Abstract
Baculoviruses are food-borne microbial pathogens that are ingested by insects on contaminated foliage. Oxidation of plant-derived phenolics, activated by insect feeding, can directly interfere with infections in the gut. Since phenolic oxidation is an important component of plant resistance against insects, baculoviruses are suggested to be incompatible with plant defences. However, plants among and within species invest differently in a myriad of chemical and physical defences. Therefore, we hypothesized that among eight soybean genotypes, some genotypes would be able to maintain both high resistance against an insect pest and high efficacy of a baculovirus. Soybean constitutive (non-induced) and jasmonic acid (JA)-induced (anti-herbivore response) resistance was measured against the fall armyworm Spodoptera frugiperda (weight gain, leaf consumption and utilization). Indicators of phenolic oxidation were measured as foliar phenolic content and peroxidase activity. Levels of armyworm mortality inflicted by baculovirus (SfMNPV) did not vary among soybean genotypes when the virus was ingested with non-induced foliage. Ingestion of the virus on JA-induced foliage reduced armyworm mortality, relative to non-induced foliage, on some soybean genotypes. Baculovirus efficacy was lower when ingested with foliage that contained higher phenolic content and defensive properties that reduced armyworm weight gain and leaf utilization. However, soybean genotypes that defended the plant by reducing consumption rate and strongly deterred feeding upon JA-induction did not reduce baculovirus efficacy, indicating that these defences may be more compatible with baculoviruses to maximize plant protection. Differential compatibility of defence traits with the third trophic level highlights an important cost/trade-off associated with plant defence strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can influence the interactions between herbivores and their natural enemies (Bailey et al. 2006; Gruner and Taylor 2006; Ode 2006; Gols 2014). It is well established that plants, among species and even among genotypes within a species, can vary in their resistance and tolerance to herbivory (Strauss and Agrawal 1999; Núñez-Farfán et al. 2007). Recent studies have revealed that intraspecific genetic variation in plants can influence the structure of insect herbivore communities (Johnson and Agrawal 2005; Bangert et al. 2006; Bukovinszky et al. 2008) and directly or indirectly affect higher trophic levels (Bailey et al. 2006; Gruner and Taylor 2006; Bukovinszky et al. 2008). Variation in plant defensive traits is well-known to influence the development and searching behaviour of parasitoids and predators and consequently affect insect herbivores (Poelman et al. 2008; Gols 2014). Far less is known how variation in plant defensive traits influences the microbial members of the third trophic level.
Studies of plant–insect-microbe interactions indicate that one participant can profoundly influence the adaptation and evolution of two-way interactions between the other participants (Biere and Bennett 2013; Biere and Tack 2013). For example, mutualistic symbionts of insects can affect insect–plant interactions by enabling insects to manipulate plant physiology for their own benefit (Frago et al. 2012; Chung et al. 2013). In the context of plant-mediated effects on insect pathogens (entomopathogens), the extent to which plants can influence insect-entomopathogen interactions depends on whether the plants directly or indirectly alter key processes, such as infection or environmental persistence (Cory and Hoover 2006). In the present study, we focus on plant-mediated effects at the point of infection, where it is clear that variation among plants can dramatically influence the susceptibility of herbivorous insects (Cory and Hoover 2006).
Baculoviruses have been the focus of many studies on plant–insect-pathogen interactions (Cory and Hoover 2006). They are food-borne pathogens that occur widely among lepidopteran insects, and can cause epizootics in outbreak populations of some forest and agricultural insect species (Cory and Myers 2003). Plants play an important role in insect–baculovirus interactions, because the insects acquire infective virus transmission stages (viral occlusion bodies; OBs) by feeding on virus-contaminated plant tissues. Phytochemicals that are biologically activated through herbivory can directly affect virus entry at the point of infection in the insect gut. Viral OBs ingested by insects on foliage dissolve in the alkaline pH of the host midgut to release infective virions that bind and infect midgut epithelial cells. The infection must then quickly progress to the tracheal system to initiate a systemic, irreversible infection (lethal infection) before infected midgut cells are sloughed and cleared. Most baculoviruses must kill their hosts to release viral progeny to infect new hosts (Engelhard et al. 1994). Strong plant-dependent variation in baculovirus-induced mortality rates have been reported in a number of herbivorous insects, including the corn earworm (Helicoverpa zea; Hoover et al. 1998b; Ali et al. 1998), tobacco budworm (Heliothis virescens; Hoover et al. 1998b, c; Ali et al. 1998), beet armyworm (Spodoptera exigua; Farrar and Ridgway 2000) and gypsy moth larvae (Lymantria dispar; Keating et al. 1988).
In cotton and tomato, plant-mediated alteration of lethal baculovirus infection can occur when maceration of plant tissue leads to the formation of free radicals by the redox cycling of plant-derived phenolics, catalyzed in part by oxidative enzymes (Hoover et al. 1998a, b; but see Keating et al. 1988). In vitro treatment of viral OBs with macerated tomato foliage reduced the infectivity of the treated OBs to H. zea larvae. This likely resulted from the binding of oxidized phenolics to OBs, which prevented the dissolution of the OBs and consequently the release of infective virions in the insect midgut (Felton and Duffey 1990). H. virescens larvae were less likely to die from baculovirus-challenge when the OBs were ingested on cotton foliage. In vitro experiments suggested that this was due to the generation of free radicals during redox cycling in the larval gut, which damaged midgut cells and increased the rate of sloughing of infected cells before the virus progressed beyond the midgut epithelium (Hoover et al. 2000). The products and intermediates of redox cycling of phenolics also play important roles in direct plant resistance against herbivores, by reducing the digestibility of dietary protein and inflicting cytotoxic effects on gut tissue (Felton et al. 1992; Appel 1993; Summers and Felton 1994). These phytochemicals are often induced to higher levels upon herbivory, which can enhance direct resistance against the herbivore (Bi and Felton 1995; Hoover et al. 1998b) but can also have additional inhibiting effects on lethal infection by baculoviruses (Hunter and Schultz 1993; Hoover et al. 1998b; Ali et al. 1998). This negative effect of a key plant resistance mechanism on mortality by virus has led to the suggestion that baculoviruses may be incompatible with plants that are high in constitutive and inducible defences (Felton et al. 1987; Hunter and Schultz 1993; Hoover et al. 1998b; Hoover et al. 1998c). However, whether ecologically relevant measures of plant resistance against insects, such as the inhibition of insect growth and feeding, are also associated with lower baculovirus efficacy has not been tested.
The outcome of plant defences against herbivores depends on a complex network of interactions among numerous plant resistance mechanisms, including a myriad of primary and secondary chemical metabolites, physical resistance, gross morphology, life-history and physiology (Carmona et al. 2011; Rasmann et al. 2015). Thus, the degree to which phenolic oxidation contributes to resistance against insect herbivores may depend on the effectiveness of other resistance mechanisms. Since plant phenolic content (Lege et al. 1995; Malencic et al. 2007) and activities of oxidative enzymes such as peroxidases (Wood and Barbara 1971; Sreenivasulu et al. 1999) can vary among genotypes within a species, some genotypes that have lower constitutive and induced levels of these phytochemicals may retain resistance against herbivores through other resistance mechanisms. Consequently, these genotypes may be able to strongly resist herbivory through mechanisms other than phenolic oxidation while conserving additional protection from baculoviruses.
Here, we investigated the impact of constitutive and inducible defences in eight soybean genotypes on the efficacy of the baculovirus Spodoptera frugiperda multi-nucleocapsid nucleopolyhedrovirus (SfMNPV), which is an important natural mortality factor of the fall armyworm, S. frugiperda (Fuxa and Geaghan 1983). Soybean genotypes can vary in their levels of constitutive and induced resistance against herbivores (Underwood et al. 2000). Similar variation in resistance also exists in wild plants, which makes soybeans a suitable system to examine the impact of intraspecific genetic variation in plant resistance on insect–baculovirus interactions. We expected phenolic oxidation by peroxidases in soybean (one of the resistance mechanisms against insect herbivory) to interfere with SfMNPV efficacy. Our objective was to determine whether more ecologically relevant measures of plant resistance against insect herbivory, through reduced insect growth, feeding and utilization of ingested foliage, would also be associated with reduced SfMNPV efficacy. If strong soybean resistance against S. frugiperda larvae is associated with high levels of foliar phenolic content and peroxidase activity, then we would expect lower SfMNPV efficacy on soybean genotypes that are strongly resistant to S. frugiperda. However, if strong resistance against S. frugiperda is not associated with high levels of phenolic content and peroxidase activity, it would suggest that some soybean genotypes can be both highly resistant to insect pests and maintain high baculovirus efficacy. Based on this framework, we proposed three hypotheses. (1) SfMNPV-induced mortality in S. frugiperda will be lower when the virus is ingested with foliage from soybean genotypes that are high in constitutive and induced phenolic content and peroxidase activity. (2) However, levels of virus-induced mortality will not be associated with levels of soybean resistance against S. frugiperda because, (3) measures of soybean resistance against S. frugiperda will not depend on just phenolic content and peroxidase activity, thereby demonstrating the possibility for soybean genotypes to be both highly resistant to S. frugiperda and maintain high SfMNPV efficacy.
Materials and methods
Plants and jasmonic acid application
Soybean, Glycine max (Fabaceae), seeds were obtained from the National Plant Germplasm System, United States Department of Agriculture, Agricultural Research Service (USDA-ARS). Genotypes were selected based on previously demonstrated differences in constitutive and inducible defences (Underwood et al. 2000, 2002). The genotypes (maturity group, stem termination) were Bragg (VII, determinate), Braxton (VII, determinate), Davis (VI, determinate), Gasoy 17 (VII, determinate), Johnston (VIII, determinate), Lamar (VI, determinate), Stonewall (VII, determinate) and Williams (III, indeterminate). Plants were grown in 10 cm plastic pots in professional growing mix (Sunshine Mix 4 Aggregate Plus), watered daily and maintained in a walk-in growth chamber at 25 °C daylight (16 h) and 22 °C dark (8 h). Both fluorescent and halogen lights were used to provide a wide spectrum of wavelengths. Plants were fertilized with 3 g of Osmocote Plus (15-9-12; Scotts) when the first true leaves were fully expanded. 10–16 plants of each genotype were grown and divided equally into jasmonic acid or control treatments.
We exogenously applied jasmonic acid (JA), a phytohormone that upregulates plant defences against chewing herbivores (Thaler et al. 1996), to induce an anti-herbivore response in soybeans as previously demonstrated (Zhao et al. 1996; Accamando and Cronin 2012). Plants in the V3 stage were sprayed with a 2 mM solution of jasmonic acid until runoff (~5 ml per plant), and then air-dried. JA was dissolved in 1 ml of 95% ethanol, and dispersed in ultrapure water to the desired concentration. Control plants were sprayed with the same concentration of ethanol in ultrapure water. Since induced resistance in soybean reaches maximal levels within 3 days of herbivory and subsequently declines (Underwood 1998), plants were used in bioassays 48 h after JA application. Only the youngest, fully opened trifoliate leaf was used. To assess the relationships between soybean resistance traits and mortality by baculovirus, leaf tissue for all feeding bioassays (choice and no choice experiments), single dose virus bioassays and chemical analyses were obtained from the same plants. Cork borers were used to cut leaf disks. Due to space and time constraints, different genotypes were tested in three separate trials of experiments conducted in 2-week intervals (trial 1: Gasoy, Lamar, Stonewall; trial 2: Davis, Johnston, Williams; trial 3: Bragg, Braxton). The virus bioassays were repeated for five of the soybean genotypes (Bragg, Braxton, Gasoy, Lamar, Stonewall) using a dose response.
Insects and baculovirus
Fall armyworm eggs were obtained from Benzon Research (Carlisle, PA, USA) and reared on artificial diet (Southland Products Inc., Lake Village, AR, USA) at 29 °C and 16L:8D in 128-well insect rearing trays (Frontier Agricultural Sciences, Newark, DE, USA) until head capsule slippage in the late third instar. These larvae were transferred to individual wells of a 12-well cell culture plate without food to complete their molt prior to the fourth instar to use in the experiments.
Occlusion bodies (OB) of wild-type SfMNPV-B (Nicaragua) were obtained from Dr. James Slavicek, USDA Forest Service (Delaware, OH). OBs were quantified under a phase contrast microscope using an improved Neubauer brightline haemocytometer. Serial dilutions were performed to produce the doses used in the virus bioassays. For the single-dose bioassay, multiple aliquots from the same suspension were stored at –20 °C for use in the three trials of virus bioassays.
Foliar peroxidase activity and phenolic content
Leaf samples (~200 mg) were collected in 2 ml microcentrifuge tubes, immediately frozen in liquid nitrogen and stored for three weeks at –80 °C. Frozen leaf samples were homogenized to a fine powder using a GenoGrinder 2000 (OPS Diagnostics, Bridgewater, NJ, USA). Approximately 100 and 30 mg of the homogenized frozen powder were weighed and transferred to individual cooled tubes for peroxidase activity and phenolic content assays, respectively.
Peroxidase activity
A modification of the technique by Bi and Felton (1995) was used to measure peroxidase activity. Briefly, 500 µl of 0.1 M potassium phosphate buffer (pH 7.0) containing 0.5 mM EDTA and 1% polyvinyl pyrrolidone extraction buffer was added to the powdered leaf samples and vortexed for 10 s, kept on ice for 5 min, then centrifuged at 11,000×g for 10 min at 4 °C. 5 µl of supernatant were combined with 190 µl of 3 mM guaiacol and 10 µl of 1 mM hydrogen peroxide in 0.1 M potassium phosphate buffer. The change in absorbance was measured in a 96-well microplate at 450 nm using the Spectra Max 190 microplate reader (Molecular Devices, Silicon Valley, CA, USA). Peroxidase activity (mOD/min) per mg tissue was then calculated.
Total phenolic content
Ainsworth and Gillespie’s (2007) Folin-Ciocalteu (F–C) method was used to quantify total phenolics. To each powdered sample, 1.5 ml of ice-cold 95% methanol was added. Samples were vortexed for 15 s, incubated at room temperature for 48 h in the dark and centrifuged at 13,000×g for 5 min at room temperature. 100 µl of the supernatant were combined with 200 µl of F–C reagent and 800 µl of 700 mM Na2CO3, and incubated at room temperature for 2 h. 200 µl of the mixture was loaded into a 96-well microplate and the absorbance measured at 765 nm. A standard curve of absorbance measurements at 765 nm was obtained from gallic acid concentrations ranging from 0.005 to 0.04 mg/ml. The regression equation from the standard curve was used to calculate the total phenolic content as mM of phenolics (gallic acid equivalents) per fresh weight of the powdered leaf sample.
Larval weight gain, consumption and leaf utilization (no-choice experiment)
The degrees to which JA-treated (induced) plants from each genotype inhibited larval feeding and growth compared to control (constitutive) plants were assessed using a no-choice feeding experiment. A single leaf disk (3.14 cm2) was placed in the center of a 6 cm diameter petri dish lined with moist paper towel. A single newly moulted, unfed larva was weighed to the nearest 0.1 mg and placed in the dish, which was then sealed with parafilm and maintained at 29 °C and 16L:8D. After 14 h, the larva was removed and starved for 4–5 h to empty the gut and reweighed to determine weight gain. The remaining leaf disk was digitally scanned and the leaf area was measured using ImageJ 1.48 (NIH, http://imagej.nih.gov/ij/). We also calculated the conversion of ingested leaf area into larval body mass (leaf utilization = larval weight gain/leaf area consumed), because impairing digestion is an important component of plant resistance (Waldbauer 1968). For instance, on a plant that impairs digestion, an insect may gain little weight even though it consumes a large quantity of foliage. But on a plant that deters feeding without impairing digestion, the insect may gain the same amount of weight while consuming less foliage. There were two replicates from each plant, except for a few plants where only one leaf disk was obtained. Three dishes with control and JA-treated disks were set up for each genotype but without larvae to estimate a mean initial disk size. To determine if differences in larval consumption, growth and utilization were due to genotypic differences in leaf weight (or thickness), we obtained the average weight of three leaf disks (1.77 cm2) from five plants per genotype. The disks were then dried at 80 °C for 72 h and reweighed.
Since larvae used in each trial could differ in consumption and growth rate, we conducted the same experiment using a pre-weighed block of artificial diet. Larval weight gain was measured after 14 h of feeding and the remaining diet was dried to constant mass at 29 °C for 6 days and reweighed. Twelve dishes with pre-weighed diet blocks were set up without larvae. A regression equation of wet and dry weights for the diet blocks was used to estimate the initial dry mass of the diet. We then calculated the amount of dry diet consumed (diet consumed = estimated initial dry diet – dry diet remaining).
JA-induced feeding deterrence (choice experiment)
The level of JA-induced feeding deterrence was measured by giving S. frugiperda larvae a choice between a JA-treated and a control leaf disk. We placed one JA-treated and one control leaf disk (1.77 cm2) from the same genotype approximately 1 cm away from each other (secured with pins) in a 6 cm diameter petri dish lined with a moist paper towel. A single larva was placed on the edge of the petri dish equidistant from the two leaf disks, and the dish was sealed with parafilm and maintained at 29 °C and 16L:8D. Larvae fed on the leaf disks for 10 h and the remaining leaf disks were digitally scanned and leaf area measured. One to two leaf disks were used from each plant and no plant pairing combination was repeated. Three JA-treated and control leaf disks without larvae were measured to estimate the initial size of leaf disks for each genotype.
Larval susceptibility to baculovirus
To assess the effects of constitutive and JA-induced soybean defences on baculovirus efficacy, larvae were fed virus and foliage simultaneously. Comparable numbers of leaf disks (0.64 cm2) were removed from each plant, and placed on wet paper towels. There were 17–24 control disks and 36 leaf disks to be treated with virus for each plant treatment per genotype. A 2 µl droplet of ultrapure water containing 6000 OBs of SfMNPV was applied to each disk. Control disks were treated with just ultrapure water. When the droplet dried completely, leaf disks were placed individually in wells of a 12-well cell culture plate lined with moist filter paper. Newly moulted, unfed larvae were placed individually in the wells. Moist paper towelling was placed under the lid and the plate was maintained at 29 °C and 16L:8D for 22 h. Larvae that consumed the entire leaf disk were transferred to individual 30 ml plastic containers with ad libitum artificial diet until death or pupation. Mortality was recorded every 8 h.
To assess potential differences in baculovirus susceptibility among larvae used in the three separate trials, additional larvae from each bioassay were fed 6000 OBs of SfMNPV on a piece of artificial diet, which weighed approximately the same as a leaf disk (10 mg). Control diets were treated with water only. Larvae were handled thereafter as described above.
Finally, since we only conducted one replicate of the virus bioassay at a single virus dose for each soybean genotype, we repeated the bioassay for five of the genotypes at multiple doses to determine if any genotype and JA treatment effects on virus-induced mortality were consistent across replicates and doses. Dose-response bioassays for each genotype were conducted at different times due to logistical constraints. Lamar and Stonewall genotypes were grown in a walk-in growth chamber as described above. Bragg, Braxton and Gasoy genotypes were grown in a temperature-controlled greenhouse at 25 °C and 16L:8D supplemented with high pressure sodium lights, and using the same pots, growing mix and fertilizer as described above. 30–40 plants of each genotype were grown and divided equally into jasmonic acid or control treatments. Virus bioassays were conducted as described above, using 5–6 SfMNPV doses (ranging from 500 to 45,000 OBs suspended in a 2 µl droplet of ultrapure water) and a water-only control.
Statistical analyses
No-choice experiment and soybean phenolic content and peroxidase activity
We examined if larvae used in the three trials differed in their weight gain, consumption and diet utilization on artificial diet using analyses of covariance (ANCOVA) with initial larval weight as the covariate. There were no significant differences between larvae used in the three trials (weight gain, F 2,32 = 0.14, p = 0.87; consumption, F 2,32 = 1.15, p = 0.33; diet utilization, F 2,32 = 1.28, p = 0.29). Thus, we pooled data from all soybean genotypes for analysis. Additionally, we used the area of leaf disk ingested, rather than weight, for both larval consumption and utilization measurements because leaf disks from different soybean genotypes did not differ significantly in fresh or dry weight (fresh weight, F 7,32 = 0.66, p = 0.71; dry weight, F 7,32 = 1.67, p = 0.15). Since up to two leaf disks were used from each plant, we used restricted maximum likelihood (REML) with plant included as a random effect, and initial larval weight as a covariate. The denominator degrees of freedom are fractional as they are based on approximation of the distribution of the statistic from when the covariance matrix is adjusted using the Kenward–Roger correction. Phenolic content and peroxidase activity were analyzed by ANCOVA with the microplate included as a covariate. Phenolic content was square root transformed to achieve normality. Variation in constitutive resistance among genotypes was analyzed using data on only the control plants. To assess the impact of JA-induction, we added JA-treatment and the interaction between genotype and JA-treatment as fixed effects. Tukey HSD tests were performed to compare differences in constitutive resistance among genotypes and means contrasts were performed to determine the effect of JA-treatment on each genotype.
Choice experiment
The feeding deterrent effect induced by JA treatment was assessed by the ratio of areas of control and JA-treated disks consumed [c/(c + i), where c and i indicate the ingested areas of control and JA-treated (induced) disks]. The distributions of these ratios were normalized by an arcsine transformation and tested using one-sample, one-tailed t tests for each individual soybean genotype. Results are presented as a deterrence index [DI = 2(c/(c + i)] (Underwood et al. 2000). DI = 1 indicates no preference for either disk, while DI >1 indicates preference for the control disk (i.e., deterred by JA-treated disk). Feeding deterrence (DI >1) was tested at α = 0.0063 (multiple comparisons corrected by the Bonferroni method). Differences in induced feeding deterrence among genotypes were analyzed by ANOVA.
Mortality from virus
First, we assessed if there were differences in susceptibility among larvae used in the three trials. We used a generalized linear model (GLM) with a binomial error distribution and logit link function; we found that the larvae from the three trials were equally susceptible to the virus when the virus was ingested on a piece of artificial diet (0.80 ± 0.03 (mean proportional mortality ± SE); \(X_{1}^{2}\) = 0.94, p = 0.33). Thus, mortality data from all soybean genotypes were analyzed together using GLM and time to death was analyzed using a Cox proportional hazards model. Variation in mortality of larvae that ingested virus on different soybean genotypes expressing constitutive resistance was analyzed using data from only the control plants. To assess the impact of induced soybean resistance in the single dose bioassay, we added JA-treatment and the interaction between genotype and JA-treatment as fixed effects. For the dose-response analysis, log10 dose and its interactions were added to the model.
Relationship between larval susceptibility to baculovirus and soybean resistance traits
The relationship between virus infectivity (mean mortality and time to death from single dose bioassay) and each measure of soybean resistance (least squared mean larval weight gain, consumption, leaf utilization, phenolic content and peroxidase activity) was analyzed using multiple linear regression, with JA-treatment and the interaction between JA-treatment and the soybean resistance measure included as predictor variables. Additionally, we used linear regression to assess how the relative change in virus-induced mortality and peroxidase activity [(c − i)/c, where c and i indicate mortality or peroxidase activity on control and JA-treated foliage, respectively] relates to JA-induced feeding deterrence.
Results
Phenolic content and peroxidase activity
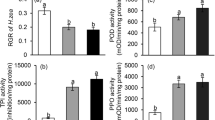
Soybean genotypes varied markedly in constitutive phenolic content (Fig. 1a; F 7,42 = 5.76, p = 0.0001), but not in constitutive peroxidase activity (Fig. 1b; F 7,37 = 1.13, p = 0.36). JA-treatment did not significantly affect total phenolic content (JA-treatment, F 1,90 = 0.17, p = 0.68; genotype, F 7,90 = 9.33, p < 0.0001; JA-treatment by genotype, F 7,90 = 0.61, p = 0.75). Peroxidase activity was marginally higher in JA-treated plants than control plants (F 1,76 = 3.95, p = 0.050), but the only genotype driving this difference was Gasoy (means contrast); although there was no JA-treatment by genotype interaction (F 7,76 = 1.57, p = 0.16).
a Phenolic content (mM gallic acid equivalents/g tissue) and b peroxidase activity (mOD/min/mg tissue) in JA-treated or control plants from eight soybean genotypes used in the larval feeding and virus bioassays. Values represent least squared means (±SE). Different letters above grey bars indicate significant differences in constitutive resistance (Tukey HSD test). Asterisk above bars indicate significant differences in peroxidase activity between control and JA-induced treatments for each genotype (means contrasts; α < 0.05)
No-choice experiment—insect feeding and growth
Constitutive resistance against S. frugiperda varied among soybean genotypes (Fig. 2; larval weight gain, F 7,48.6 = 7.04, p < 0.0001; consumption, F 7,45.6 = 2.38, p = 0.037; utilization, F 7,46.5 = 2.71, p = 0.019). Larvae feeding on leaf disks from JA-treated plants gained significantly less weight (F 1,93.8 = 300, p < 0.0001; Fig. 2a), ate less (F 1,91.5 = 169, p < 0.0001; Fig. 2b) and produced less body mass per area of disk consumed (F 1,91.4 = 20.04, p < 0.0001; Fig. 2c). The direction and magnitude of the JA-induced effects were similar among genotypes (no interaction effect; weight gain, F 7,93.6 = 1.59, p = 0.15; consumption, F 7,91.3 = 1.42, p = 0.21; utilization, F 7,91.4 = 1.10, p = 0.37).
a Larval weight gain, b leaf area consumed and c leaf utilization on control and JA-treated leaf disks from eight soybean genotypes (no-choice experiment). Values represent least squared means (±SE). Different letters above grey bars indicate significant differences in constitutive resistance. Asterisks indicate significant differences between control and JA-induced treatments for each genotype (means contrast; †α < 0.10, *α < 0.05 and **α < 0.0063)
Choice experiment—JA-induced feeding deterrence (DI)
Soybean genotypes differed in the level of JA-induced feeding deterrence (F 7,93 = 3.79, p = 0.001; Fig. 3a). Less leaf area was consumed of the JA-treated leaf disks from all genotypes, except Bragg, compared with control leaf disks (t test). Greater JA-induced feeding deterrence was associated with higher peroxidase activity in JA-treated plants relative to control plants (Fig. 3b).
a Levels of induced feeding deterrence (±SE) in the choice experiment. The deterrence index (DI) ranges from 0 to 2, where 1 means that both control and JA-treated disks are equally preferred, and values >1 indicate deterrence from JA-treated disk (or preference for control disk). Asterisks indicate significant induced deterrence for each genotype [DI >1, one-tailed t test, α = 0.0063 (corrected for eight comparisons)]. b Induced feeding deterrence plotted against the times increase in peroxidase activity in JA-treated foliage relative to control foliage, calculated as [1 + (i – c)/c] where c and i are the peroxidase activity in control and JA-treated foliage, respectively. Values >1 indicate JA-induced increase in peroxidase activity
Relationships between the indicators of phenolic oxidation and soybean resistance against the insect
Higher phenolic content in the foliage was associated with reduced larval weight gain (Table 1). There was no significant relationship between phenolic content and leaf area consumed or leaf utilization, and peroxidase activity was not significantly associated with any of the insect resistance measures (Table 1).
Virus-induced mortality
Mortality did not differ significantly among soybean genotypes when larvae ingested the virus on leaf disks expressing constitutive resistance (\(X_{7}^{2}\) = 8.41, p = 0.30; Fig. 4a). However, adding mortality of larvae that ingested the virus on JA-treated plants to the analysis resulted in a strong main effect of soybean genotypes (\(X_{7}^{2}\) = 21.50, p = 0.003). This occurred because virus-induced mortality was significantly lower when the virus was ingested on leaf disks from JA-treated plants compared to non-induced plants (\(X_{1}^{2}\) = 6.60, p = 0.010), particularly for the Bragg and Braxton genotypes (24 and 31% lower mortality, respectively), although there was no JA-treatment by genotype interaction (\(X_{7}^{2}\) = 7.87, p = 0.34). No control larvae (i.e., larvae not exposed to virus) died before pupation. In contrast to mortality, the speed at which the virus killed its host was strongly influenced by the constitutive resistance of soybean genotypes ingested with the virus (\(X_{7}^{2}\) = 18.38, p = 0.01; Fig. 4b), differing by more than 22 h between the fastest (Davis) and slowest (Bragg) plant genotypes. JA-treatment did not significantly affect time to death (JA-treatment, \(X_{1}^{2}\) = 1.75, p = 0.19; genotype, \(X_{7}^{2}\) = 27.58, p = 0.0003; JA-treatment by genotype, \(X_{7}^{2}\) = 5.82, p = 0.56).
a Proportional mortality and b time to death of larvae fed virus (6000 OBs of SfMNPV) on JA-treated or control leaf disks from eight soybean genotypes. Different letters above bars indicate significant differences in time to death among soybean genotypes expressing constitutive resistance (based on risk ratios; α < 0.0063, corrected for eight comparisons)
The dose-response bioassay also showed that virus-induced mortality did not differ significantly among soybean genotypes when larvae ingested the virus on leaf disks expressing constitutive resistance (genotype: \(X_{4}^{2}\) = 8.41, p = 0.30; dose: \(X_{1}^{2}\) = 355.76, p < 0.0001; genotype by dose: \(X_{4}^{2}\) = 5.11, p = 0.28; Online resource 1). Similar to the single dose bioassay, adding mortality of larvae that ingested the virus doses on JA-treated plants to the analysis resulted in a strong main effect of soybean genotype (\(X_{4}^{2}\) = 13.89, p = 0.008). The effect of JA-treatment on virus-induced mortality varied depending on the soybean genotype (genotype by JA-treatment: \(X_{4}^{2}\) = 11.95, p = 0.018; JA-treatment: \(X_{1}^{2}\) = 20.68, p < 0.0001; Online resource 2). Specifically, mortality was significantly lower when the virus was ingested on leaf disks from JA-treated plants compared to non-induced plants for the Bragg and Braxton genotypes (Bragg, \(X_{1}^{2}\) = 13.28, p = 0.0003; Braxton, \(X_{1}^{2}\) = 15.15, p < 0.0001), but not for the Gasoy, Lamar or Stonewall genotypes (Gasoy, \(X_{1}^{2}\) = 1.48, p = 0.22; Lamar, \(X_{1}^{2}\) = 0.03, p = 0.87; Stonewall, \(X_{1}^{2}\) = 1.15, p = 0.28). The dose-response of mortality was consistent among genotypes and JA-treatment (genotype by dose: \(X_{4}^{2}\) = 6.45, p = 0.17; JA-treatment by dose: \(X_{1}^{2}\) = 1.97, p = 0.16; genotype by JA-treatment by dose: \(X_{4}^{2}\) = 2.89, p = 0.58).
Relationship between insect susceptibility to virus and indicators of phenolic oxidation in soybean foliage
Virus-induced mortality was lower when the virus was ingested with foliage containing higher phenolic content (Fig. 5a; Table 2). Mortality was lower in JA-treated plants at any given phenolic content, and the slope of the relationship was the same between control and JA-treated plants (no phenolic content by JA treatment interaction). Increasing phenolic content was also associated with slower time to death, but this did not vary with JA-treatment (Fig. 5c; Table 2).
The impact of phenolic content (mM gallic acid equivalents/g tissue) and peroxidase activity (mOD/min/mg tissue) in the foliage of control and JA-treated soybean genotypes on (a, b) virus-induced mortality and (c, d) time to death. The lines represent fitted lines from the final minimal model. Two lines are presented if there was a significant effect of JA-treatment, (long dashed control plants, short dashed JA-treated plants)
Virus-induced mortality was higher when the virus was ingested with foliage containing greater peroxidase activity (Fig. 5b; Table 2), and mortality was lower when virus was ingested on JA-treated plants at any level of peroxidase activity. There was no relationship between peroxidase activity and time to death (Fig. 5d; Table 2).
Relationship between insect susceptibility to virus and soybean resistance against insect
We assessed the relationship between the resistance levels of soybean genotypes against S. frugiperda in the no-choice feeding experiment and the susceptibility of S. frugiperda to virus when it ingested the virus on a leaf disk from the same plants used in the no-choice experiment. Virus-induced mortality was lower when the virus was ingested with leaf disks from soybean genotypes that more strongly inhibited larval weight gain and from genotypes that more effectively reduced the ability of larvae to convert ingested leaf area into body mass (Fig. 6a, c; Table 3), and these effects were not influenced by JA-treatment. Larvae took longer to die when they ingested virus on soybean genotypes that more strongly inhibited larval weight gain and leaf utilization (Fig. 6d, f; Table 3). These relationships were consistent on control and JA-treated plants (i.e., same slope), even though larval weight gain was significantly lower on JA-treated plants.
The impact of soybean resistance (mean insect weight gain, area of leaf disk ingested and leaf utilization), measured for control and JA-treated soybean genotypes in the no-choice experiment, on (a, b, c) virus-induced mortality and (d, e, f) time to death. The lines represent fitted lines from the final minimal model. Two lines are presented if there was a significant effect of JA-treatment, (long dashed control plants, short dashed JA-treated plants)
Ingestion of virus with leaf disks from soybean genotypes that reduced the feeding rate of larvae in the no-choice experiment was not associated with virus-induced mortality (Fig. 6b; Table 3) or time to death (Fig. 6e; Table 3).
Higher JA-induced feeding deterrence in the choice experiment was associated with lower JA-induced inhibition of virus-induced mortality (Fig. 7). JA-induced changes in virus-induced mortality were not associated with changes in larval weight gain, consumption, leaf utilization, or peroxidase activity (Online resource 3).
The relative difference in mortality between larvae challenged with virus on control and JA-treated disks plotted against JA-induced feeding deterrence. Mortality values >0 indicate that virus-induced mortality was lower on JA-treated leaf disks than control disks. The line represents a significant relationship. Bg Bragg, Bx Braxton, D Davis, G Gasoy 17, J Johnston, L Lamar, S Stonewall, W Williams
Discussion
Plants with high levels of constitutive and induced resistance against insect pests are suggested to be incompatible for baculoviruses to serve as effective “plant bodyguards” (Felton et al. 1987; Hunter and Schultz 1993; Hoover et al. 1998b, c), because plant chemical defenses can inhibit lethal infection by baculoviruses (Hoover et al. 1998a, b). Here, we showed that the differential effect of JA-induction, but not constitutive resistance, among soybean genotypes on baculovirus infectivity was associated with the genotypic variation in the levels of baculovirus-induced mortality (Fig. 4a). Soybean plants with higher phenolic content, and those that inhibited the growth and leaf utilization of fall armyworm larvae, were associated with lower baculovirus lethality. However, the inhibition of larval feeding (leaf area consumed), which is probably the most ecologically and economically relevant measure of plant resistance, was not associated with levels of virus-induced mortality (Fig. 6). Moreover, greater JA-induced feeding deterrence in the choice experiment was associated with less JA-induced inhibition of virus-induced mortality (Fig. 7). Higher peroxidase activity was only associated with deterring feeding and not other measures of soybean resistance (reduced insect weight gain, consumption and leaf utilization) and phenolic content was only associated with one measure of resistance against fall armyworm (reduced insect weight gain). These suggest that there is a complex network of interactions among numerous resistance mechanisms, rather than a single mechanism, in determining the outcome of plant resistance (Carmona et al. 2011; Rasmann et al. 2015). Together, these findings suggest that plant resistance can be compatible with baculoviruses, provided that phenolic content is limited. The findings also suggest that the plant-mediated inhibition of baculovirus infectivity may be a cost or trade-off associated with different resistance strategies of plants against insect herbivores.
Interestingly, virus-induced mortality was reduced when the virus was ingested on JA-induced foliage even though there was no JA-induced increase in total phenolic content. This may have resulted from a JA-induced change in the composition of different classes of phenolic substrates. Different classes of phenolic substrates have been linked to differing levels of baculovirus-induced mortality (Hoover et al. 1998a, b), because phenolic substrates, mainly some of the catecholic phenolics, have a variety of activities depending on the absence or presence of enzymes, such as peroxidase and polyphenol oxidase, their relative concentrations and chemical context (summarized in Duffey et al. 1995). Additionally, phenolic species can act as anti-oxidants or pro-oxidants, depending on redox potential. Thus, the interactions that occur in vivo (within the insect gut) depend on phytochemical mixture, as well as physiological and chemical properties of the gut, such as pH and digestive enzymes, which together determine the final outcome of herbivore responses to pathogens and plant defences (Duffey et al. 1995; Salminen and Karonen 2011). Moreover, induced peroxidase activity in cotton and tomato plants has been implicated in the reduced infectivity of baculoviruses (Hoover et al. 1998a, b, c). However, peroxidase activity in most soybean genotypes was unaffected by JA-treatment, and virus-induced mortality increased when the virus was ingested with foliage containing higher levels of peroxidase activity (Fig. 5b). Thus, other JA-induced phytochemicals may have influenced baculovirus-induced mortality in some soybean genotypes. Together, these highlight complex interactions among soybean resistance mechanisms and the consequences it can have for plant–insect and plant–insect-pathogen interactions.
Bottom-up plant effects on mortality by pathogens can occur by direct effects on the infectivity of virions, but can also occur indirectly prior to and after pathogen challenge by influencing the physiology of the host. For example, the quality of plants eaten before exposure to pathogens may influence virus entry and/or establishment of infections by altering host immune functioning (Shikano et al. 2010; Martemyanov et al. 2012, 2015; Shikano et al. 2015) and physical barriers to infection, such as the thickness of the peritrophic matrix (Plymale et al. 2008) and the rate of sloughing of infected midgut cells (Hoover et al. 2000). Consumption of higher protein diets after baculovirus-challenge are associated with a higher probability of survival in noctuid larvae (Lee et al. 2006; Povey et al. 2013; Shikano and Cory 2015).
To better predict the effects of plants on the outcome of insect–pathogen interactions, we need to determine how the multitude of indirect plant effects work in concert with direct phytochemical effects. Ali et al. (1998) showed that when H. zea and H. virescens larvae were reared throughout development on non-induced or herbivore-induced plants, their susceptibility to H. zea NPV was the same, and even higher on some induced plants. This lack of an induced plant effect on baculovirus lethality could simply result from the use of different plant genotypes and/or cultivars. However, reduced immune functioning (reduced encapsulation of parasitoid eggs) has been demonstrated in Pieris rapae larvae fed induced foliage (Bukovinszky et al. 2009), although increased lysozyme-like activity has been recorded in Lymantria dispar larvae fed leaves of silver birch that had previous defoliation history (delayed induced resistance) (Martemyanov et al. 2012). Moreover, induced plant resistance can reduce leaf consumption rate of herbivores. Reduced consumption rate can decrease the probability of ingesting virus-contaminated foliage within a given amount of time (Elderd and Reilly 2014), but it could also slow growth and decrease the rate of sloughing of infected gut cells, thereby delaying developmental resistance (Shikano and Hoover, manuscript in preparation). Indirect negative effects of induced plant resistance on herbivore immune functioning and other physiological and behavioural resistance mechanisms might offset the direct phytochemical inhibition of baculovirus infectivity. Plant quality can also influence several other aspects of baculovirus fitness, such as speed of kill (Hoover et al. 2000; Raymond et al. 2002), yield (Ali et al. 2002; Raymond and Hails 2007) and infectivity of the progeny virus (Cory and Myers 2004), which will impact the sustained protection of plants.
The compatibility of certain plant resistance traits with baculovirus efficacy may have significant implications for the evolution of plant–insect-baculovirus interactions. Much progress has been made regarding our understanding of the influence of variation in plant resistance on herbivore-natural enemy interactions, and the reciprocal selection pressures exerted by natural enemies on plant resistance traits against herbivores (Ode 2006). While the idea of insect pathogens acting as plant bodyguards and potentially exerting selection pressure on plant defence have been proposed (Elliot et al. 2000; Cory and Hoover 2006), empirical evidence is still lacking. Baculoviruses have tremendous potential in this respect because they persist in soil reservoirs and are continuously transported to plant surfaces, through wind and rain, where host insects can contact them (Fuxa and Richter 2001). A theoretical model suggests that pathogens, such as baculoviruses, that can translocate from pathogen reservoirs to host habitats, can regulate host populations to low and relatively constant densities (Hochberg 1989). During baculovirus epizootics, infections can be widespread among populations, and infection rates within a host population can exceed 90% (Cory and Myers 2003). Moreover, induced resistance in soybean plants can be costly and may not translate into fitness benefits for the plants (Accamando and Cronin 2012). Since our findings indicate that some plant genotypes with low phenolic content can resist herbivory without inhibiting baculovirus efficacy, we hypothesize that the fitness of these plants would benefit in the presence of herbivorous insects and their baculoviruses. Thus, in plant populations where insect pressure is high and effective viral reservoirs are available, there may be selective pressure for plants to reallocate investments in resistance mechanisms from those that inhibit virus efficacy to those that do not. Identifying selectable plant traits that improve the compatibility between plant resistance and insect pathogens could also be valuable for agriculture, as these selectable traits could become targets to develop new agricultural varieties.
References
Accamando AK, Cronin JT (2012) Costs and benefits of jasmonic acid induced responses in soybean. Environ Entomol 41:551–561
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. doi:10.1038/nprot.2007.102
Ali MI, Felton GW, Meade T, Young SY (1998) Influence of interspecific and intraspecific host plant variation on the susceptibility of Heliothines to a baculovirus. Biol Control 12:42–49. doi:10.1006/bcon.1998.0619
Ali MI, Young SY, Felton GW, McNew RW (2002) Influence of the host plant on occluded virus production and lethal infectivity of a baculovirus. J Invertebr Pathol 81:158–165
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19:1521–1552. doi:10.1007/BF00984895
Bailey JK, Wooley SC, Lindroth RL, Whitham TG (2006) Importance of species interactions to community heritability: a genetic basis to trophic-level interactions. Ecol Lett 9:78–85. doi:10.1111/j.1461-0248.2005.00844.x
Bangert RK, Allan GJ, Turek RJ et al (2006) From genes to geography: a genetic similarity rule for arthropod community structure at multiple geographic scales. Mol Ecol 15:4215–4228. doi:10.1111/j.1365-294X.2006.03092.x
Bi JL, Felton GW (1995) Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J Chem Ecol 21:1511–1530. doi:10.1007/BF02035149
Biere A, Bennett AE (2013) Three-way interactions between plants, microbes and insects. Funct Ecol 27:567–573. doi:10.1111/1365-2435.12100
Biere A, Tack AJM (2013) Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Funct Ecol 27:646–660. doi:10.1111/1365-2435.12096
Bukovinszky T, Poelman EH, Gols R et al (2009) Consequences of constitutive and induced variation in plant nutritional quality for immune defence of a herbivore against parasitism. Oecologia 160:299–308. doi:10.1007/s00442-009-1308-y
Bukovinszky T, van Veen FJF, Jongema Y, Dicke M (2008) Direct and indirect effects of resource quality on food web structure. Science 319:804–807. doi:10.1126/science.1148310
Carmona D, Lajeunesse MJ, Johnson MT (2011) Plant traits that predict resistance to herbivores. Funct Ecol 25:358–367. doi:10.1111/j.1365-2435.2010.01794.x
Chung SH, Rosa C, Scully ED et al (2013) Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci USA 110:15728–15733. doi:10.1073/pnas.1308867110
Cory JS, Hoover K (2006) Plant-mediated effects in insect–pathogen interactions. Trends Ecol Evol 21:278–286. doi:10.1016/j.tree.2006.02.005
Cory JS, Myers JH (2003) The ecology and evolution of insect baculoviruses. Annu Rev Ecol Evol Syst 34:239–272. doi:10.1146/132402
Cory JS, Myers JH (2004) Adaptation in an insect host-plant pathogen interaction. Ecol Lett 7:632–639. doi:10.1111/j.1461-0248.2004.00617.x
Duffey SS, Hoover K, Bonning B, Hammock BD (1995) The impact of host plant on the efficacy of baculoviruses. In: Roe RM, Kuhr RJ (eds) Reviews in pesticide toxicology (USA). Toxicology Communications Inc., Raleigh, pp 137–275
Elderd BD, Reilly JR (2014) Warmer temperatures increase disease transmission and outbreak intensity in a host-pathogen system. J Anim Ecol 83:838–849. doi:10.1111/1365-2656.12180
Elliot SL, Sabelis MW, Janssen A et al (2000) Can plants use entomophagens as bodyguards? Ecol Lett 3:228–235
Engelhard EK, Kam-Morgan LN, Washburn JO, Volkman LE (1994) The insect tracheal system: a conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc Natl Acad Sci USA 91:3224–3227
Farrar RR, Ridgway RL (2000) Host plant effects on the activity of selected nuclear polyhedrosis viruses against the corn earworm and beet armyworm (Lepidoptera: Noctuidae). Environ Entomol 29:108–115. doi:10.1603/0046-225X-29.1.108
Felton GW, Donato KK, Broadway RM, Duffey SS (1992) Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol 38:277–285. doi:10.1016/0022-1910(92)90128-Z
Felton GW, Duffey SS (1990) Inactivation of baculovirus by quinones formed in insect-damaged plant tissues. J Chem Ecol 16:1221–1236. doi:10.1007/BF01021021
Felton GW, Duffey SS, Vail PV et al (1987) Interaction of nuclear polyhedrosis virus with catechols: potential incompatibility for host-plant resistance against noctuid larvae. J Chem Ecol 13:947–957
Frago E, Dicke M, Godfray HCJ (2012) Insect symbionts as hidden players in insect–plant interactions. Trends Ecol Evol 27:705–711. doi:10.1016/j.tree.2012.08.013
Fuxa JR, Geaghan JP (1983) Multiple-regression analysis of factors affecting prevalence of nuclear polyhedrosis virus in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations. Environ Entomol 12:311–316
Fuxa JR, Richter AR (2001) Quantification of soil-to-plant transport of recombinant nucleopolyhedrovirus: effects of soil type and moisture, air currents, and precipitation. Appl Environ Microbiol 67:5166–5170. doi:10.1128/AEM.67.11.5166-5170.2001
Gols R (2014) Direct and indirect chemical defences against insects in a multitrophic framework. Plant Cell Environ 37:1741–1752. doi:10.1111/pce.12318
Gruner DS, Taylor AD (2006) Richness and species composition of arboreal arthropods affected by nutrients and predators: a press experiment. Oecologia 147:714–724. doi:10.1007/s00442-005-0337-4
Hochberg ME (1989) The potential role of pathogens in biological control. Nature 337:262–265. doi:10.1038/337262a0
Hoover K, Kishida KT, DiGiorgio LA et al (1998a) Inhibition of baculoviral disease by plant-mediated peroxidase activity and free radical generation. J Chem Ecol 24:1949–2001
Hoover K, Stout MJ, Alaniz SA et al (1998b) Influence of induced plant defenses in cotton and tomato on the efficacy of baculoviruses on noctuid larvae. J Chem Ecol 24:253–271
Hoover K, Washburn JO, Volkman LE (2000) Midgut-based resistance of Heliothis virescens to baculovirus infection mediated by phytochemicals in cotton. J Insect Physiol 46:999–1007
Hoover K, Yee JL, Schultz CM et al (1998c) Effects of plant identity and chemical constituents on the efficacy of a baculovirus against Heliothis virescens. J Chem Ecol 24:221–252
Hunter MD, Schultz JC (1993) Induced plant defenses breached? Phytochemical induction protects an herbivore from disease. Oecologia 94:195–203
Johnson MTJ, Agrawal AA (2005) Plant genotype and environment interact to shape a diverse arthropod community on evening primrose (Oenothera biennis). Ecology 86:874–885
Keating ST, Yendol WG, Schultz JC (1988) Relationship between susceptibility of gypsy moth larvae (Lepidoptera: Lymantriidae) to a baculovirus and host plant foliage constituents. Environ Entomol 17:952–958
Lee KP, Cory JS, Wilson K et al (2006) Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc R Soc B Biol Sci 273:823–829. doi:10.1098/rspb.2005.3385
Lege KE, Cothren JT, Smith CW (1995) Phenolic acid and condensed tannin concentrations of six cotton genotypes. Environ Exp Bot 35:241–249. doi:10.1016/0098-8472(94)00051-6
Malenčić D, Popović M, Miladinović J (2007) Phenolic content and antioxidant properties of soybean (Glycine max (L.) Merr.) seeds. Molecules 12:576–581. doi:10.3390/12030576
Martemyanov VV, Dubovskiy IM, Rantala MJ et al (2012) The effects of defoliation-induced delayed changes in silver birch foliar chemistry on gypsy moth fitness, immune response, and resistance to baculovirus infection. J Chem Ecol 38:295–305. doi:10.1007/s10886-012-0090-1
Martemyanov VV, Pavlushin SV, Dubovskiy IM et al (2015) Asynchrony between host plant and insects-defoliator within a tritrophic system: the role of herbivore innate immunity. PLoS One 10:e0130988. doi:10.1371/journal.pone.0130988
Núñez-Farfán J, Fornoni J, Valverde PL (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38:541–566. doi:10.1146/annurev.ecolsys.38.091206.095822
Ode PJ (2006) Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy interactions. Annu Rev Entomol 51:163–185. doi:10.1146/annurev.ento.51.110104.151110
Plymale R, Grove MJ, Cox-Foster D et al (2008) Plant-mediated alteration of the peritrophic matrix and baculovirus infection in lepidopteran larvae. J Insect Physiol 54:737–749. doi:10.1016/j.jinsphys.2008.02.005
Poelman EH, van Loon JJA, Dicke M (2008) Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant Sci 13:534–541. doi:10.1016/j.tplants.2008.08.003
Povey S, Cotter SC, Simpson SJ, Wilson K (2013) Dynamics of macronutrient self-medication and illness-induced anorexia in virally infected insects. J Anim Ecol 83:245–255. doi:10.1111/1365-2656.12127
Rasmann S, Chassin E, Bilat J et al (2015) Trade-off between constitutive and inducible resistance against herbivores is only partially explained by gene expression and glucosinolate production. J Exp Bot 66:2527–2534. doi:10.1093/jxb/erv033
Raymond B, Hails RS (2007) Variation in plant resource quality and the transmission and fitness of the winter moth, Operophtera brumata nucleopolyhedrovirus. Biol Control 41:237–245. doi:10.1016/j.biocontrol.2007.02.005
Raymond B, Vanbergen A, Pearce I et al (2002) Host plant species can influence the fitness of herbivore pathogens: the winter moth and its nucleopolyhedrovirus. Oecologia 131:533–541. doi:10.1007/s00442-002-0926-4
Salminen J-P, Karonen M (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25:325–338. doi:10.1111/j.1365-2435.2010.01826.x
Shikano I, Cory JS (2015) Impact of environmental variation on host performance differs with pathogen identity: implications for host-pathogen interactions in a changing climate. Sci Rep 5:15351. doi:10.1038/srep15351
Shikano I, Ericsson JD, Cory JS, Myers JH (2010) Indirect plant-mediated effects on insect immunity and disease resistance in a tritrophic system. Basic Appl Ecol 11:15–22. doi:10.1016/j.baae.2009.06.008
Shikano I, Olson GL, Cory JS (2015) Impact of non-pathogenic bacteria on insect disease resistance: importance of ecological context. Ecol Entomol 40:620–628. doi:10.1111/een.12235
Sreenivasulu N, Ramanjulu S, Ramachandra-Kini K et al (1999) Total peroxidase activity and peroxidase isoforms as modified by salt stress in two cultivars of fox-tail millet with differential salt tolerance. Plant Sci 141:1–9. doi:10.1016/S0168-9452(98)00204-0
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Summers CB, Felton GW (1994) Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochem Mol Biol 24:943–953. doi:10.1016/0965-1748(94)90023-X
Thaler JS, Stout MJ, Karban R, Duffey SS (1996) Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J Chem Ecol 22:1767–1781. doi:10.1007/BF02028503
Underwood N (1998) The timing of induced resistance and induced susceptibility in the soybean-Mexican bean beetle system. Oecologia 114:376–381
Underwood N, Morris W, Gross K, Lockwood JR III (2000) Induced resistance to Mexican bean beetles in soybean: variation among genotypes and lack of correlation with constitutive resistance. Oecologia 122:83–89. doi:10.1007/PL00008839
Underwood N, Rausher M, Cook W (2002) Bioassay versus chemical assay: measuring the impact of induced and constitutive resistance on herbivores in the field. Oecologia 131:211–219. doi:10.1007/s00442-002-0867-y
Waldbauer GP (1968) The consumption and utilization of food by insects. Adv In Insect Phys 5:229–288. doi:10.1016/S0065-2806(08)60230-1
Wood KR, Barbara DJ (1971) Peroxidase isoenzymes in leaves of cucumber (Cucumis sativus L.) cultivars systemically infected with the W strain of cucumber mosaic virus. Physiol Plant Pathol 1:73–81
Zhao Y, Botella MA, Subramanian L et al (1996) Two wound-inducible soybean cysteine proteinase inhibitors have greater insect digestive proteinase inhibitory activities than a constitutive homolog. Plant Physiol 111:1299–1306
Acknowledgements
We thank Bret Elderd and Michael Stout for helpful discussions of the study system and experimental design, Morgan Kimmel for laboratory assistance, and Jennifer Thaler and two anonymous referees for their constructive comments on the manuscript. This work was supported by a National Science Foundation Grant (1316334) to K.H.
Author contribution statement
IS, KH, GWF conceived and designed the experiments. IS, KLS, MP performed the experiments. IS analyzed the data. IS, KLS, KH wrote the manuscript; MP, GWF provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jennifer Thaler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shikano, I., Shumaker, K.L., Peiffer, M. et al. Plant-mediated effects on an insect–pathogen interaction vary with intraspecific genetic variation in plant defences. Oecologia 183, 1121–1134 (2017). https://doi.org/10.1007/s00442-017-3826-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3826-3