Abstract
Species invading new habitats experience novel selection pressures that can lead to rapid evolution, which may contribute to invasion success and/or increased impact on native community members. Many studies have hypothesized that plants in the introduced range will be larger than those in the native range, leading to increases in competitive ability. There is mixed support for evolution of larger sizes in the introduced range, but few studies have explicitly tested whether evolutionary changes result in decreased competitive responses or increased competitive effects on other species in the community. Here, we show that introduced Medicago polymorpha genotypes produced 14% more aboveground and 41% more belowground biomass than genotypes from the native range, suggesting that evolutionary changes in size occurred after introduction. However, these size differences were only observed in the absence of competition. The competitive effects of introduced and native range genotypes on three species that commonly co-occur with Medicago in invaded regions were remarkably similar. These results suggest that evolutionary increases in size during biological invasions do not necessarily alter the competitive effects of the invader on other community members, but may increase invasion success in disturbed or low competition environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid evolution occurs when species experience strong selection that drives evolutionary changes in traits quickly enough to alter the outcome of ecological interactions (Hairston et al. 2005; Strauss et al. 2008). One scenario in which species may evolve rapidly is when they invade new ranges and experience different selection pressures than they experienced in their native range. Comparisons of species in their invasive and native ranges suggest that this is common; 16 of 23 studies of introduced species detected significant evolutionary change in at least one trait after invasion (Buswell et al. 2011).
Although the majority of explanations for invasion success focus on ecological mechanisms (Catford et al. 2009), rapid evolution has the potential to influence invasion success, particularly when evolutionary changes occur in traits known to predict invasive species spread (Dlugosch and Parker 2008; Ebeling et al. 2008; Whitney and Gabler 2008). For example, evolutionary increases in dispersal ability at invasion fronts have accelerated the spread of invasive cane toads in Australia (Phillips et al. 2010). Similarly, a rapid adaptive shift in flowering time is the main contributor to increased reproductive success, and, therefore, potentially the northern spread, of invasive purple loosestrife (Lythrum salicaria) in North America (Colautti and Barrett 2013). Evolutionary changes may occur early in the invasion process, because genotypes with particular traits are better colonizers, or at later invasion stages due to the novel environmental conditions that impose selection in the invasive range (Sakai et al. 2001).
Many studies of evolution during invasion have focused on size as an important trait. The Evolution of Increased Competitive Ability (EICA) hypothesis posits that individuals in the invasive range escape natural enemies, and individuals that reallocate resources from defense to growth and competitive ability are more successful (Harper 1964; Blossey and Notzold 1995). EICA predicts larger and more competitive individuals in the invaded range, relative to individuals from the native range. Conversely, the Evolution of Reduced Competitive Ability Hypothesis (ERCA; Bossdorf et al. 2004) predicts that individuals that invest less in competitive ability, and more in reproduction, will be more successful in the invaded range. Several studies have confirmed the predictions of EICA, finding larger sized individuals in the invasive range (Blossey and Notzold 1995; Colautti et al. 2009; Beaton et al. 2011; Huang et al. 2012; Yang et al. 2014). However, other studies have found no difference in size when comparing invasive and native populations (Meyer and Hull-Sanders 2008; Colautti et al. 2009; Gonzalez-Teuber et al. 2017; reviewed in Felker-Quinn et al. (2013)).
Although several studies have examined differences in size between individuals in the invasive and native ranges, fewer have examined whether evolutionary changes in size translate to differences in competitive ability (e.g., Vila et al. 2003; He et al. 2009; Ridenour et al. 2008; Joshi et al. 2014). Competitive ability can be measured as either competitive response, the ability of a focal species to maintain growth and fitness in response to competition from other species, or competitive effect, the reductions in growth or fitness other species exhibit when growing in the presence of the focal species (Goldberg and Werner 1983; Miller and Werner 1987). Decreased competitive responses of potential invaders to other species could allow invaders to overcome biotic resistance imposed by competition from other species. Increased competitive effects of potential invaders may allow species to overcome biotic resistance, but are also likely to result in negative consequences of invasion for the native community.
Some studies demonstrate that evolutionary changes in size increased the competitive effects of the invaders on other species (Ridenour et al. 2008; Joshi et al. 2014), while others detected no difference in competitive effect (Vila et al. 2003; He et al. 2009). Two studies investigating whether evolutionary changes in size affected the competitive response of invaders to native species found decreased competitive response in invasive range genotypes (Ridenour et al. 2008; Joshi et al. 2014). Relatively few studies have examined whether evolutionary changes in traits affect both competitive effect and response, and most have used only a few populations or genotypes for comparison.
To distinguish between evolutionary changes in plant size, competitive effect, and competitive response, we conducted two experiments on a large number of Medicago polymorpha genotypes collected from both the native range and introduced regions on five continents (Table 1). We asked (1) has Medicago size evolved in the introduced range? (2) Has the response of Medicago to competition evolved in the introduced range? (3) Has the competitive effect of Medicago on coexisting plants in the introduced range evolved?
Methods
Study system
Medicago polymorpha (hereafter “Medicago”) is native to the Mediterranean and Middle East, but is a common invader in grasslands around the world, including Asia, Australia, South America, northern Europe, Africa, and the United States. At the McLaughlin Reserve (Lake County, California), where we have performed field experiments on Medicago in the past (Lau and Strauss 2005; Lau 2008; terHorst and Lau 2012; Bayliss et al. 2017), Medicago competes primarily with other legumes and grasses, including Vicia villosa, introduced in the early 1900s, Trifolium hirtum, introduced in the early 1800s, Bromus hordeaceus, introduced in the mid-to-late 1800s (Heady 1977), and the native Acmispon wrangelianus (formerly Lotus wrangelianus). Acmispon, in particular, suffers large reductions in fitness as a result of Medicago invasion (Lau and Strauss 2005; terHorst and Lau 2012), but it is unclear whether different genotypes affect Acmispon differently.
Because Medicago is a common invader around the world and is also valued for its potential as a forage crop, the National Plant Germplasm System at the United States Department of Agriculture (USDA) maintains a collection of Medicago genotypes. We obtained many genotypes from the USDA and also collected several genotypes from the McLaughlin Natural Reserve, Napa County, California (Table 1). Medicago is almost entirely selfing (Vitale et al. 1998), and because strong inbreeding leads to homozygosity, seeds from a single plant are effectively a single genotype. Hereafter, we refer to accessions as genotypes. Each genotype was grown in a common garden greenhouse environment at the W. K. Kellogg Biological Station for at least one generation to reduce maternal effects due to historical environmental differences.
Experiment 1: evolutionary changes in size and competitive response
To test for evolutionary changes in biomass production, fecundity, and competitive response, we compared introduced and native range Medicago genotypes grown in three interspecific competition treatments (0, 4, or 8 competitors). We used 16 genotypes that were collected from the native range of Medicago, and 21 genotypes collected from introduced regions around the world, including six genotypes from different populations within the McLaughlin Reserve (Table 1). Seeds were physically scarified and germinated in water-filled petri dishes. A single Medicago seedling was planted into each 620 ml pot filled with potting media (SunGro LP5: Canadian Sphagnum peat moss, fine perlite, low nutrient charge with Gypsum and dolomitic limestone; SunGro Horticulture Canada Ltd., Alberta, Canada) and watered every 3 days or as needed. We chose a low nutrient substrate to ensure nutrient limitation and competition and to better represent the nutrient poor field conditions. The competitive environment was manipulated by sowing either 0, 4, or 8 Bromus hordeaceus seeds into the appropriate pots. Bromus spp., are the most common plant species in many sites at the McLaughlin Reserve, where Medicago is found. B. hordeaceus is also native to the Mediterranean and likely co-occurs with Medicago in its native range. B. hordeaceus seeds were obtained from L.A. Hearne Company (King City, CA, USA). The experiment initially had 555 replicate pots (37 Medicago genotypes × 3 competition treatments × 5 replicates), though 12 plants died shortly after transplant and were removed from the data set. Replicate pots were randomly distributed across the greenhouse. After 70 days, some plants were beginning to show signs of senescence, so all above- and belowground Medicago biomasses were harvested, and dried at 65 °C for > 24 h prior to weighing. We used biomass as an estimate of plant performance and also separately examined above- and belowground biomass as estimates of investment in shoots or roots. We counted the number of fruits on each plant at the time of harvest, though this measure is biased against genotypes with later phenologies. We present the analysis of fruit number in the online supplement (Table S1), but do not interpret those results here.
We tested for differences in biomass of Medicago from the introduced and native ranges with generalized linear mixed models, using proc glimmix in SAS (version 9.4) and using AIC to find the best error distribution (gamma distribution in all cases; ΔAIC > 175 for all variables, compared to gaussian and log-normal distributions). We included range, competition treatment, and their interaction as fixed factors, and genotype (nested within range) and the genotype x competition interaction as random factors. We used backwards stepwise model selection to find the model with the lowest AIC value, using ΔAIC > 2 as the criteria for retaining a model. We used separate models to analyze aboveground, belowground, and total biomass as response variables. We tested the significance of random effects with likelihood ratio tests.
Experiment 2: evolutionary changes in competitive effects
To test for differences in competitive effect between introduced and native range genotypes, we grew one individual of each of 34 genotypes (15 native range, 19 introduced range, Table 1) in interspecific competition with one individual of either Acmispon wrangelianus, Vicia villosa or Trifolium hirtum (each collected from the McLaughlin Reserve in Napa County, California, USA). These other species are common competitors with Medicago in the McLaughlin Reserve. This design allows us to examine relative differences in competitive effect between genotypes, but does not provide an estimate of the absolute competitive effect, because we did not grow each species without competition. The experiment included three replicates per genotype per competition treatment (N = 306; 34 genotypes × 3 treatments, n = 3). Plants were germinated and grown in 164 ml Containers™ (Stuewe and Sons Inc., Corvallis, OR, USA) filled with the same potting media used in Experiment 1. Replicate pots were randomly distributed across the greenhouse. On day 40, we measured the height of the competitor. After 43 days, the above- and belowground biomass of all Medicago and competitor plants were harvested separately and dried at 65 °C for 24 h prior to weighing.
We tested for the effects of introduced and native genotypes of Medicago on the biomass and height of competitor species with general linear mixed models (proc glimmix, SAS version 9.4). We log-transformed the dependent variables to meet assumptions of normality and homogeneity of variance. We included Medicago range and competitor identity, and their interaction, as fixed factors and Medicago genotype (nested within range) and the genotype x competitor interaction as random factors. We used separate models to analyze height and aboveground, belowground, and total biomass as response variables. We tested the significance of random effects with likelihood ratio tests.
Results
Experiment 1: evolutionary changes in size and competitive response
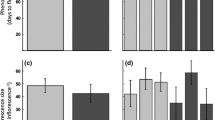
We detected a significant interaction between Medicago range (introduced or native) and the B. hordeaceus competition treatment on Medicago total biomass production (P = 0.005, Table 2). This interaction was significant for belowground biomass (P = 0.007), but not aboveground biomass (P = 0.32) (Table 2). In the absence of competition, the total biomass of introduced range genotypes was 27% larger than native range genotypes; aboveground and belowground biomasses were 14 and 41% greater, respectively (Fig. 1). In the presence of competition, Medicago was much smaller and introduced and native range genotypes did not differ significantly in size (Fig. 1).
Mean ± SE total biomass, aboveground biomass, and belowground biomass of native range (open bars) and introduced range (gray bars) Medicago genotypes grown in the absence of competition, or in the presence of four or eight B. hordeaceus competitors. Note the broken scale of the y-axis, as biomass in the presence of competitors was quite low
We detected significant variation among genotypes, beyond that explained by range, in aboveground biomass (P < 0.001), belowground biomass (P < 0.001), and total biomass (P < 0.001) and variation among genotypes in aboveground biomass responses to competition [significant genotype x competition interaction (P = 0.015), Table 2, Fig. S1].
Experiment 2: evolutionary changes in competitive effects
Even though Medicago genotypes collected from the introduced range tended to be larger than genotypes from the native range when grown in the absence of competition (Fig. 1), the increased size of introduced range genotypes did not translate into increased competitive effects on co-occurring plants in the invaded range (Fig. 2). Competitors had similar total biomass in the presence of invasive range and native range genotypes (P = 0.45, Fig. 2, Table 3). This result was consistent for all three competitors (species × range effect P = 0.81, Fig. 2). The effects of different Medicago genotypes on competitor biomass were remarkably similar (genotype effect P = 0.97). However, genotypes varied in their effects on competitor height, although the magnitude of this effect differed across competing species [significant genotype x competitor species interaction (P = 0.013), Table 3, Figs. S2–S4]. We found the most variation in the effect of Medicago genotypes on Trifolium height (coefficient of variation = 1.2, Fig. S4), followed by Vicia (CV = 0.41, Fig. S3) and Acmispon (CV = 0.21) (Fig. S2).
Discussion
Medicago polymorpha has evolved increased size in its introduced range, although this evolutionary effect was only evident in the absence of competition. The evolutionary increase in plant size did not translate to decreased competitive response to, or increased competitive effects on, other species. These results suggest that, although evolution occurred during or after invasion, it is unlikely to help Medicago overcome the biotic resistance imposed by competitors, or lead to increased negative effects on other community members in the introduced range.
Many invasive species undergo rapid evolution in novel habitats (Buswell et al. 2011). Some have evolved decreased defenses, while others have evolved increased size or height or altered leaf morphologies, or some combination of the four (Buswell et al. 2011). The fact that introduced genotypes outperform native range genotypes only in treatments without competition (Fig. 1) supports the previous studies demonstrating that increases in size are only observed in low stress environments (e.g., Leger and Rice 2003; Blumenthal and Hufbauer 2007). However, our results do not support the predictions of EICA, which presume that increased size should lead to increased competitive ability. Few studies have shown that rapid evolution can increase competitive effects [but see Ridenour et al. (2008), Uesugi and Kessler (2013), Joshi et al. (2014)], and a recent review by Felker-Quinn et al. (2013) suggests that there is little support for the EICA Hypothesis in its entirety, despite strong evidence for rapid evolution of invasive species. Their review and other recent studies (Vila et al. 2003; He et al. 2009) support our conclusion that rapid evolution does not necessarily affect competitive ability despite increases in vegetative growth. Other hypotheses may also explain evolutionary changes in size [reviewed in Atwood and Meyerson (2011)], such as release from natural enemies (Blumenthal 2006; Van Grunsven et al. 2009), plasticity leading to local adaptation (Sexton et al. 2002), and multiple introductions and hybridization (Roman 2006). Therefore, we urge caution in assuming that morphological evolution necessarily leads to increased invasiveness in competitive environments or increased competitive effects on native species.
The fact that we did not observe any evolution of competitive ability was not due to a lack of opportunity for selection. Although we did not observe differences in responses to competition between native and introduced range genotypes, we found significant variation among genotypes within each range. We found variation among genotypes in their response to competition from B. hordeaceus (Fig. S1) and in their effect on competitor height (but not biomass), although the extent of this variation was dependent on the competitor species (Figs. S2–S4). This genotypic variation in competitive effect and response likely results from differences in morphology and growth, including plant height, degree of lateral spread, root:shoot ratio, and growth rate. The presence of such genetic variation also indicates that these traits have the capacity to evolve in response to divergent competitive environments between introduced and native ranges. The success of some genotypes may be explained by variation in environmental parameters in their collection range. We tested this hypothesis for genotypes for which climate data was available, but found no effect when regressing plant biomass on the latitude, elevation, or annual precipitation of the collection range (all P > 0.38). However, exact geography of collection locations was often limited to broad geographic regions (e.g., large states or countries) and average climate data for the region may not represent the climate at a particular collection site; thus, lack of evidence for associations with climate should be interpreted with caution.
Our experiments collectively measured competitive effect and response in pairwise competition. However, evolution in a community context may differ from evolution in pairwise competition (terHorst et al. 2015), and evolution in response to other selective agents may explain the evolution of increased size (Atwood and Meyerson 2011). For example, invasive range genotypes of Brassica nigra are better competitors when surrounded by a natural community, but native range genotypes are better competitors in pairwise scenarios (Oduor et al. 2013). Evolution may also occur in response to selective agents other than competitors or other natural enemies, and the ecological consequences of evolutionary effects may be context dependent (Ellner et al. 2011; terHorst et al. 2014). For example, the observed evolutionary difference in competitive ability between invasive and native range genotypes of Trifolium spp. was largely dependent on the soil biota (Shelby et al. 2016). Because most studies investigating evolutionary changes in size and competitive ability are conducted in relatively simplistic greenhouse environments or heavily manipulated (e.g., weeded or tilled) field environments, how evolution and the ecological effects of evolution play out in natural diverse communities requires further exploration.
The evolutionary increase in size in the introduced range was almost entirely due to increases in belowground biomass, indicating the need to study both above- and belowground processes. Had we only examined aboveground biomass, as many studies do, we would have found scant evidence for evolutionary changes in size. This pattern indicates that genotypes in the introduced range have increased investment in roots relative to shoots. Such an investment may increase competitive ability for water or nutrients, although the lack of difference in competitive response and effect between introduced and native range genotypes observed here suggests that this is unlikely. Instead the increased root:shoot ratios observed in introduced genotypes may be a response to direct selection by abiotic conditions, such as the dry conditions, these plants experience in many parts of the introduced range (Schenk and Jackson 2002) or potentially even a lack of compatible rhizobium resource mutualists (terHorst et al., in review). Introduced Medicago genotypes receive fewer fitness benefits from rhizobia, potentially favoring increased investment in belowground biomass that allows them to better forage for nutrients directly from the soil (terHorst et al., in review).
This finding is consistent with other hypotheses for biological invasions that predict that invasive species will be those best able to take full advantage of high resource, low competition environments, in part because they are well-adapted to productive or human-altered environments in their native ranges (Gray 1879; Baker 1974; Dostal et al. 2013). While these hypotheses are typically invoked to explain which species are likely to be invasive, similar arguments may be made within species—those genotypes that are best able to invade may be those genotypes best able to take full advantage of disturbed, low competition conditions, possibly because they originate from disturbed or human-altered landscapes in the native range (Hufbauer et al. 2012). Local adaptation to disturbed environments in the native range may more generally lead to exaptation to low competition environments in novel invaded habitats.
Medicago was initially introduced to many locations through both accidental transports of the seeds and deliberately as a cover or fodder crop. It is possible that such agricultural practices resulted in artificial selection before or after introduction. This may account for the success of Medicago in disturbed and human-altered landscapes. Alternatively, invading populations may rapidly adapt to low competition environments in the introduced range. Either way, genotypes that are able to produce high biomass in low competition environments are likely to produce more seeds and, therefore, may have higher population growth rates than smaller genotypes. Greater seed production and higher population growth rates would allow these genotypes to more effectively overcome the problems associated with small populations (e.g., demographic stochasticity) that are known to limit the reproduction and spread of invasive species (Firestone and Jasieniuk 2013). Although each of these scenarios seem plausible, the previous work on this system failed to find evidence that disturbance increased the fitness of introduced genotypes more than native genotypes, although substantial genetic variation in response to disturbance was detected (Bayliss et al. 2017).
Caveats and future directions
The densities of the common competitor used in experiment 1 are similar to those experienced by Medicago in natural California grasslands. However, not all genotypes may have experienced this competitor in their evolutionary history. Similarly, although we examined competitive effects on three species in experiment 2, competition with these other species may not be relevant in the region in which they were collected. Three of the four competitors in our experiment were also non-native species; because there are so many invasive species in California grasslands, a potential invader is more likely to interact with invasive than native species. These results are worth considering in that context, but also in the context that most ecologists consider biotic resistance to invasion as arising from communities of native species. Future work should consider how Medicago genotypes respond to other species and levels of competition in these communities. The strong effects of only a few B. hordeaceus individuals on Medicago biomass, for example, left little scope for genotypic variation that may have been more obvious in less competitive environments. In addition, we measured competition in terms of biomass production, but ultimately what determines invasion success is fecundity and recruitment.
We demonstrated that the evolution of increased size during biological invasions did not increase competitive effects on other species or minimize the response to competitors. We are, therefore, left to speculate on the origins and advantages of increased size. Measuring natural selection on size in different environmental contexts, ideally in both the introduced and native ranges would help identify the environments in which increased size, and particularly increased belowground biomass, is adaptive. Unfortunately, few studies have measured natural selection on exotic species in their introduced environment and even fewer have done so in both introduced and native regions (Colautti and Lau 2015). Furthermore, all but one of the competitors used in our experiments were other invasive species. Invaders now make up the bulk of plants observed in California grasslands, so this is a realistic contemporary scenario for Medicago invasion, but it may not reflect conditions of the past, or those in other geographical regions. Other species that are native to California may respond differently to potential invaders.
Conclusions
Our study, combined with the previous conflicting findings that characterize tests of evolutionary changes in competitive ability, points to the need for further work investigating the selective agents acting on exotic and invasive species. While enemy escape is one potential selective agent causing the evolution of increased size (assuming growth-defense trade-offs), strong selection for increased growth in low competition environments, whether because of disturbance, drought, or other biotic or abiotic stressors, is an alternative hypothesis that warrants exploration and seems to be consistent with the patterns observed in this study. Regardless of when or why the evolution of increased size occurred, the global dispersal of Medicago into new locations is leading to introduced populations composed of plants capable of growing to larger sizes. Fortunately, for the communities Medicago invades, the evolution of increased size has not been accompanied by evolutionary increases in competitive effect or response that would exacerbate the ecological impacts of this invasive species, although the presence of genetic variation for both competitive response and competitive effect suggests that such evolutionary effects are possible.
References
Atwood JP, Meyerson LA (2011) Beyond EICA: understanding post-establishment evolution requires a broader evaluation of potential selection pressures. NeoBiota 10:7–25. https://doi.org/10.3897/neobiota.10.954
Baker HG (1974) The evolution of weeds. Ecol Evol Syst 5:1–24
Bayliss SLJ, terHorst CP, Lau JA (2017) Testing genotypic variation of an invasive plant species in response to soil disturbance and herbivory. Oecologia 183:1135–1141. https://doi.org/10.1007/s00442-017-3820-9
Beaton LL, Van Zandt PA, Esselman EJ, Knight TM (2011) Comparison of the herbivore defense and competitive ability of ancestral and modern genotypes of an invasive plant, Lespedeza cuneata. Oikos 120:1413–1419. https://doi.org/10.1111/j.1600-0706.2011.18893.x
Blossey B, Notzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889. https://doi.org/10.2307/2261425
Blumenthal DM (2006) Interactions between resource availability and enemy release in plant invasion. Ecol Lett 9:887–895. https://doi.org/10.1111/j.1461-0248.2006.00934.x
Blumenthal DM, Hufbauer RA (2007) Increased plant size in exotic populations: a common-garden test with 14 invasive species. Ecology 88:2758–2765. https://doi.org/10.1890/06-2115.1
Bossdorf O, Prati D, Auge H, Schmid B (2004) Reduced competitive ability in an invasive plant. Ecol Lett 7:346–353. https://doi.org/10.1111/j.1461-0248.2004.00583.x
Buswell JM, Moles AT, Hartley S (2011) Is rapid evolution common in introduced plant species? J Ecol 99:214–224. https://doi.org/10.1111/j.1365-2745.2010.01759.x
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40. https://doi.org/10.1111/j.1472-4642.2008.00521.x
Colautti RI, Barrett SCH (2013) Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342:364–366. https://doi.org/10.1126/science.1242121
Colautti RI, Lau JA (2015) Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Mol Ecol 24:1999–2017. https://doi.org/10.1111/mec.13162
Colautti RI, Maron JL, Barrett SCH (2009) Common garden comparisons of native and introduced plant populations: latitudinal clines can obscure evolutionary inferences. Evol Appl 2:187–199. https://doi.org/10.1111/j.1752-4571.2008.00053.x
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449. https://doi.org/10.1111/j.1365-294X.2007.03538.x
Dostal P, Allan E, Dawson W et al (2013) Enemy damage of exotic plant species is similar to that of natives and increases with productivity. J Ecol 101:388–399. https://doi.org/10.1111/1365-2745.12037
Ebeling SK, Welk E, Auge H, Bruelheide H (2008) Predicting the spread of an invasive plant: combining experiments and ecological niche model. Ecography 31:709–719. https://doi.org/10.1111/j.1600-0587.2008.05470.x
Ellner SP, Geber MA, Hairston NG (2011) Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol Lett 14:603–614. https://doi.org/10.1111/j.1461-0248.2011.01616.x
Felker-Quinn E, Schweitzer JA, Bailey JK (2013) Meta-analysis reveals evolution in invasive plant species but little support for evolution of increased competitive ability (EICA). Ecol Evol 3:739–751. https://doi.org/10.1002/ece3.488
Firestone JL, Jasieniuk M (2013) Small population size limits reproduction in an invasive grass through both demography and genetics. Oecologia 172:109–117. https://doi.org/10.1007/s00442-012-2465-y
Goldberg D, Werner P (1983) Equivalence of competitors in plant communities: a null hypothesis and a field experimental approach. Am J Bot 70:1098–1104. https://doi.org/10.2307/2442821
Gonzalez-Teuber M, Quiroz CL, Concha-Bloomfield I, Cavieres LA (2017) Enhanced fitness and greater herbivore resistance: implications for dandelion invasion in an alpine habitat. Biol Invasions 19:647–653. https://doi.org/10.1007/s10530-016-1309-9
Gray A (1879) The pertinacity and predominance of weeds. Am J Sci 18:161–167
Hairston NG, Ellner SP, Geber MA et al (2005) Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett 8:1114–1127. https://doi.org/10.1111/j.1461-0248.2005.00812.x
Harper JL (1964) The individual in the population. J Ecol 52:149–159
He W-M, Feng Y, Ridenour WM et al (2009) Novel weapons and invasion: biogeographic differences in the competitive effects of Centaurea maculosa and its root exudate (±)-catechin. Oecologia 159:803–815. https://doi.org/10.1007/s00442-008-1234-4
Heady HF (1977) Valley grassland. In: Barbour MG, Major J (eds) Terrestrial vegetation of California. Wiley, New York, pp 491–514
Huang W, Carrillo J, Ding J, Siemann E (2012) Interactive effects of herbivory and competition intensity determine invasive plant performance. Oecologia 170:373–382. https://doi.org/10.1007/s00442-012-2328-6
Hufbauer RA, Facon B, Ravigne V et al (2012) Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl 5:89–101. https://doi.org/10.1111/j.1752-4571.2011.00211.x
Joshi S, Gruntman M, Bilton M et al (2014) A comprehensive test of evolutionarily increased competitive ability in a highly invasive plant species. Ann Bot 114:1761–1768. https://doi.org/10.1093/aob/mcu199
Lau JA (2008) Beyond the ecological: biological invasions alter natural selection on a native plant species. Ecology 89:1023–1031. https://doi.org/10.1890/06-1999.1
Lau JA, Strauss SY (2005) Insect herbivores drive important indirect effects of exotic plants on native communities. Ecology 86:2990–2997. https://doi.org/10.1890/04-1779
Leger EA, Rice KJ (2003) Invasive California poppies (Eschscholzia californica Cham.) grow larger than native individuals under reduced competition. Ecol Lett 6:257–264. https://doi.org/10.1046/j.1461-0248.2003.00423.x
Meyer GA, Hull-Sanders HM (2008) Altered patterns of growth, physiology and reproduction in invasive genotypes of Solidago gigantea (Asteraceae). Biol Invasions 10:303–317. https://doi.org/10.1007/s10530-007-9131-z
Miller T, Werner P (1987) Competitive effects and responses between plant species in a 1st-year old-field community. Ecology 68:1201–1210. https://doi.org/10.2307/1939204
Oduor AMO, Strauss SY, Garcia Y et al (2013) Herbivores mediate different competitive and facilitative responses of native and invader populations of Brassica nigra. Ecology 94:2288–2298. https://doi.org/10.1890/12-2021.1
Phillips BL, Brown GP, Shine R (2010) Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. J Evol Biol 23:2595–2601. https://doi.org/10.1111/j.1420-9101.2010.02118.x
Ridenour WM, Vivanco JM, Feng Y et al (2008) No evidence for trade-offs: centaurea plants from America are better competitors and defenders. Ecol Monogr 78:369–386. https://doi.org/10.1890/06-1926.1
Roman J (2006) Diluting the founder effect: cryptic invasions expand a marine invader’s range. Proc R Soc B Biol Sci 273:2453–2459. https://doi.org/10.1098/rspb.2006.3597
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90:480–494. https://doi.org/10.1046/j.1365-2745.2002.00682.x
Sexton JP, McKay JK, Sala A (2002) Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol Appl 12:1652–1660. https://doi.org/10.2307/3099929
Shelby N, Hulme PE, van der Putten WH et al (2016) No difference in the competitive ability of introduced and native Trifolium provenances when grown with soil biota from their introduced and native ranges. Aob Plants 8:plw016. https://doi.org/10.1093/aobpla/plw016
Strauss SY, Lau JA, Schoener TW, Tiffin P (2008) Evolution in ecological field experiments: implications for effect size. Ecol Lett 11:199–207. https://doi.org/10.1111/j.1461-0248.2007.01128.x
terHorst CP, Lau JA (2012) Direct and indirect transgenerational effects alter plant-herbivore interactions. Evol Ecol 26:1469–1480. https://doi.org/10.1007/s10682-012-9560-8
terHorst CP, Lennon JT, Lau JA (2014) The relative importance of rapid evolution for plant-microbe interactions depends on ecological context. Proc R Soc B Biol Sci 281:20140028. https://doi.org/10.1098/rspb.2014.0028
terHorst CP, Lau JA, Cooper IA et al (2015) Quantifying nonadditive selection caused by indirect ecological effects. Ecology 96:2360–2369. https://doi.org/10.1890/14-0619.1
Uesugi A, Kessler A (2013) Herbivore exclusion drives the evolution of plant competitiveness via increased allelopathy. New Phytol 198:916–924. https://doi.org/10.1111/nph.12172
Van Grunsven RHA, Bos F, Ripley BS et al (2009) Release from soil pathogens plays an important role in the success of invasive Carpobrotus in the Mediterranean. S Afr J Bot 75:172–175. https://doi.org/10.1016/j.sajb.2008.09.003
Vila M, Gomez A, Maron JL (2003) Are alien plants more competitive than their native conspecifics? A test using Hypericum perforatum L. Oecologia 137:211–215. https://doi.org/10.1007/s00442-003-1342-0
Vitale M, Pupilli F, Labombarda P, Arcioni S (1998) RAPD analysis reveals a low rate of outcrossing in burr medic (Medicago polymorpha L.). Genet Resour Crop Evol 45:337–342. https://doi.org/10.1023/A:1008686721385
Whitney KD, Gabler CA (2008) Rapid evolution in introduced species, “invasive traits” and recipient communities: challenges for predicting invasive potential. Divers Distrib 14:569–580. https://doi.org/10.1111/j.1472-4642.2008.00473.x
Yang X, Huang W, Tian B, Ding J (2014) Differences in growth and herbivory damage of native and invasive kudzu (Peuraria montana var. lobata) populations grown in the native range. Plant Ecol 215:339–346. https://doi.org/10.1007/s11258-014-0304-4
Acknowledgements
We thank Mark Hammond for greenhouse assistance and T. Bassett, K. Keller, E. Schultheis, T. Suwa, and several anonymous reviewers for commenting on previous drafts of this manuscript. This work was supported by funding from the National Science Foundation to JAL (DEB-0918963) and to CPT (DMS-1312490, OCE-1559105), and the Kellogg Biological Station REU Program. This is KBS publication #1904. Data from these experiments will be archived at Dryad Repository and will be publicly available (https://doi.org/10.5061/dryad.pv1kc45).
Author information
Authors and Affiliations
Contributions
ZLGP designed and performed Expt. 2 and wrote the manuscript. CPT designed Expt. 2, analyzed the data, and wrote the manuscript. SMM designed and performed Expt. 1. JAL conceived and designed both experiments and wrote the manuscript.
Corresponding author
Additional information
Communicated by Wayne Dawson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Getman-Pickering, Z.L., terHorst, C.P., Magnoli, S.M. et al. Evolution of increased Medicaco polymorpha size during invasion does not result in increased competitive ability. Oecologia 188, 203–212 (2018). https://doi.org/10.1007/s00442-018-4168-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4168-5