm from the centre. In monocultures, soils were cored at the plot centre and on either side at 100 cm from the plot centre. These positions corresponded to a central point between four trees to maximize species interactions among local tree neighbourhoods. Soil cores were taken using a sledgehammer and a custom-made soil auger which consisted of a steel cylinder (ø = 7 cm) with a sharpened edge to cut the roots and an opening in the side to allow for the sectioning of samples.
Once the soil auger was extracted, each core was segmented into one of six soil layers (0–5, 5–10, 10–15, 15–20, 20–30 and 30–40 cm) and transferred into plastic bags. This division allowed for a better representation of the root distribution in a given volume of soil along the profile. All samples (one layer from one core) were immediately stored in a cold room after sampling in the field and frozen at − 25 °C within a few days, until processing. The washing and sorting of the roots were carried out during the summer and fall of 2013. A total of 624 soil cores were taken that comprised monocultures (144 cores) and each of the two- and four-species mixtures (280 and 200 cores, respectively). Each core was divided into six layers for a total of 3744 samples. Once the soil samples were thawed, roots were manually removed from the soil with a 4-mm sieve stacked over a 1-mm sieve where roots were then washed (Fisher brand). The fine roots that were recovered (dead and alive) were washed with clean water and again with distilled water to remove any residue or remaining soil. All 3744 samples were separated according to diameter (< and > 2 mm). Roots recovered from the cores were dried at 60 °C and weighed (0.001 g) to calculate root distribution (vertical organization) and root density of the belowground biomass. For this study, only fine roots (< 2 mm) were reported since they comprised the vast majority of the roots sampled and were found in all vertical layers. Coarse roots (> 2 mm) occurred in only 29% of the samples and both their distributions within the soil core and across treatments were highly unbalanced. Dead and live fine roots were not differentiated, but the majority of fine roots recovered were intact, tough and flexible, indicating that they were mostly alive.
Root productivity
Fine root productivity (≤ 2 mm diameter) was measured over 1 year using a modified ingrowth core method (Lund et al. 1970). In each plot in early June 2012, two cores (8 cm diameter, 15 cm depth) were sampled randomly in a central point between four trees. The cores were then refilled with sieved, root-free soil. The exact position of ingrowth core boundaries was marked with flags to ensure sampling of the same placement when retrieving the cores. In early June 2013, the ingrowth cores were retrieved and all live and dead roots were manually removed from the soil samples, washed and then dried in a forced-air oven at 65 °C to constant weight. New root production was estimated as the sum of live and dead roots present in the ingrowth core in June 2013.
Data analysis
The effect of species richness (SR) was first tested on the four response variables: percentiles (cm), i.e. the depth at which 10, 25, 50, 75 and 90% of the total fine root biomass was found; vertical dispersion index (cm), i.e. the vertical spread of the central portion of fine root biomass (difference between 75th and 25th percentile); weighted mean depth, i.e. the vertical centre of gravity of the fine roots (cm; Eq. 1); and fine root mean density (mean density; mg cm−3) of every layer. For ease of interpretation and because mean depth provides more information, the vertical dispersion index and percentiles, for which no diversity effect was found, are not presented.
where M is the mass of fine roots (mg) at a given layer (cm), weighted by the centre of that layer.
To test for an effect of species richness on our responses variables (Y) for mean rooting depth, mean root density, root productivity and below:above allocation ratio (n = 144), a simple generalized mixed effects model with restricted maximum likelihood estimation (REML) was applied (Eq. 2), with SR, initial (planted) proportion of evergreen species (“RatioEvergreen”), texture (% sand, “Sand”) and elevation microtopography (cm, “Elevation”) as fixed effects. Sand and elevation were included as covariates to account for small differences in soil texture and drainage at the plot scale that had a significant effect on predicting aboveground growth (Tobner et al. 2016). Random effects were blocks, that integrated a broader spatial scale of environmental variation, and plots that were nested within SR levels (1, 2 and 4), to account for the intended wide range of species compositions within each level by design (Tobner et al. 2016).
To test if there was a net diversity effect associated with the fine root systems of our tree communities (n = 96), the proportional deviation was calculated (Eq. 3; Loreau 1998):
where DT is the proportional deviation of the total fine root biomass (0–40 cm) of each mixture (i.e. the net biodiversity effect), OT is the observed fine root biomass in each mixture, and ET is the expected value estimated from the weighted average yields of the component monocultures (weighted by the initial relative abundance of species in mixture). A net diversity effect occurs if the species in mixtures have a higher or lower yield than their respective single-species plots. DT was validated by testing a 95% confidence interval to see if there was a significant deviation from zero (no effect of diversity).
Statistical analyses were performed using JMP 10.0 software (SAS Institute Inc., Cary, NC, USA).
Results
Mean fine root depth, density, productivity and below:aboveground biomass ratio among tree communities
Mean fine rooting depth of tree communities ranged from 9.6 to 18.8 cm overall (Fig. 1). Species richness had no effect on mean rooting depth, which was 13.6 ± 0.3 cm across all treatments (Table 1; Fig. 1). Maximum rooting depth could not be identified to the nearest cm because the soil core was sectioned into depth layers. However, we observed that very few trees rooted in the 30- to 40-cm layer (on average, less than 3% of fine root biomass across all treatments).
Vertical root distribution (mean ± SEM, n = 4 for each community type) for a monocultures (N = 48), b two-species (N = 56) and c four-species (N = 40) mixtures comprising pure evergreen (blue), mixed evergreen–deciduous (grey) and pure deciduous (red) communities. The overall weighted mean depth for all communities was 13.6 cm. ABBA—Abies balsamea, LALA—Larix laricina, PIGL—Picea glauca, PIRE—Pinus resinosa, PIRU—Picea rubens, PIST—Pinus strobus, THOC—Thuja occidentalis, ACRU—Acer rubrum, ACSA—Acer saccharum, BEAL—Betula alleghaniensis, BEPA—Betula papyrifera and QURU—Quercus rubra (colour figure online)
Mean fine root densities of trees were not affected by species richness (Table 1, Fig. 2). Overall, mean fine root densities ranged from 0.74 to 1.73 mg cm−3 with mean fine root densities of 0.81 ± 0.04 mg cm−3, 0.73 ± 0.04 mg cm−3 and 0.78 ± 0.04 mg cm−3 for monocultures, two- and four-species mixtures, respectively. We observed a strong and significant increase in fine root density with an increasing proportion of evergreen species in the mixture (Table 1). Density was highest in pure evergreen (mean 1.02 ± 0.03 mg cm−3; either monocultures or mixtures containing only evergreen species) compared to pure deciduous (0.59 ± 0.03 mg cm−3) tree communities (Fig. 2). Among conifer species, T. occidentalis had the highest fine root density (1.48 mg cm−3), while L. laricina, a deciduous gymnosperm, had the lowest (0.73 mg cm−3). As for deciduous broadleaved tree species, Q. rubra had the highest fine root density (0.82 mg cm−3), while A. saccharum had the lowest (0.40 mg cm−3). Fine root density for the majority of species was two times higher at 0–15 cm depth than at 15–30 cm, except for two notable exceptions: T. occidentalis which was three times higher in the 0–15 cm depth than at 15–30 cm depth, while Q. rubra showed no significant difference between depth layers.
Mean root density (± SEM, n = 4 for each community type) for a monocultures (N = 48), b two-species (N = 56) and c four-species (N = 40) tree communities shown by rooting depth layer (from left to right with decreasing colour saturation: 0–5, 5–10, 10–15, 15–20, 20–30, 30–40 cm depth). The overall mean fine root density for pure evergreen (blue), mixed deciduous–evergreen (grey) and pure deciduous (red) was 1.02 mg cm−3, 0.71 mg cm−3 and 0.59 mg cm−3, respectively. See Fig. 1 legend for species abbreviations (colour figure online)
Species richness did not affect fine root productivity (Table 1, Fig. 3). Mean fine root productivity of tree communities ranged from 2.46 to 19.91 g m−2 year−1 with mean fine root productivity of 9.99 ± 0.89 g m−2 year−1, 10.45 ± 0.81 g m−2 year−1 and 9.49 ± 0.83 g m−2 year−1 for monocultures, two- and four-species mixtures, respectively. We observed a significant increase in fine root productivity with an increasing proportion of evergreen species in the mixture (Table 1). Fine root productivity was higher in pure evergreen (11.99 ± 2.64 g m−2 year−1) compared to pure deciduous (7.87 ± 2.03 g m−2 year−1) tree communities. A notable exception was L. laricina (the deciduous gymnosperm) which had the second highest fine root productivity (Fig. 3). Among monoculture conifer species, P. rubens had the highest fine root productivity (19.91 g m−2 year−1), while P. resinosa had the lowest (8.09 g m−2 year−1). For monoculture deciduous broadleaved tree species, A. rubrum had the highest fine root productivity (9.35 g m−2 year−1), while A. saccharum had the lowest (2.46 g m−2 year−1).
Mean root productivity (± SEM, n = 4 for each community type) for a monocultures (N = 48), b two-species (N = 56) and c four-species (N = 40) tree communities. The overall mean root density for pure evergreen (blue), mixed deciduous–evergreen (grey) and pure deciduous (red) was 12.01 g m−2 year−1, 9.57 g m−2 year−1 and 8.48 g m−2 year−1, respectively. See Fig. 1 legend for species abbreviations (colour figure online)
Biomass preferential allocation to roots or aboveground parts (below:aboveground ratio) was the most responsive response variable, with the greatest variance explained. Species richness had a significant positive effect demonstrating greater allocation to roots (P = 0.016; Table 1), but the most important fixed effect was the proportion of evergreen species (P < 0.0001).
There was also a small, but significant positive effect of elevation on root density (P = 0.049), and a stronger effect on biomass preferential allocation to roots (P < 0.001). Blocks were never significant, but the other random effect plot[SR], was always important, accounting for variation in composition within each SR level (Tobner et al. 2014).
Net diversity effect on fine root density
There was an overall significant negative diversity effect (DT = − 0.076, P < 0.05; Fig. 4), which translated to an average 7.6% reduction in total fine root density (0–40 cm) in mixtures compared to monocultures. However, this effect was not different among species richness levels (P = 0.83). One mixture presented positive deviance and four P < 0.0001 mixtures negative deviance in fine root density from that expected for their component monocultures (Fig. 4). The community composed of A. rubrum, Q. rubra, P. strobus and T. occidentalis produced significantly (P ≤ 0.01) less (− 21%) root density than expected. Similarly, the community composed of Q. rubra, L. laricina, P. strobus and T. occidentalis (-25%), A. rubrum and T. occidentalis (− 33%) and B. papyrifera and P. strobus (− 27%), all mixtures of deciduous and evergreen species, produced marginally significantly (P ≤ 0.1) less root density than expected. In contrast, when two pine species were growing together, P. strobus and P. resinosa, we observed a significant (P ≤ 0.05) positive fine root overyielding (+ 20%).
Net effect of biodiversity (DT) on total fine root biomass (0–40 cm) by tree community for two- and four-species communities. Bars are ± SEM (n = 4 for each community type). Levels of significance are **P ≤ 0.01; *P ≤ 0.05; °P ≤ 0.1. The overall net diversity effect is shown with a dashed line. See Fig. 1 legend for species abbreviations (colour figure online)
Below- versus aboveground biomass in relation to evergreenness
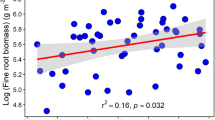
We found a strong and significant negative correlation between above- and belowground parts (r = − 0.4670; P < 0.0001), and this negative relationship was strongly dependent on the level of evergreenness of the tree communities, i.e. the more evergreen species present in the tree community, the greater the proportion of belowground compared to aboveground biomass (Fig. 5; Table 1).
Relationship between total above- and belowground biomass per plot in 2012, for all plots, according to the planted proportion of evergreen species (r = − 0.4670, N = 144). Aboveground biomass data provided by Tobner et al. (2016) (colour figure online)
Discussion
Belowground tree diversity effects
To explain the overall significant and positive effect of tree diversity on aboveground biomass yield reported by Tobner et al. (2016) for the same experiment and growing season (2012), our working hypotheses were that trees had increased soil resource extraction by (1) increasing the soil volume being prospected through differential rooting depths and/or increasing rooting density, or alternatively (2) a more efficient extraction of soil resources per unit root with similar or fewer roots. Our results clearly refute the first hypothesis as neither vertical segregation of roots, nor increasing root density from 0 to 40 cm soil depth, was found along the species richness gradient (Table 1; Figs. 1 and 2). In terms of niche partitioning, our results contradict those observed in pure and mixed temperate forest stands varying from 55 to 152 years of age (Hendriks and Bianchi 1995; Schmid and Kazda 2002; Bolte and Villanueva 2006), but are in agreement with Valverde-Barrantes et al. (2015), who also failed to find any vertical spatial segregation in roots of different tree species growing together in a diverse natural temperate deciduous forest. However, since we could not identify fine roots of specific species, it is possible that although there was no difference in the total distribution of roots within the soil profile with diversity, stratification between species still occurred (e.g. one species grew at the surface while the other used deeper layers). Further investigation using methods that allow us to discriminate species fine roots will be useful to test this hypothesis. In terms of investment towards total root biomass (measured here as fine root density), our lack of an observed response supports results from other young tree biodiversity experimental plantations (Bauhus et al. 2000; Lei et al. 2012b; Domisch et al. 2015), as well as observations of pure and mixed mature forest stands (Meinen et al. 2009a, b; Jacob et al. 2013; Finér et al. 2017), all predominantly in temperate forests. However, other studies in naturally establishing post-fire boreal forest stands (Brassard et al. 2011, 2013; Ma and Chen 2017), as well as in a young subtropical biodiversity experimental plantation (Sun et al. 2017) have reported positive effects on fine root biomass with increasing species richness, suggesting contrasting responses associated with differences in climatic conditions, site fertility and species identity.
Instead, our results are supported by our alternate hypothesis which suggests that the observed aboveground positive diversity effect may be due to increased efficiency of resource extraction, resulting in less allocation towards fine roots with increasing diversity. We reported an overall small, but significant, negative diversity effect on fine root density (Fig. 4). Similar negative tree diversity effects have been reported by McKay and Malcolm (1988) and Bolte and Villanueva (2006) in varying forest types. Such reduction in fine root biomass in mixed forests, that are otherwise overyielding aboveground compared to monocultures, could be explained by a more efficient soil resource uptake per unit of fine root biomass in these mixed tree communities. Although not investigated here, multiple mechanisms could be invoked, such as a more efficient prospection of soil by roots through a greater diversity of soil mycorrhizae and bacteria in and around the roots, or complementarity in root traits among species (Chen et al. 2016b; Bu et al. 2017). For the same study site and field season (2012), Khlifa et al. (2017) found that the soil microbial community associated with mixtures of higher levels of tree diversity used a higher number of soil carbon sources than monocultures, which may indicate greater efficiency. Since the soil of our research site was not nitrogen limited (C:N of 11:13) and no summer drought occurred for 3 out of 4 years of the experiment, it is plausible that any additional biomass production due to tree species mixing was directed not towards the roots, but aboveground. In other words, after 4 years of growth, our high-density tree communities probably competed for light more than they did for belowground resources, although this trend could change as the stand develops and belowground resources become more limited. Spatial complementarity in tree crowns was shown to explain overyielding in species mixtures for the same IDENT site and year (Williams et al. 2017).
As mentioned earlier, when comparing monocultures and mixtures of two species, several studies have found differences in the vertical distribution of fine roots between species, and have attributed this to competition for limited soil resources (Hendriks and Bianchi 1995; Rust and Savill 2000; Schmid and Kazda 2005). It can thus be expected that the intensity of competition is less in soils with a high nutrient availability. Such conditions may be the case in our experiment, as the site is located on former high-yield agricultural soils (relatively low C:N, under corn cultivation just before establishment), possibly explaining the overall net negative diversity effect on root biomass (mean − 7.6%, Fig. 4). However, competition dynamics for water and nutrients in the future may very well change this portrait.
A possible alternative hypothesis for the small but significant overall net negative diversity effect belowground is that root turnover in diverse communities might be faster compared to monocultures. If this is the case, for a similar annual fine root production, there would be less standing fine root biomass at any point during the year in mixtures compared to monocultures. However, in our case, species richness did not affect fine root productivity either (Fig. 3), which does not support this alternative hypothesis. In contrast, Mommer et al. (2015) studied an experimental grassland and found exactly the opposite, i.e. root production was greater and root turnover lower in mixtures compared to monocultures, which explained the reported overyielding observed in this experimental grassland.
Effect of evergreenness
The strong positive relationship found between the proportion of evergreen species and the increased proportion of biomass allocated to fine roots over aboveground growth, and that between proportion of evergreen species and root productivity, confirms our hypothesis and is rather striking. To date, only a handful of studies comparing relatively few tree species have compared fine root biomass for deciduous and evergreen tree species growing in similar conditions, and results vary. A recent paper by Chen et al. (2016a) reported four to five times higher fine root biomass in 10-year-old plantations of Pinus tabulaeformis compared to Robinia pseudoacacia having a similar height and diameter. Interestingly, these differences tended to disappear with age in stands with similar slope and elevation within the study area. In another study, Domisch et al. (2015) reported contrasting results between two sites where mixtures of deciduous and evergreen species were planted in monoculture or in various mixtures in southern Finland. In the richer site, deciduous species produced more fine root biomass per unit tree basal area, whereas the reverse was reported for the poorer site. One possible problem with the latter study is that no control of the competing herbaceous vegetation was done and so, on the richer sites, the slower growing evergreen tree species may simply have suffered more severe competition. Finér et al. (2017), studying the effects of tree species diversity, evergreen proportion, stand basal area and soil properties on fine root biomass across several major European forest types, reported a slight increase in fine root biomass in relation to evergreen proportion only in the organic horizon, but this effect disappeared within the first 20 cm of the mineral soil.
To our knowledge, our common garden experiment is the first to demonstrate for 36 different tree communities with varying proportions of evergreenness that evergreen gymnosperm tree species allocate a larger proportion of their biomass to roots in the early years of life. The question is why? One explanation could be that evergreen species have a lower fine root turnover rate compared to deciduous species, favouring the accumulation at any given point in time of a larger root standing biomass. However, support for this explanation is limited since no clear differences in fine root turnover rates have been reported for evergreen versus deciduous species (Brunner et al. 2013; Augusto et al. 2015). McCormack et al. (2014) even found a positive relationship between root turnover rate and total root production. An alternative explanation could be that since evergreen tree species have evolved to grow on poorer soils, they have less plasticity and are unable to allocate fewer resources to roots on richer sites than deciduous tree species, at least in the early years. Further efforts to test these patterns on a broader gradient of soil fertility and to better understand the role of biotic interactions with tree roots may help elucidate the mechanisms at play.
Data availability statement
The data generated during the current study are not yet publicly available but are available from the corresponding author on reasonable request.
References
Augusto L, De Schrijver A, Vesterdal L, Smolander A, Prescott C, Ranger J (2015) Influences of evergreen gymnosperm and deciduous angiosperm tree species on the functioning of temperate and boreal forests. Biol Rev 90:444–466. https://doi.org/10.1111/brv.12119
Bardgett RD, Mommer L, De Vries FT (2014) Going underground: root traits as drivers of ecosystem processes. Tree 29:692–699. https://doi.org/10.1016/j.tree.2014.10.006
Bauhus J, Khanna PK, Menden N (2000) Aboveground and belowground interactions in mixed plantations of Eucalyptus globulus and Acacia mearnsii. Can J For Res 30:1886–1894. https://doi.org/10.1139/x00-141
Bolte A, Villanueva I (2006) Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur J Forest Res 125:15–26. https://doi.org/10.1007/s10342-005-0075-5
Brassard BW, Chen HYH, Bergeron Y, Paré D (2011) Differences in fine root productivity between mixed- and single-species stands. Funct Ecol 25:238–246. https://doi.org/10.1111/j.1365-2435.2010.01769.x
Brassard BW, Chen HYH, Cavard X, Laganière J, Reich PB, Bergeron Y, Paré D, Yuan Z (2013) Tree species diversity increases fine root productivity through increased soil volume filling. J Ecol 101:210–219. https://doi.org/10.1111/1365-2745.12023
Brunner I, Bakker MR, Björk RG, Hirano Y, Lukac M, Aranda X, Børja I, Eldhuset TD, Helmisaari HS, Jourdan C, Konôpka B, López BC, Miguel Pérez C, Persson H, Ostonen I (2013) Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362:357–372. https://doi.org/10.1007/s11104-012-1313-5
Bu W, Schmid B, Liu X, Härdtle W, von Oheimb G, Liang Y, Sun Z, Huang Y, Bruelheide H, Ma K (2017) Interspecific and intraspecific variation in specific root length drives aboveground biodiversity effects in young experimental forest stands. J Plant Ecol 10:158–169. https://doi.org/10.1093/jpe/rtw096
Burke MK, Raynal DJ (1994) Fine root growth phenology, production, and turnover in a northern hardwood forest ecosystem. Plant Soil 162:135–146. https://doi.org/10.1007/bf01416099
Cadotte MW (2011) The new diversity: management gains through insights into the functional diversity of communities. J Appl Ecol 48:1067–1069. https://doi.org/10.1111/j.1365-2664.2011.02056.x
Cairns MA, Brown S, Helmer EH, Baumgardner GA (1997) Root biomass allocation in the world’s upland forests. Oecologia 111:1–11. https://doi.org/10.1007/s004420050201
Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, Weis JJ (2007) Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci 104:18123–18128. https://doi.org/10.1073/pnas.0709069104
Cardinale BJ, Emmett Duffy J, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature 486:59–67. https://doi.org/10.1038/nature11148
Chen L, Mu X, Yuan Z, Deng Q, Chen Y, Yuan LY, Ryan LT, Kallenbach RL (2016a) Soil nutrients and water affect the age-related fine root biomass but not production in two plantation forests on the Loess Plateau, China. J Arid Environ 135:173–180. https://doi.org/10.1016/j.jaridenv.2016.09.003
Chen W, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016b) Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc Natl Acad Sci 113:8741–8746. https://doi.org/10.1073/pnas.1601006113
Comeau PG, Kimmins JP (1989) Above- and below-ground biomass and production of lodgepole pine on sites with differing soil moisture regimes. Can J For Res 19:447–454. https://doi.org/10.1139/x89-070
Domisch T, Finér L, Dawud S, Vesterdal L, Raulund-Rasmussen K (2015) Does species richness affect fine root biomass and production in young forest plantations? Oecologia 177:581–594. https://doi.org/10.1007/s00442-014-3107-3
Ewel JJ, Mazzarino MJ (2008) Competition from below for light and nutrients shifts productivity among tropical species. Proc Natl Acad Sci 105:18836–18841. https://doi.org/10.1073/pnas.0807216105
Fargione J, Tilman D (2005) Niche differences in phenology and rooting depth promote coexistence with a dominant C4 bunchgrass. Oecologia 143:598–606. https://doi.org/10.1007/s00442-005-0010-y
Fargione J, Tilman D, Dybzinski R, Hille Ris Lambers J, Clark C, Harpole WS, Knops JMH, Reich PB, Loreau M (2007) From selection to complementarity: shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc R Soc B 274:871–876. https://doi.org/10.1098/rspb.2006.0351
Finér L, Helmisaari HS, Lõhmus K, Majdi H, Brunner I, Børja I, Eldhuset T, Godbould D, Grebenc T, Konôpka B, Kraigher H, Möttönen MR, Ohashi M, Oleksyn J, Ostonen I, Uri V, Vanguelova E (2007) Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosystems 141:394–405. https://doi.org/10.1080/11263500701625897
Finér L, Domisch T, Dawud MS, Rauland-Rasmussen K, Vesterdal L, Bouriaud O, Bruelheide H, Jaroszewicz B, Selvi F, Valladares F (2017) Conifer proportion explains fine root biomass more than tree species diversity and site factors in major European forest types. For Ecol Manage 406:330–350. https://doi.org/10.1016/j.foreco.2017.09.017
Grime JP (1998) Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol 86:902–910. https://doi.org/10.1046/j.1365-2745.1998.00306.x
Handa IT, Aerts R, Berendse F, Berg MP, Bruder A, Butenschoen O, Chauvet E, Gessner MO, Jabiol J, Makkonen M, McKie BG, Malmqvist B, Peeters ETHM, Scheu S, Schmid B, van Ruijven J, Vos VCA, Hättenschwiler S (2014) Consequences of biodiversity loss for litter decomposition across biomes. Nature 509:218–221. https://doi.org/10.1038/nature13247
Hendrick RL, Pregitzer KS (1993) Patterns of fine root mortality in two sugar maple forests. Nature 361:59–61
Hendrick RL, Pregitzer KS (1996) Temporal and depth-related patterns of fine root dynamics in northern hardwood forests. J Ecol 84:167–176
Hendriks C, Bianchi F (1995) Root density and root biomass in pure and mixed forest stands of Douglas-fir and beech. NJAS Wageningen J Life Sci 43:321–331
Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, Gonzalez A, Emmett Duffy J, Gamfeldt L, O’Connor MI (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108. https://doi.org/10.1038/nature11118
Iversen CM, McCormack ML, Powell AS, Blackwood CB, Freschet GT, Kattge J, Roumet C, Stover DB, Soudzilovskaia NA, Valverde-Barrantes OJ, van Bodegom PM, Violle C (2017) A global Fine Root Ecology Database to address belowground challenges in plant ecology. New Phytol 215:15–26. https://doi.org/10.1111/nph.14486
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze E-D (1996) Maximum rooting depth of vegetation types at the global scaleMaximum rooting depth of vegetation types at the global scale. Oecologia 108:583–595. https://doi.org/10.1007/BF00329030
Jacob A, Hertel D, Leuschner C (2013) On the significance of belowground overyielding in temperate mixed forests: separating species identity and species diversity effects. Oikos 122:463–473. https://doi.org/10.1111/j.1600-0706.2012.20476.x
Jones A (2015) Belowground fine root productivity, traits, and trees. New Phytol 205:461–462. https://doi.org/10.1111/nph.13222
Khlifa R, Paquette A, Messier C, Reich P, Munson AD (2017) Do temperate tree species diversity and identity influence soil microbial community function and composition? Ecol Evol 7:7965–7974. https://doi.org/10.1002/ece3.3313
Kikuzawa K, Onoda Y, Wright Ian J, Reich Peter B (2013) Mechanisms underlying global temperature related patterns in leaf longevity. Glob Ecol Biogeogr 22:982–993. https://doi.org/10.1111/geb.12042
Lehtonen A, Palviainen M, Ojanen P, Kalliokoski T, Nöjd P, Kukkola M, Penttilä T, Mäkipää R, Leppälammi-Kujansuu J, Helmisaari HS (2016) Modelling fine root biomass of boreal tree stands using site and stand variables. For Ecol Manage 359:361–369. https://doi.org/10.1016/j.foreco.2015.06.023
Lei P, Scherer-Lorenzen M, Bauhus J (2012a) The effect of tree species diversity on fine-root production in a young temperate forest. Oecologia 169:1105–1115. https://doi.org/10.1007/s00442-012-2259-2
Lei P, Scherer-Lorenzen M, Bauhus J (2012b) Belowground facilitation and competition in young tree species mixtures. For Ecol Manage 265:191–200. https://doi.org/10.1016/j.foreco.2011.10.033
Loreau M (1998) Separating sampling and other effects in biodiversity experiments. Oikos 82:600–602. https://doi.org/10.2307/3546381
Loreau M, Hector A (2001) Partitioning selection and complementarity in biodiversity experiments. Nature 412:72–76. https://doi.org/10.1038/35083573
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808. https://doi.org/10.1126/science.1064088
Loreau M, Sapijanskas J, Isbell F, Hector A (2012) Niche and fitness differences relate the maintenance of diversity to ecosystem function: comment. Ecol 93:1482–1487. https://doi.org/10.1890/11-0792.1
Lund ZF, Pearson RW, Buchanan GA (1970) An implanted soil mass technique to study herbicide effects on root growth. Weed Sci 18:279–281
Ma Z, Chen HYH (2016) Effects of species diversity on fine root productivity in diverse ecosystems: a global meta-analysis. Glob Ecol Biogeo 25:1387–1396. https://doi.org/10.1111/geb.12488
Ma Z, Chen HYH (2017) Effects of species diversity on fine root productivity increase with stand development and associated mechanisms in a boreal forest. J Ecol 105:237–245. https://doi.org/10.1111/1365-2745.12667
Mamolos AP, Veresoglou DS (2000) Patterns of root activity and responses of species to nutrients in vegetation of fertile alluvial soil. Plant Ecol 148:245–253. https://doi.org/10.1023/A:1009890630391
Mamolos AP, Elisseou GK, Veresoglou DS (1995) Depth of root activity of coexisting grassland species in relation to N and P additions, measured using nonradioactive tracers. J Ecol 83:643–652. https://doi.org/10.2307/2261632
McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM (2014) Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecol 95:2224–2235. https://doi.org/10.1890/13-1942
McKay HM, Malcolm DC (1988) A comparison of the fine root component of a pure and a mixed coniferous stand. Can J For Res 18:1416–1426. https://doi.org/10.1139/x88-220
Meinen C, Hertel D, Leuschner C (2009a) Biomass and morphology of fine roots in temperate broad-leaved forests differing in tree species diversity: is there evidence of below-ground overyielding? Oecologia 161:99–111. https://doi.org/10.1007/s00442-009-1352-7
Meinen C, Leuschner C, Ryan NT, Hertel D (2009b) No evidence of spatial root system segregation and elevated fine root biomass in multi-species temperate broad-leaved forests. Trees 23:941–950. https://doi.org/10.1007/s00468-009-0336-x
Mommer L, Padilla FM, van Ruijven J, de Caluwe H, Smit-Tiekstra A, Berendse F, de Kroon H (2015) Diversity effects on root length production and loss in an experimental grassland community. Funct Ecol 29:1560–1568. https://doi.org/10.1111/1365-2435.12466
Mueller K, Tilman D, Fornara D, Hobbie S (2013) Root depth distribution and the diversity-productivity relationship in a long-term grassland experiment. Ecology 94:787–793. https://doi.org/10.2307/23436291
Nadrowski N, Wirth C, Scherer-Lorenzen M (2010) Is forest diversity driving ecosystem function and service? Curr Opin Environ Sustain 2:75–79. https://doi.org/10.1016/j.cosust.2010.02.003
Paquette A, Messier C (2011) The effect of biodiversity on tree productivity: from temperate to boreal forests. Glob Ecol Biogeogr 20:170–180. https://doi.org/10.1111/j.1466-8238.2010.00592.x
Pate JS, Bell TL (1999) Application of the ecosystem mimic concept to the species-rich Banksia woodlands of Western Australia. Agrofor Syst 45:303. https://doi.org/10.1023/a:1006218310248
Potvin C, Gotelli NJ (2008) Biodiversity enhances individual performance but does not affect survivorship in tropical trees. Ecol Lett 11:217–223. https://doi.org/10.1111/j.1461-0248.2007.01148.x
Ravenek J, Bessler H, Engels C, Scherer-Lorenzen M, Gessler A, Gockele A, De Luca E, Temperton VM, Ebeling A, Roscher C, Schmid B, Weisser WW, Wirth C, de Kroon H, Weigelt A, Mommer L (2014) Long-term study of root biomass in a biodiversity experiment reveals shifts in diversity effects over time. Oikos 123:1528–1536. https://doi.org/10.1111/oik.01502
Reich PB (2014) The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301. https://doi.org/10.1111/1365-2745.12211
Rust S, Savill P (2000) The root systems of Fraxinus excelsior and Fagus sylvatica and their competitive relationships. Forestry 73:499–503. https://doi.org/10.1093/forestry/73.5.499
Sapijanskas J, Paquette A, Potvin C, Kunert N, Loreau M (2014) Tropical tree diversity enhances light capture through crown plasticity and spatial and temporal niche differences. Ecology 95:2479–2492
Schmid I, Kazda M (2002) Root distribution of Norway spruce in monospecific and mixed stands on different soils. For Ecol Manage 159:37–47. https://doi.org/10.1016/S0378-1127(01)00708-3
Schmid I, Kazda M (2005) Clustered root distribution in mature stands of Fagus sylvatica and Picea abies. Oecologia 144:25–31. https://doi.org/10.1007/s00442-005-0036-1
Sun Z, Liu X, Schmid B, Bruelheide H, Bu W, Ma K (2017) Positive effects of tree species richness on fine-root production in a subtropical forest in SE-China. J Plant Ecol 10:146–157. https://doi.org/10.1093/jpe/rtw094
Tobner CM, Paquette A, Messier C (2013a) Interspecific coordination and intraspecific plasticity of fine root traits in North American temperate tree species. Front Plant Sci 4:242. https://doi.org/10.3389/fpls.2013.00242
Tobner CM, Paquette A, Reich PB, Gravel D, Messier C (2013b) Advancing biodiversity-ecosystem functioning science using high-density tree-based experiments over functional diversity gradients. Oecologia 174:609–621. https://doi.org/10.1007/s00442-013-2815-4
Tobner CM, Paquette A, Reich PB, Gravel D, Messier C (2014) Advancing biodiversity—ecosystem functioning science with the use of high-density tree-based experiments. Oecologia 174:609–621. https://doi.org/10.1007/s00442-013-2815-4
Tobner CM, Paquette A, Gravel D, Reich PB, Williams LJ, Messier C (2016) Functional identity is the main driver of diversity effects in young tree communities. Ecol Lett 19:638–647. https://doi.org/10.1111/ele.12600
Valverde-Barrantes OJ, Smemo KA, Feinstein LM, Kershner MW, Blackwood CB (2015) Aggregated and complementary: symmetric proliferation, overyielding, and mass effects explain fine-root biomass in soil patches in a diverse temperate deciduous forest landscape. New Phytol 205:731–742. https://doi.org/10.1111/nph.13179
Williams LJ, Paquette A, Cavender-Bares J, Messier C, Reich PB (2017) Spatial complementarity in tree crowns drives overyielding in species mixtures. Nat Ecol Evol 1:0063. https://doi.org/10.1038/s41559-016-0063
Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci 96:1463–1468. https://doi.org/10.1073/pnas.96.4.1463
Zhang Y, Chen HYH, Reich PB (2012) Forest productivity increases with evenness, species richness and trait variation: a global meta-analysis. J Ecol 100:742–749. https://doi.org/10.1111/j.1365-2745.2011.01944.x
Acknowledgements
We are indebted to numerous field and lab assistants who helped with the intense root sampling and washing campaign, and the weeding of the experiment over 3 years. This research was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant and Fonds de recherche du Québec equipment grant to ITH, a Natural Sciences and Engineering Research Council Collaborative Research Development Grant to CM, a Natural Sciences and Engineering Research Council Discovery Grant to AM, and a Natural Sciences and Engineering Research Council Collaborative Research and Training Experience scholarship in Forest Complexity Modeling to CA. We thank other collaborators in the IDENT network for stimulating exchanges through various stages of the project.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study. CA and RK led the field and lab work. AP, CA and RK analysed the data. RK, CA, AP and ITH prepared the figures. ITH, AP and CM led the writing of the manuscript assisted by CA. RK and AM provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Brian J. Wilsey.
Rights and permissions
About this article
Cite this article
Archambault, C., Paquette, A., Messier, C. et al. Evergreenness influences fine root growth more than tree diversity in a common garden experiment. Oecologia 189, 1027–1039 (2019). https://doi.org/10.1007/s00442-019-04373-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-019-04373-5