Abstract
Investigating factors which affect the decline in survival with age, i.e. actuarial senescence, is important in order to understand how demographic rates vary in wild populations. Although the evidence for the occurrence of actuarial senescence in wild populations is growing, very few studies have compared actuarial senescence rates between wild populations of the same species. We used data from a long-time study of demography of house sparrows (Passer domesticus) to investigate differences in rates of actuarial senescence between habitats and sub-populations. We also investigated whether rates of actuarial senescence differed between males and females. We found that rates of actuarial senescence showed large spatial variation. We also found that the onset of actuarial senescence varied between sub-populations. However, these differences were not significantly explained by a general difference in habitat type. We also found no significant difference in actuarial senescence rates between males and females. This study shows that senescence rates in natural populations may vary significantly between sub-populations and that failing to account for such differences may give a biased estimate of senescence rates of a species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evidence supporting the hypothesis that senescence (e.g. decline in survival and/or reproduction with age) occurs in wild populations has become substantial in recent decades. This has also led to an increasing interest in the underlying mechanisms that may influence senescence (see Nussey et al. 2013 for a review). The fundamental evolutionary mechanism(s) explaining the occurrence of actuarial senescence (i.e. survival senescence) has been attributed to the decline of natural selection with age (Medawar 1952; Hamilton 1966). Williams (1957) expanded this work and provided the antagonistic pleiotropy theory of ageing, which states that an allele with a positive effect on reproduction early in life may be selected even if it has a negative effect on survival later in life. Subsequently, Kirkwood (1977) proposed the disposable soma theory of ageing. Both the antagonistic pleiotropy theory and the disposable soma theory share the same prediction of a trade-off between reproduction and/or growth during early life and intensity of ageing later in life (Nussey et al. 2013; Lemaitre et al. 2015). Because the energy available to an individual is finite, senescence may be expected to start at the age of maturity (but see Brunet-Rossinni and Austad 2006) and manifest itself within the normal lifespan of the species (Nussey et al. 2013).
Rates of actuarial senescence (hereafter senescence) in wild populations may be significantly influenced by the environment. Specifically, if a population is exposed to environments that increase mortality, this may amplify the rate of senescence under particular circumstances (Caswell 2007). For instance, it has been shown that a high level of predation (e.g. Dhondt et al. 1998) or an increased competition between individuals for resources (i.e. density dependence; e.g. Altwegg et al. 2003) may increase the rate of senescence (Nussey et al. 2007). Predation and density may even interact with each other in affecting senescence rates (e.g. Balbontin and Møller 2015). Because these sources of mortality may vary among populations, one may also expect senescence rates to vary among populations. For example, Kawasaki et al. (2008) found that the rate of ageing in stalk-legged flies (Telostylinus angusticollis) in wild populations was significantly faster compared to laboratory populations founded from the same wild population. Similarly, Austad (1993) found that an island population of Virginia opossums (Didelphis virginiana) had a shallower senescence slope compared to the mainland population. This difference coincided with a lack of predators on the island (Austad 1993). Despite this empirical foundation, there has been a lack of studies investigating intraspecific spatial variation in senescence rates between different habitats/populations in the wild (but see Austad 1993 and Baker and Thompson 2007; see also Bouwhuis et al. 2010 and Balbontin et al. 2012 for studies of inter-population variation in rates of reproductive senescence). The lack of studies may be caused by the requirement for long-term monitoring of known-aged animals from different populations of the same species.
The rate of senescence may vary among groups in a given population (e.g. males and females). The difference in senescence rates between males and females has become a topic of increased interest in evolutionary biology (Maklakov and Lummaa 2013; Regan and Partridge 2013). Life-history theory predicts that the sex with the higher mortality rates should be the one exhibiting the higher rates of senescence (Williams 1957). Therefore, as males from polygynous and dimorphic species suffer from high mortality rates during the mating season due for instance to male–male combat, they should exhibit higher senescence rates than females (Bonduriansky et al. 2008; Festa-Bianchet 2012). An interspecific comparison of 35 vertebrate species (Clutton-Brock and Isvaran 2007) provided an overall support for that prediction, finding that, in general, males had faster rates of senescence than females. As expected in socially monogamous species, such a difference in senescence rates between males and females appeared to be less pronounced (Clutton-Brock and Isvaran 2007). However, it is noteworthy that, until now, many of the studies investigating senescence patterns in the wild have focused solely on females (but see Reed et al. 2008; Brown and Roth 2009; Nussey et al. 2009; Pardo et al. 2013; Cornwallis et al. 2014; Gamelon et al. 2014; Hayward et al. 2015; Zhang et al. 2015), and evidence for sex differences in rates of senescence in the wild remains somewhat scarce in the literature (Clutton-Brock and Isvaran 2007; Bonduriansky et al. 2008; Balbontin and Møller 2015).
Here, we aimed at filling these gaps in our knowledge by investigating intraspecific spatial variation and also sex differences in rates and onset of senescence in a wild metapopulation of house sparrows (Passer domesticus) in a Norwegian archipelago (66.5°N, 12.5°E). This metapopulation has been intensively monitored by annual capture, mark and resighting of both males and females since 1993. An important feature of this metapopulation is that some islands contain farms where the birds had the option of sheltering inside cattle-farm buildings whenever the weather is harsh (e.g. during winter). In contrast, other islands do not have any cattle-farms and the birds have to find shelter around the human settlements. Therefore, according to the current evolutionary theory of senescence, we expected: (1) inter-population variation in rates and onset of senescence with faster and/or earlier senescence in the populations inhabiting the islands free of cattle-farms compared to the populations living in more sheltered environments; and (2) no sex difference in senescence rates within a given population for this socially monogamous species (Anderson 2006).
Materials and methods
Study area and habitats
The study was carried out in an archipelago consisting of 18 islands covering ca. 1600 km2 in the Helgeland district in northern Norway (see map in Baalsrud et al. 2014). The house sparrows on these islands have been systematically captured, marked and resighted several times during their lifetime since 1993 (e.g. Ringsby et al. 2002; Jensen et al. 2008; Pärn et al. 2012). In this study, we compared two sets of islands which differed in habitat: two islands with cattle farms (Gjerøy and Hestmannøy) and two islands without cattle farms (Selvær and Træna). On the farm islands, house sparrows lived in association with dairy farms where they reproduced, foraged and sheltered (under harsh weather conditions) inside barns and cow-sheds. On these farm islands, the cattle food and seeds from cultivated crops were readily available for house sparrows throughout the year. On the non-farm islands, where house sparrows live in association with small human settlements, the shelter provided by the barns was lacking. In addition, the main food resource on the non-farm islands was seeds from birdfeeders provided by the local human inhabitants. Although we focus on four islands, observations from the other islands were used to identify and exclude emigrants and immigrants from the dataset (n = 330). This was done to ensure that the effect of habitat/island on individual survival remained as constant as possible throughout the lifespan of individuals. We were thus also able to separate mortality from migration in our analyses.
Field work and datasets
Field work was carried out during the summer (1 May–15 August) and autumn (1 September–1 November). During field work, house sparrows were captured using mist nets. Upon first capture, they were banded with a metal ring engraved with a unique id-number and three plastic color rings (two rings on each tarsus). In addition, we visited nests (nest boxes or under barn roofs) and marked fledglings (age = 8–14 days old). Thus, after individuals had been marked they could be resighted by capturing them, or by observing their unique combination of color rings through a telescope or binoculars. For detailed description on field work, see Ringsby et al. (1998), Sæther et al. (1999) and Pärn et al. (2009).
Our datasets only included individuals that had a known age (i.e. individuals marked as fledglings or juveniles during May–August). The dataset from farm islands included the years 1993–2013 and contained the resighting history of 3543 individuals (6574 observations). A continuous time series of observations from non-farm islands was only available from 2003 to 2013 (1539 individuals, 2035 observations). Before 2003, populations on the non-farm islands had experienced a severe decline in population size (Baalsrud et al. 2014). The dataset used to compare senescence rates among males and females, contained only individuals that had been resighted and sexed as adults. The sex of individuals was determined by visual inspection of plumage characteristics. This dataset contained the resighting history of 1005 individuals (1715 observations).
Survival analyses
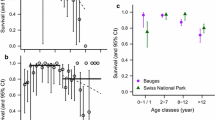
We estimated survival probabilities with capture–mark–recapture (CMR) models (Lebreton et al. 1992; Kéry and Schaub 2011). Previous studies have found that resighting probabilities may vary between islands and years in the metapopulation (Ringsby et al. 1999; Holand et al. 2014). We therefore included island, year and the interaction between islands and years in all models of resighting probability. To account for temporal variation in survival estimates, we included the effect of years as a random factor in all survival models. An investigation by Jones et al. (2008) indicated that senescence in house sparrows at Helgeland started at the mean age of first reproduction (age = 1). However, as the onset of senescence may occur later than the age of first reproduction (e.g. Weimerskirch 1992; Nussey et al. 2008; Peron et al. 2010), we tested for linear effect of age and also non-linear (i.e. quadratic) change in survival probability with increasing ages (i.e. senescence) either starting at age = 1, 2, 3 or 4 in separate models. Due to low sample sizes at ages >4 (see Fig. 2), we did not test for onsets starting at later ages. In detail, our analyses were divided into three parts. First, at the metapopulation level (i.e. all four islands pooled together), we investigated the relationship between survival probability (on the logit-scale) and age. Secondly, we investigated the difference in senescence rates (i.e. difference in slopes) between the two habitats (farm- vs. non-farm islands). Thirdly, we investigated if there were significant differences in senescence rates between islands (Gjerøy, Hestmanøy, Selvær and Træna) in the metapopulation. The rates of senescence were thus estimated separately for each habitat/island. To illustrate how survival probability varied among age classes, we used age as a factor instead of a continuous variable (see Figs. 1, 2).
The mean survival probability of age classes in a metapopulation of house sparrows (Passer domesticus) on four islands in the Helgeland archipelago, northern Norway. Age = 0 denotes the mean survival of fledglings on the four islands. Lines indicate upper and lower limits of a 95 % Bayesian credibility interval of the mean value. Numbers along the top of the figure indicate observed sample sizes for each age class
The change in survival probability with age in four island populations of house sparrows in the Helgeland archipelago, northern Norway (1993–2013). The dashed line indicates the predicted linear decline in survival probability (i.e. actuarial senescence) starting at age = 1 (Træna) or age = 2 (Gjerøy). Solid lines indicate upper and lower limits of a 95 % Bayesian credibility Interval of the mean values (open symbols). Numbers along the top of the figure indicate observed sample sizes for each age class
Our ancillary analysis investigated the difference in senescence rates between males and females. These differences were estimated in the same models as in our main analyses, with the addition of “sex” as a factor (male/female). We thus tested if there were significant differences in senescence rates between males and females in general, within habitats or within islands.
We used the model fitting options provided by the programing language BUGS (Lunn et al. 2000). This language offers several options for creating CMR models in a Bayesian framework using MCMC simulations to obtain posterior stationary distributions of parameters (Kéry and Schaub 2011). The models were run in JAGS (v.3.2.0; Plummer 2003) controlled from R (v.3.1.1, R Core Team 2014) using the package “JagsUI” (v.1.1). This package allows for easy parallel computation of multiple chains on computers using a cpu with multiple cores. For all models, we used three chains each with 120,000 iterations and a thinning rate of 6; the first 90,000 iterations were discarded (“burn-in”). Mixing and convergence of chains to a stationary distribution was evaluated by visual inspection of time-series plots produced by JAGS and by the Brooks–Gelman–Rubin criterion (R-hat; Brooks and Gelman 1998). Parameter estimates were obtained as the mean from the respective stationary posterior distributions and lower/upper limits of the 95 % Bayesian credibility interval (BCI). We applied vague priors for all parameters (see Kéry and Schaub 2011). We considered respective slope estimates obtained within habitats/islands as significantly different from each other if the 95 % BCI of their difference (Δβ) did not include zero (Kéry and Schaub 2011; Holand et al. 2014). Subtracting/adding parameter estimates (while obtaining a 95 % BCI of the sum) is a common feature of the BUGS language (Kéry and Schaub 2011).
Results
The first part of our main analysis did not indicate a general, significant linear or non-linear decline in survival probability with age in the metapopulation (see Fig. 1; Table 1; Electronic Supplementary Material Table 1A). We also found no significant difference in senescence rates (linear or non-linear) between males and females at the metapopulation level (Electronic Supplementary Material Table 2A and 3A).
The second part of our main analysis indicated that linear senescence rates were not significantly different between the two habitat types (see Table 1), starting at age = 1 [Δβ = −0.01 BCI: (−0.25, 0.22)], age = 2 [Δβ = −0.18 BCI: (−0.61, 0.22)], age = 3 [Δβ = −0.35 BCI: (−1.25, 0.414)] or age = 4 [Δβ = −1.29 BCI: (−2.99, 0.311)]. We also found no significant difference in non-linear senescence rates between habitats (Electronic Supplementary Material, Table 1A). In addition, we did not detect a significant difference in senescence rates (linear or non-linear) between males and females either on farm islands or non-farm islands (Electronic Supplementary Material, Tables 2A and 3A).
The results from the third part of the main analysis indicated that there were significant differences in linear senescence rates between islands (see Fig. 2 and Electronic Supplementary Material, Tables 4A and 5A). Specifically, the senescence rates on Gjerøy and Træna were found to be significantly steeper compared to Hestmannøy. There was a significant linear decline in survival probability on Træna starting at age = 1 and on Gjerøy starting at age = 2 (see Table 1). We found no significant difference in non-linear senescence rates between islands (Electronic Supplementary Material, Table 1A).We also found no significant difference in senescence rates (linear or non-linear) between males and females on any of the four islands (Electronic Supplementary Material, Tables 2A and 3A).
Discussion
This study has shown that senescence rates and onset of senescence may vary spatially in a wild metapopulation. Although the lack of mean difference in senescence rates between habitats did not support our initial hypothesis, the results of this study suggest that local environmental conditions may have an important effect on the ageing patterns of wild animals. Failure to account for such variation may lead to an oversimplified view of senescence rates of a species (Fig. 1 vs. Fig. 2). Although the specific cause of heterogeneous senescence rates between populations may be difficult to detect, the resulting effect on local demography may influence the population dynamics of the sub-population (Gaillard et al. 2000) and the metapopulation as a whole. Accounting for such heterogeneities may be important for predicting future population fluctuations of fragmented populations (i.e. metapopulations) in the wild.
Although the pattern found on one non-farm island fitted the expected pattern of faster senescence rates and earlier onset, this was not the case on the other non-farm island (see Fig. 2). In addition, the rates of senescence on the two farm islands were significantly different (see Table 1 and Electronic Supplementary Material, Tables 4A and 5A) even though these islands are only ca. 11 km apart. A distinct difference between the two farm islands was the mean survival probabilities of the first two adult age classes (see Fig. 2; Table 1). The relatively high survival probability of these age classes on the farm island of Gjerøy may point to a difference in investment strategies between the two islands. Individuals that invest a relatively large amount of energy in early reproduction and/or survival may also be expected to suffer more pronounced senescence in later life (McCleery et al. 1996; Orell and Belda 2002; Reid et al. 2003; Reed et al. 2008; Hammers et al. 2013). However, the lack of differences in fledgling survival probability among populations (see Fig. 2) appears to exclude the possibility that the variation observed was caused by a substantial difference in mortality before maturation (e.g. stronger selection for quality individuals). Alternatively, differences in natal environments may have caused subsequent changes in the senescence pattern between the islands that manifested in the adult age classes (Nussey et al. 2007; Reed et al. 2008; Millon et al. 2011; Cartwright et al. 2014). However, the specific cause for the within habitat variation found in our study is not known as we have not observed an obvious source for adult mortality that may differ in strength within habitats. These differences may be subtle and very difficult to observe in the wild (Nussey et al. 2013).
As the house sparrow is a socially monogamous species (Anderson 2006), the lack of difference in senescence found between adult males and adult females appears to support the pattern found by Clutton-Brock and Isvaran (2007). Although one might expect a general female-biased mortality pattern in birds (Liker and Szekely 2005) to cause a different senescence rate in females, this did not appear to be the case in our populations (see Electronic Supplementary Material, Tables 2A and 3A). Indeed, the overall result from our analysis did not support the notion of a general pattern of female-biased mortality in house sparrows. Previous studies on house sparrows have also not detected a general sex bias in adult survival probability (for review, see Anderson 2006).
References
Altwegg R, Roulin A, Kestenholz M, Jenni L (2003) Variation and covariation in survival, dispersal, and population size in barn owls Tyto alba. J Anim Ecol 72:391–399
Anderson TR (2006) Biology of the ubiquitous house sparrow: from genes to populations. Oxford University Press, New York
Austad SN (1993) Retarded senescence in an insular population of Virginia opossums (Didelphis virginiana). J Zool 229:695–708
Baalsrud HT et al (2014) Effects of population characteristics and structure on estimates of effective population size in a house sparrow metapopulation. Mol Ecol 23:2653–2668
Baker JD, Thompson PM (2007) Temporal and spatial variation in age-specific survival rates of a long-lived mammal, the Hawaiian monk seal. Proc R Soc Lond B 274:407–415
Balbontin J, Møller AP (2015) Environmental conditions during early life accelerate the rate of senescence in a short-lived passerine bird. Ecology 96:948–959
Balbontin J, Møller AP, Hermosell IG, Marzal A, Reviriego M, de Lope F (2012) Geographical variation in reproductive ageing patterns and life-history strategy of a short-lived passerine bird. J Evol Biol 25:2298–2309
Bonduriansky R, Maklakov A, Zajitschek F, Brooks R (2008) Sexual selection, sexual conflict and the evolution of ageing and life span. Funct Ecol 22:443–453
Bouwhuis S, van Noordwijk AJ, Sheldon BC, Verhulst S, Visser ME (2010) Similar patterns of age-specific reproduction in an island and mainland population of great tits Parus major. J Avian Biol 41:615–620
Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7:434–455
Brown WP, Roth RR (2009) Age-specific reproduction and survival of individually marked Wood Thrushes, Hylocichla mustelina. Ecology 90:218–229
Brunet-Rossinni AK, Austad SN (2006) Senescence in wild populations of mammals and birds. Handb Biol Aging 6:243–266
Cartwright SJ, Nicoll MAC, Jones CG, Tatayah V, Norris K (2014) Anthropogenic natal environmental effects on life histories in a wild bird population. Curr Biol 24:536–540
Caswell H (2007) Extrinsic mortality and the evolution of senescence. Trends Ecol Evol 22:173–174
Clutton-Brock TH, Isvaran K (2007) Sex differences in ageing in natural populations of vertebrates. Proc R Soc Lond B 274:3097–3104
Core Team R (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cornwallis CK, Dean R, Pizzari T (2014) Sex-specific patterns of aging in sexual ornaments and gametes. Am Nat 184:E66–E78
Dhondt AA, Kempenaers B, Clobert J (1998) Sparrowhawk Accipiter nisus predation and blue tit Parus caeruleus adult annual survival rate. Ibis 140:580–584
Festa-Bianchet M (2012) The cost of trying: weak interspecific correlations among life-history components in male ungulates. Can J Zool 90:1072–1085
Gaillard JM, Festa-Bianchet M, Yoccoz NG, Loison A, Toigo C (2000) Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Syst 31:367–393
Gamelon M et al (2014) Do age-specific survival patterns of wild boar fit current evolutionary theories of senescence? Evolution 68:3636–3643
Hamilton WD (1966) Moulding of senescence by natural selection. J Theor Biol 12:12–45
Hammers M, Richardson DS, Burke T, Komdeur J (2013) The impact of reproductive investment and early-life environmental conditions on senescence: support for the disposable soma hypothesis. J Evol Biol 26:1999–2007
Hayward AD et al (2015) Asynchrony of senescence among phenotypic traits in a wild mammal population. Exp Gerontol 71:56–68
Holand H et al (2014) Lower survival probability of house sparrows severely infected by the gapeworm parasite. J Avian Biol 45:365–373
Jensen H, Steinsland I, Ringsby TH, Sæther B-E (2008) Evolutionary dynamics of a sexual ornament in the house sparrow (Passer domesticus): the role of indirect selection within and between sexes. Evolution 62:1275–1293
Jones OR et al (2008) Senescence rates are determined by ranking on the fast–slow life-history continuum. Ecol Lett 11:664–673
Kawasaki N, Brassil CE, Brooks RC, Bonduriansky R (2008) Environmental effects on the expression of life span and aging: an extreme contrast between wild and captive cohorts of Telostylinus angusticollis (Diptera: Neriidae). Am Nat 172:346–357
Kéry M, Schaub M (2011) Bayesian population analysis using WinBUGS. Academic, London
Kirkwood TBL (1977) Evolution of aging. Nature 270:301–304
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals—a unified approach with case-studies. Ecol Monogr 62:67–118
Lemaitre JF et al (2015) Early-late life trade-offs and the evolution of ageing in the wild. Proc R Soc Lond B 282:10
Liker A, Szekely T (2005) Mortality costs of sexual selection and parental care in natural populations of birds. Evolution 59:890–897
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337
Maklakov AA, Lummaa V (2013) Evolution of sex differences in lifespan and aging: causes and constraints. BioEssays 35:717–724
McCleery RH, Clobert J, Julliard R, Perrins CM (1996) Nest predation and delayed cost of reproduction in the great tit. J Anim Ecol 65:96–104
Medawar PB (1952) An unsolved problem of biology. Lewis, London
Millon A, Petty SJ, Little B, Lambin X (2011) Natal conditions alter age-specific reproduction but not survival or senescence in a long-lived bird of prey. J Anim Ecol 80:968–975
Nussey DH, Kruuk LEB, Morris A, Clutton-Brock TH (2007) Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr Biol 17:R1000–R1001
Nussey DH, Coulson T, Festa-Bianchet M, Gaillard JM (2008) Measuring senescence in wild animal populations: towards a longitudinal approach. Funct Ecol 22:393–406
Nussey DH, Kruuk LEB, Morris A, Clements MN, Pemberton JM, Clutton-Brock TH (2009) Inter- and intrasexual variation in aging patterns across reproductive traits in a wild red deer population. Am Nat 174:342–357
Nussey DH, Froy H, Lemaitre JF, Gaillard JM, Austad SN (2013) Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res Rev 12:214–225
Orell M, Belda EJ (2002) Delayed cost of reproduction and senescence in the willow tit Parus montanus. J Anim Ecol 71:55–64
Pardo D, Barbraud C, Weimerskirch H (2013) Females better face senescence in the wandering albatross. Oecologia 173:1283–1294
Pärn H, Jensen H, Ringsby TH, Sæther B-E (2009) Sex-specific fitness correlates of dispersal in a house sparrow metapopulation. J Anim Ecol 78:1216–1225
Pärn H, Ringsby TH, Jensen H, Sæther B-E (2012) Spatial heterogeneity in the effects of climate and density-dependence on dispersal in a house sparrow metapopulation. Proc R Soc Lond B 279:144–152
Peron G, Gimenez O, Charmantier A, Gaillard JM, Crochet PA (2010) Age at the onset of senescence in birds and mammals is predicted by early-life performance. Proc R Soc Lond B 277:2849–2856
Plummer M (2003) JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In: Proceedings of the 3rd international workshop on distributed statistical computing, Vienna, Austria
Reed TE, Kruuk LEB, Wanless S, Frederiksen M, Cunningham EJA, Harris MP (2008) Reproductive senescence in a long-lived seabird: rates of decline in late-life performance are associated with varying costs of early reproduction. Am Nat 171:E89–E101
Regan JC, Partridge L (2013) Gender and longevity: Why do men die earlier than women? Comparative and experimental evidence. Best Pract Res Clin Endocrinol Metab 27:467–479
Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P (2003) Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J Anim Ecol 72:765–776
Ringsby TH, Sæther B-E, Solberg EJ (1998) Factors affecting juvenile survival in House Sparrow Passer domesticus. J Avian Biol 29:241–247
Ringsby TH, Sæther B-E, Altwegg R, Solberg EJ (1999) Temporal and spatial variation in survival rates of a house sparrow, Passer domesticus, metapopulation. Oikos 85:419–425
Ringsby TH, Sæther B-E, Tufto J, Jensen H, Solberg EJ (2002) Asynchronous spatiotemporal demography of a house sparrow metapopulation in a correlated environment. Ecology 83:561–569
Sæther B-E, Ringsby TH, Bakke O, Solberg EJ (1999) Spatial and temporal variation in demography of a house sparrow metapopulation. J Anim Ecol 68:628–637
Weimerskirch H (1992) Reproductive effort in long-lived birds—age-specific patterns of condition, reproduction and survival in the wandering albatross. Oikos 64:464–473
Williams GC (1957) Pleiotropy, natural-selection, and the evolution of senescence. Evolution 11:398–411
Zhang H, Rebke M, Becker PH, Bouwhuis S (2015) Fitness prospects: effects of age, sex and recruitment age on reproductive value in a long-lived seabird. J Anim Ecol 84:199–207
Acknowledgments
We would like to thank everyone involved in the House Sparrow Project for help with fieldwork. We are also grateful to everyone at Centre for Biodiversity Dynamics at the Department of Biology, NTNU, for helpful comments and help with statistics in R. This study was supported by Grants from the Research Council of Norway (FRIMEDBIO 204303 and 221956, SFF 223257), the European Research Council (ERC-2010-AdG 268562), and NTNU. The research was carried out in accordance with permits from the Norwegian Environment Agency and the Bird Ringing Centre at Stavanger Museum, Norway.
Author contribution statement
H. H wrote the manuscript. H. H, T. K, H. J, H. P, T. H. R contributed to field work and data collection. H. H, T. K, J. T conducted the analysis. H. H, T. H. R, B. E. S conceived the study. All authors contributed to the interpretation of results and revisions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Christopher Whelan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Holand, H., Kvalnes, T., Gamelon, M. et al. Spatial variation in senescence rates in a bird metapopulation. Oecologia 181, 865–871 (2016). https://doi.org/10.1007/s00442-016-3615-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3615-4