Abstract
Current climate change affects the competitive ability and reproductive success of many species, leading to local extinctions, adjustment to novel local conditions by phenotypic plasticity or rapid adaptation, or tracking their optima through range shifts. However, many species have limited ability to expand to suitable areas. Altitudinal gradients, with abrupt changes in abiotic conditions over short distances, represent “natural experiments” for the evaluation of ecological and evolutionary responses under scenarios of climate change. Nothofagus pumilio is the tree species which dominates as pure stands the montane forests of Patagonia. We evaluated the adaptive value of variation in quantitative traits of N. pumilio under contrasting conditions of the altitudinal gradient with a long-term reciprocal transplant experimental design. While high-elevation plants show little response in plant, leaf, and phenological traits to the experimental trials, low-elevation ones show greater plasticity in their responses to changing environments, particularly at high elevation. Our results suggest a relatively reduced potential for evolutionary adaptation of high-elevation genotypes, and a greater evolutionary potential of low-elevation ones. Under global warming scenarios of forest upslope migration, high-elevation variants may be outperformed by low-elevation ones during this process, leading to the local extinction and/or replacement of these genotypes. These results challenge previous models and predictions expected under global warming for altitudinal gradients, on which the leading edge is considered to be the upper treeline forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern climate change is producing radical changes in species geographical distribution, timing of growth, and reproduction (Easterling et al. 2000; Hoffmann and Sgro 2011). As a result species will respond by adjusting to novel local conditions by phenotypic plasticity or rapid adaptation; shifting their ranges tracking environmental optimums; or become locally extinct (Chevin et al. 2010; Nicotra et al. 2010). The sessile nature of tree species, particularly those taxa with restricted seed dispersal, makes them more prone to suffer from climatic changes that occur in their natural environments. Particularly, tree species with long generation times and narrow ecological amplitudes usually harbor low levels of plasticity, and may not be able to withstand climatic changes. Therefore, migration capacity will act as a limiting factor in their response to such climate extremes. Those species able to track their bioclimatic envelope will expand towards coolest range margins (leading edge) while retracting at the warmest opposite ends (rear edge) (Hill et al. 2011). Nonetheless, the ability of species to track future climate change is limited (Pearson 2006). In recent years, an upward movement has been reported for many species as a response to climate warming to escape rising temperatures (Lenoir et al. 2008; Jump et al. 2009). However, the migration rates estimated for several tree species since the last glacial maximum (Lenoir et al. 2008; McLachlan et al. 2005; Mathiasen and Premoli 2010) are slower than the rates required to track the predicted warming of the twenty-first century, as the required migratory responses far exceed maximum post-glacial rates (Aitken et al. 2008). Therefore, as many species are unlikely to migrate fast enough to track the rapidly changing climate of the future, adaptation must play an increasingly important role in their response (Jump and Peñuelas 2005). Adaptation to novel environments may occur through selection on pre-existing genetic variation or on new mutations, leading the former to faster evolutionary change (Barrett and Schluter 2008). In particular, under sustained directional change in the optimum phenotype, a small amount of additive genetic variance always greatly reduces the total genetic load (Lande and Shannon 1996). Therefore, the maintenance of genetic variation in quantitative traits is a first priority as a mechanism of adaptation and population persistence in a changing environment.
Range margins are currently the main focus of evolutionary research due to the fact that most expansions of populations searching for preferred habitats under changing climates will probably occur from edges [see review of evolution and ecology at range margins in Sexton et al. (2009)]. Different types of edges have been identified: the rear edge, where populations persist throughout climate oscillations (Hampe and Petit 2005), and the leading edge, where populations expand from following climate changes (Parisod and Joost 2010). Rear edges may consist of stable relict populations, older than others of the range, and usually genetically depauperated due to isolation and small population size (Hampe and Petit 2005). On the other hand, the leading edge model of colonization states that range expansions involve mostly populations from the colonization front (Hampe and Petit 2005). Although these hypotheses were put forward in relation to latitude, few empirical examples analyze these models in elevation gradients.
In montane habitats environmental conditions may vary widely along the altitudinal gradient over a few hundred meters, leading to major changes in the selection pressures acting on plant life history traits. Thus, altitudinal gradients are among the most powerful “natural experiments” for testing ecological and evolutionary processes linked to geophysical influences (Körner 2007). It is now clear that upward shifts of species ranges have occurred across a wide range of taxonomic groups and geographical locations during the twentieth century in response to warming (Walther et al. 2002). Nonetheless, responses to contrasting environmental settings along elevation gradients may differ between species (Vitasse et al. 2009; Bresson et al. 2011) which may be the result of adjustments via phenotypic plasticity (adjustment to different environments) or adaptation (selection of genotypes) to particular local conditions (Vitasse et al. 2014). While several studies have analyzed genetic and phenotypic variation of plant species along altitudinal gradients (Ohsawa and Ide 2008) only translocation experiments may aid in differentiating between the two (Clausen et al. 1940; Huenneke 1991; Linhart and Grant 1996; Joshi et al. 2001).
South American temperate forests occur along a narrow but latitudinally extensive strip of land between 32 and 55°S considered a biogeographic island (Armesto et al. 1998). High-altitude temperate forests of Patagonia are dominated by a single tree species that forms continuous stands, Nothofagus pumilio (Poepping et Endlicher) Krasser. To decipher how this species will respond to climate change is a first priority because it is the only tree species that dominates ca. 2500 km of montane forest environments. N. pumilio presents cline variation in several plant architectural and ecological traits, as well as genetic variation across the altitudinal gradient (Barrera et al. 2000; Cuevas 2000; Premoli 2004). Common garden experiments of N. pumilio plants of contrasting elevation provenances demonstrated that some of these traits have a genetic basis (Premoli and Brewer 2007; Premoli et al. 2007). However, none of these studies has evaluated whether such differences are adaptive, nor predicted how these populations would respond to changing environments through long-term experiments. The aim of this study is to evaluate the extent of phenotypic plasticity and/or adaptive responses of N. pumilio under a reciprocal transplant experiment at contrasting elevations of the altitudinal gradient. Because many plant traits are triggered by temperature-related cues, changes may relate to the timing of events (phenology) and growth features (leaf and whole plant levels). We tested the hypothesis that rapid responses to contrasting environmental conditions may be possible through phenotypic plasticity or genetic advantage by morphological and functional adjustments. On the other hand, relatively low adaptive potential and/or plasticity may impose severe limitations to plant performance under changing climates. Under scenarios of climate change better performers will be able to track their climatic optimum and hence keep pace with predicted warming trends.
Materials and methods
Study site
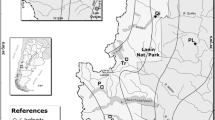
The study was carried out in two experimental sites at contrasting elevations of the altitudinal gradient (i.e., low- and high-elevation altitudinal limits) in the Challhuaco Valley (41°15′S, 71°18′W), within the Nahuel Huapi National Park in northwestern Patagonia (Bariloche, Argentina). Both experimental sites consist of pure stands located towards the northern range of N. pumilio. The study area is situated in the ecotone between humid temperate forest and the Patagonian steppe. Precipitation mainly falls in autumn and winter as rain or snow (approximately 1000 mm/year). Registered mean annual temperatures are 5–6 °C for low elevation (Kitzberger et al. 2005), and 3–4 °C for high elevation (Rusch 1993). Soil types of both sites correspond to Andisols, which are mainly derived from volcanic ash (Singer and Morello 1960; Veblen and Ashton 1979). These soils have a high capacity to retain phosphorus and to stabilize organic matter, which often leads to nitrogen and phosphorus deficiencies (Mazzarino et al. 1998). High-elevation sites inhabited by N. pumilio are deficient in available organic elements in the soil, where organic C and total N can be as low as 2.8 and 0.11 %, respectively, compared to 17 and 0.80 % at lower elevations, respectively (data extracted from Premoli et al. 2007). At the low-elevation site N. pumilio grow as erect trees. Dominant canopy trees were up to 26–28 m tall, 80–120 cm in diameter at breast height, and at least 280 years of age (Heinemann et al. 2000; Mathiasen and Premoli 2013). The high-elevation site is located below the timberline and individuals are mostly dwarf plants with small leaves, due to harsher environmental conditions, not exceeding 2 m tall, 8 cm diameter, and 62 years in age (Mathiasen and Premoli 2013).
Reciprocal transplant experiment
In November 1999, during the austral growing season, 400 randomly selected recently born seedlings (bearing cotyledons) that had germinated under natural conditions were collected from both low- and high-elevation source populations (approximately at 1200 and 1540 m a.s.l.). These were transported to a naturally lit greenhouse at 876 m a.s.l., located 10 km from source populations at the Laboratorio Ecotono (Universidad Nacional del Comahue, Bariloche, Río Negro, Argentina). Seedlings were cultivated for 5.5 years under homogeneous conditions, e.g., similarly watered and relocated periodically within the greenhouse to minimize the differences due to micro-environmental effects. In autumn 2005, a total of 220 of the remaining greenhouse-grown saplings were relocated to the field following a reciprocal transplant experimental design (Fig. 1). Experimental plots were established in suitable areas, with similar characteristics of canopy trees at their home sites in source populations, at 1258 and 1549 m a.s.l. (low- and high-elevation experimental sites, respectively) in the Challhuaco Valley. Within each experimental site a total of 110 saplings (55 from each provenance) were transplanted into the field and randomly positioned so that low and high plants were all intermixed. Transplanted saplings were fenced to exclude grazing by hare and other mammals (Fig. 1). Digital data loggers (HOBO Pro RH/Temp; Onset) were set on each site to record ambient temperature and relative humidity every 3 h during the first 4 years of measurements, to obtain biologically relevant climatic variables (bioclimatic variables). The information on the bioclimatic characterization of both experimental sites is provided in the Electronic Supplemental Material (Text S1; Table S1).
Scheme of the reciprocal transplant experimental design showing the location of source populations where Nothofagus pumilio seedlings from two provenances (source sites) were harvested (Challhuaco Valley, Patagonia, Argentina). After cultivation under controlled conditions, saplings were relocated to the field into fenced plots set up at the low- and high-elevation experimental sites, with the same characteristics of source populations where seedlings were harvested. Top left photographs show seedlings bearing cotyledons, collected at the low- and high-elevation source populations. Bottom left photograph shows saplings cultivated at the common garden at the Laboratorio Ecotono. Top right photographs show the fenced plots set up in low (below) and high (above) experimental sites (photos by P. Mathiasen and A. C. Premoli)
Measurements on seedlings
Plant architecture was evaluated in the field by size and shape traits. Plant size variables included plant height (centimeters) measured on the longest stem with a measuring tape, and the basal diameter (millimeters) with a digital electronic caliper 723Z-6/150MM (Starrett, USA). We counted the total number of branches greater than 1 cm long on the main stem, and the total number of leaves of the whole sapling. Plant shape (no. per centimeter) was calculated as the ratio between the number of branches and plant height. Initial plant height was measured in the field at the time of transplantation in order to evaluate differences in the relative growth rates (RGR) (Premoli et al. 2007). Plant mortality was recorded each year as live (1) or dead (0) plants, and quantified as the proportion of dead plants in 2010 relative to the total number of relocated plants at the beginning of the experiment in 2005. Some plants presented partial death of the main stem and/or lateral branches, while some portion of the plant remained alive, which can be commonly observed in seedling and also adult Nothofagus plants. Therefore we recorded whether plants presented partial death of the main stem (1), a secondary stem (0.5), or none (0).
Leaf measurements were performed on ten leaves randomly selected from different positions within each sapling, so as to represent a sample of the entire individual. Leaves were harvested at two different times, after the growing season in March 2008 and at the end of the experiment in March 2013. All leaves were digitalized with an Epson CX5600 Scanner (Epson America). Images were used to measure size and shape leaf traits by automatic recognition using the program SigmaScan Pro 5.0 (Systat Software). Leaf size was assessed by means of: total leaf area (squared centimeters), leaf perimeter (centimeters), and maximum leaf width and length (centimeters). Leaf shape was described as the width/length ratio, and by the shape factor, which is a measure estimated by the software of the relationship between leaf width and the length from the insertion point of the petiole to the maximum width. The dissection index was estimated dividing the perimeter by the square root of leaf area. Leaf functional traits were measured by means of leaf dry mass (grams) after drying leaves at 70 °C for 48 h, and specific leaf area (squared centimeter per gram), calculated as leaf area per unit of dry mass.
Leaf phenology observations were made during the leafing process of saplings from bud burst, at the beginning of the growing season, until full leaf expansion was completed. Measurements were carried out during the austral spring in 2005, 2006, and a final measurement in 2012, i.e., after 7 years of plant growth under the reciprocal transplant experiment. The phenological state of all surviving saplings was recorded weekly from early September to late November (i.e., from snowmelt until all saplings had fully expanded leaves) following Premoli et al. (2007). Observed phenological stages included: resting buds, swollen buds, unfolded leaves, and fully expanded leaves. Each plant was assigned to a determined phenological stage when at least one bud and/or leaf had reached a given phenophase (all buds/leaves present on each sapling were examined). We also recorded phenological periods defined as: leaf unfolding date, as the average date when leaves of saplings reached the unfolded leaf stage; leaf expansion date, as the average date on which the sapling reached the fully expanded leaf category; phenological extension, as the time elapsed since onset until total leaf expansion; and phenological development, as the time elapsed since snowmelt until total leaf expansion date.
Statistical analysis
The effects of the reciprocal transplant experiment on plant, leaf, and phenological traits were assessed by generalized linear models (GLM). The statistical model for each trait was run with the altitude of origin (source populations), the destination (experimental sites), and their interaction as fixed effects, thus each experimental site represents a different environment (different altitude). The model for all traits has normal error structure and an identity link function, except for mortality which is a binary trait (alive/dead) and has binomial error structure and a logit link function. A significant main effect of origin (O) indicates genetically driven differences among treatments; whereas a significant main effect of destination (D) suggests environmentally induced phenotypic plasticity for the studied trait; and a significant interaction (D × O) reveals home-site advantage or disadvantage (Vitasse et al. 2010). The relationship between bioclimatic variables and growth and leaf traits were also analyzed by stepwise multiple regression analyses. Phenological differences among treatments at each date were tested by χ2-tests between the frequencies of saplings at each phenological state. In addition, phenological lags of high-elevation saplings were analyzed by χ2-tests between the frequency of high-elevation plants at each phenophase at T (i) against the frequency of low-elevation saplings in the same phenophase at T (i−1), where T is time. All statistical analyses were run using the program STATISTICA version 7.0 (StatSoft).
To measure the relative performance of N. pumilio seedlings under different experimental sites for each trait we calculated Hedges’ effect size (d ) (Hedges and Olkin 1985), as the difference between the mean performance of local (L) plants compared to foreign (F) ones on each experimental site divided by their pooled SD \((d = \frac{{{\bar{\text{X}}\text{L}} - {\text{XS}}}}{dsLF})\) and multiplied by a correction term \(\left( {1 - \frac{3}{{\left( {4\left( {NL + NF - 2} \right) - 1} \right)}}} \right)\) to account for small sample bias (Møller and Jennions 2002; Leimu and Fischer 2008). Absolute values of Hedges’ d of \(|d|\) = 0.2 are considered a small effect, \(|d|\) = 0.5 an intermediate effect, and \(|d|\) = 0.8 a large effect (Cohen 1988). A positive effect size indicates that local plants outperform foreign plants in a given environment, whereas a negative effect indicates better performance of foreign plants.
Results
Plant traits
Overall, after 8 years of the reciprocal transplant experiment, plant architecture differed with elevation. The GLM analyses for plant architectural traits yielded significant results for most measured variables (Fig. 2; Table S2). No significant effect of plant provenance was observed in initial plant height between low- and high-elevation plants at each experimental site (Fig. 2a). Saplings from low elevation attained significantly larger final height and basal diameter than high-elevation ones at both experimental sites (Figs. 2b, c). This was reflected in the significant effect of origin, i.e., provenance of saplings, suggesting a better performance of thee genotypes (selective advantage) in both environments for these traits. In addition, low- and high-elevation saplings at their home environment had more branches and higher whole plant performance traits such as RGR, as well as lower partial death and mortality than the transplanted saplings. The significant destination × origin interaction for these traits indicates local adaptation (Fig. 2d, g–i). In contrast, other plant characters such as the total number of leaves, plant shape, number of branches, and basal diameter yielded a significant effect of destination, i.e., growing conditions during the experiment, which suggest plastic responses for such traits (Fig. 2c–f). The homogeneity of slopes test for RGR of seedlings yielded significant differences [F (3,53) = 7.090; P < 0.001] among slopes for the different treatments of the reciprocal transplant experiment, indicating different growth patterns of plants from contrasting origins growing at the low-elevation experimental site (Fig. S1; Table S3).
Average (SE) plant traits measured on N. pumilio saplings from different treatments [origin (O) × destination (O)] during the reciprocal transplant experiment at Challhuaco Valley. The significance of each main effect (D, O, and their interaction D × O) is indicated in the bottom left of each graph, *P < 0.05, **P < 0.01, and ***P < 0.001
Leaf traits
The GLM results for leaf traits yielded significant differences among treatments (Fig. 3; Table S4). Saplings growing at the high-elevation experimental site produced leaves that were significantly smaller, narrower, and lighter than those of the low-elevation experimental site (P < 0.001; Fig. 3a–e). The significant destination × origin interaction for most leaf size traits such as total leaf area, leaf perimeter, maximum width, maximum length, and the functional leaf trait dry mass, suggest local adaptation (Fig. 3a–e). Signals of these adaptive adjustments were found for all leaf-size traits and dry mass particularly at low elevation where local plants attained greater values than high-elevation ones. Moreover, saplings from contrasting origins attained similar leaf-size traits at the high-elevation experimental site suggesting environmental, i.e., plastic, adjustments to extreme conditions. Leaf shape characters and the functional trait SLA reached similar values under each growing condition suggesting plastic adjustments (Fig. 3f–i).
Average (SE) leaf traits measured on N. pumilio saplings from different treatments (O × D) from the reciprocal transplant experiment at the Challhuaco Valley. The significance of each main effect (D, O and D × O) is indicated in the bottom left of each graph, *P < 0.05, **P < 0.01, and ***P < 0.001. For abbreviations, see Fig. 2
Leaf phenology
Phenological patterns of N. pumilio differed with altitude. Leaf expansion of saplings at the low-elevation experimental site started 21 days earlier than that of high-elevation ones (Table 1; see Fig. S2 for detailed results of χ2-tests). A significant delay in leaf expansion of high-elevation saplings was measured within both experimental sites. Consequently, high-elevation plants presented a significant delay of 1 week in phenology initiation relative to low-elevation ones independently of the destination site (Table 1; Fig. S2).
The GLM analyses for phenological traits show a strong significant effect of altitude (destination) on leaf unfolding and expansion dates (Fig. 4a, b; Table S5). In addition, a significant main effect of provenance (origin) was found for these two variables, as evidenced by the earlier dates for the leafing process measured on plants from low-elevation origin at both experimental sites (Fig. 4a, b), indicating also genetic differences. The length of leaf unfolding period (phenology extension) showed a significant main effect of destination (i.e., altitude, environmentally induced phenotypic plasticity), while the time elapsed since snowmelt until total leaf expansion (phenological development) yielded a significant main effect of origin (i.e., source population, genetically driven differences) (Fig. 4c, d).
Average (SE) phenological traits measured on N. pumilio saplings from different treatments (O × D) from the reciprocal transplant experiment at Challhuaco Valley. The significance of each main effect (D, O and D × O) is indicated in the bottom left of each graph as: *P < 0.05, **P < 0.01, and ***P < 0.001. For abbreviations, see Fig. 2
The multiple regressions among phenological traits and climatic variables yielded significant relationships for all measured traits (Table S6). Leaf unfolding date and phenology extension are significantly correlated with the mean annual temperature, whereas the leaf expansion date and the phenological development are significantly correlated with the chilling degree days during spring.
Effect size
Effect size analysis for most plant architectural and leaf traits (Fig. 5; Tables S7, S8) show that the low-elevation site presented intermediate to large effect size whereas at high elevation the effect was small to moderate. This means that low-elevation plants have an overall better performance than high-elevation ones in both experimental sites (Fig. 5a, b). However, the effect size for specific leaf area showed that foreign plants outperformed local plants in the low-elevation site (Fig. 5b).
Effect size (Hedges’ d) of the interaction (O × D) on plant and leaf morphological traits measured on N. pumilio saplings from the reciprocal transplant experiment at Challhuaco Valley. Absolute values of Hedges’ d (|d|) = 0.2 are considered a small effect, |d| = 0.5 an intermediate effect, and |d| = 0.8 a large effect (Cohen 1988). A positive effect indicates better performance of local plants compared to foreign plants at a given site. PHi Initial plant height, PH final plant height, NB number of branches, BD basal diameter, NL number of leaves, PS plant shape, PD partial death, PM plant mortality, RGR relative growth rate, LA leaf area, LP leaf perimeter, LW maximum leaf width, LL maximum leaf length, SF shape factor,, DI dissection index, DM dry mass, SLA specific leaf area; for other abbreviations, see Fig. 2
Discussion
Plastic adjustments vs. local adaptation
Our results show a combination of adaptive and plastic responses of N. pumilio plants from different origins when growing in contrasting conditions of the altitudinal gradient. Adapted genotypes have adjusted to such different environmental settings. Thus local plants had higher growth rates and lower mortality and partial death, as well as significantly different leaf-size traits and leaf dry mass in their local environment. Physical conditions of the altitudinal gradient, such as greater chilling and water deficit degree days at high elevation, impose severe restrictions on growth. As a result, local plants outperformed foreign ones particularly at the low-elevation site, while plants of different origins growing at high elevation tend to phenotypically converge. In addition, foreign plants were able to acclimate to novel environmental settings through plasticity (e.g., total number of leaves and branches). Nonetheless, while some traits may directly adjust through plasticity, others may be the result of tradeoffs. In addition, some traits, such as total height and to some extent basal diameter, showed selective advantage in low-elevation genotypes under both growing conditions.
Common garden experiments developed for N. pumilio evidenced that differences in plant and leaf traits between low- and high-elevation saplings are under genetic control (Premoli et al. 2007). The results of the reciprocal transplant experiment presented here suggest that the norm of reaction of those genotypes may allow plastic adjustments under field conditions. This also shows that tradeoffs may exist between the number of leaves, their shape, and mass per unit area in response to contrasting physical conditions along elevation gradients. As such, low-elevation plants have a lower number of leaves but of greater size and mass. On the other hand, high-elevation plants may develop a larger number of relatively smaller leaves to avoid self-shading under increased irradiance (Givnish 1987) and in response to prevailing strong winds that may increase bud damage (Smith et al. 2003).
Phenological events, such as the initiation and the end of seasonal growth, are thought to be under strong evolutionary control because of their influence on tree fitness (Vitasse et al. 2013). Early leafing in spring may increase light capture and carbon gain prior to canopy closure (Lopez et al. 2008), thus conferring a competitive advantage to translocated plants at high elevation. It has been argued that early leafing may increase exposure to freezing, which could damage buds and leaves resulting in a maladaptive trait, the so-called frost damage hypothesis (reviewed by Martin et al. 2010). However, other studies demonstrated that frost injury varies across species and throughout their distribution, and may even decrease with climate change (Morin and Chuine 2014). Under such scenarios of global warming where less frequency of freezing events is expected, survival of early leafing genotypes may be increased, conferring on them a competitive advantage.
Leaf phenological traits of N. pumilio related to the initiation of the leafing process are genetically controlled and showed a strong relationship with the mean annual temperature. The length of the leaf unfolding process was controlled by the altitude (environmentally induced phenotypic plasticity) which is significantly related to chilling degree days, indicating a trade-off between maximizing growing season length and the avoidance of frost damage (Vitasse et al. 2013). The physiological mechanisms trees adopt to escape, avoid, and tolerate freezing temperatures include a cold acclimation in autumn, a dormancy period during winter (leafless in deciduous trees), and the maintenance of a certain freezing tolerance during dehardening in early spring (Vitasse et al. 2014). Nonetheless, the results presented here clearly show that leaf phenology, although under genetic control in response to extreme environmental conditions (Premoli et al. 2007), may also acclimate as depicted by phenological shifts in response to growing conditions.
Ecological implications of adaptive plasticity
Many taxa have had to shift their distributions to track recent climate changes. Range shifts usually take place at the leading edge of range expansion via long-distance dispersal of small numbers of migrant individuals (Hill et al. 2011). Populations inhabiting distribution margins, such as those that occur at high elevation, may be the result of former upslope migration events resulting in a genetic depauperation due to bottlenecks produced during founder events. In addition, plant taxa at high elevation may face strong directional selection which results in local adaptation with somewhat reduced acclimation potential. In particular, if genes are subjected to natural selection, high gene flow can be overcome by selection among heterogeneous environments, producing clines associated with physical gradients (Mitton 1995). Premoli (2003) found continuous clinal genetic variation in populations of N. pumilio along the altitudinal gradient, as a result of adaptive responses to ecological gradients and/or restrictions for gene flow. Other tree species have shown these same patterns of strong local adaptation to heterogeneous environments, resulting in genetically different populations (Rehfeldt 1983, 1989, 1995; Rehfeldt et al. 1999, 2002; Ishizuka and Goto 2012). Lack of genetic variation precludes adaptation to novel environmental conditions prevailing at range limits. While phenotypic plasticity (i.e., fine tuning) and adaptive (i.e., long-term) responses allow populations to cope with climate change (Salmela 2014), taxa with restricted evolutionary potential (i.e., genetically adapted to local conditions) may be at risk of not keeping pace with rapidly changing conditions, because of their relatively low plasticity (Ishizuka and Goto 2012). The evidence presented here shows that high-elevation plants present lower performance in their local environment, and also in the low-elevation experimental site (i.e., higher temperatures), being outgrown by low-elevation plants for most plant and leaf traits as shown by near zero or negative Hedges’ d effect sizes. Molecular studies on N. pumilio along elevation gradients show that genetic diversity by means of isozymes and microsatellite markers significantly decrease with elevation (Premoli 2003; Mathiasen and Premoli 2013).
At its northern distribution, N. pumilio is restricted to mountain environments that probably suffered from genetic erosion due to in situ survival as small populations within ice-free areas (Mathiasen and Premoli 2010). In addition to a complex evolutionary history at its northern range, ecological factors impact on the gene pool of N. pumilio. Reduced germination rates at high elevation (Premoli 2004) in combination with limited opportunities for seed establishment (Barrera et al. 2000; Cuevas 2000; Heinemann et al. 2000) favors vegetative propagation which results in genetically poor populations near the treeline. To some extent this may translate into limited potential responses of high-elevation individuals to changing local growing conditions. In contrast, low-elevation populations are genetically diverse (Premoli 2003), which may correlate to a greater capacity for adaptive and plastic adjustments of these plants in their home environment. For most traits high-elevation plants were outperformed by low-elevation ones even at high elevation, as shown by, e.g., greater total height and early onset of leafing phenology of low-elevation plants. Thus, under relaxed selection pressures at upper altitudinal limits such as those predicted under climate warming, high-elevation plants would be potentially outgrown by low-elevation ones.
Upslope migration
It is widely known that under global scenarios of range shifts of species, communities and ecosystems due to climate change, plants from lower elevations will be more prone to suffer from the negative consequences of such changes (see review in Parmesan and Yohe 2003). In spite of this, the results presented here suggest that N. pumilio may potentially have the ability to grow higher on mountains to endure global warming, which in turn may primarily involve populations from low elevations. The higher adaptive and plastic response of low-elevation plants will favor their persistence under environmental changing conditions allowing these populations to track their phenotypic optimum. However, the relatively lower response capacity of high-elevation plants shows that phenotypic plasticity may reach a physiological limit and saturate in extreme environments (Premoli and Mathiasen 2011). In addition, the reduced neutral genetic diversity of these plants, and thereby low evolutionary potential, suggest that they will be more prone to suffer from local extinctions under warmer trends. In contrast, low-elevation populations with higher genetic diversity may have greater plastic and adaptive potential in benign environments for establishment and growth.
Models of migration fronts may be more complex than previously thought, and should be revised. High-elevation plants adapted to harshness have potentially limited responses to changing conditions. Although little mortality has been reported for high-elevation plants, the poor performance of translocated plants in the low-elevation experimental site (i.e., higher temperature), suggests that low-elevation plants may outperform them under warmer trends (i.e., warmer high-elevation environments). The global mean temperature has risen 0.9 °C over the last 100 years (Jones et al. 2001; IPCC 2013). The projected change in the global mean surface air temperature for the near term will likely be in the range of 0.3–0.7 °C, and for the long term, if net CO2 emissions do not stop rising, the temperature is expected to rise to 2 °C, or even 3.5 °C higher than the current mean temperature (Hulme et al. 2002; IPCC 2013). The expected increases in extreme weather events under scenarios of climate change (Easterling et al. 2000) will lead to biological responses. If altitudinal shifts predicted under warming occur, it is unlikely that the migration front will take place from the expansion of local populations located in the upper altitudinal limit (Daniels and Veblen 2003), but through the gradual migration of genotypes from lower altitudes with greater fitness performance instead.
In recent years, a growing body of evidence shows that retraction of the trailing edge of the distribution of various woody species is now underway on mountains worldwide (Jump et al. 2009). Global warming has resulted in a significant upward shift in species optimum elevation averaging 29 m/decade (Lenoir et al. 2008), and the rate of northward tree migration is approaching 100 km/century for many species (McLachlan et al. 2005; Woodall et al. 2009). We estimated latitudinal postglacial migration rates of 40 m/year for pollen and 0.4 m/year for seeds (based on nuclear and chloroplast DNA markers, respectively) under optimum conditions for N. pumilio regeneration (Mathiasen and Premoli 2010), which correspond to 0.46 m/year in elevation (following Jump et al. 2009). Populations of N. pumilio occupy altitudinal gradients along which they show gradual variation in several functional and genetic traits (Rusch 1993; Premoli 2003). Given its limited dispersal ability, upslope migrations will probably occur as a slow stepping stone process from nearby low-elevation yet genetically distinct populations that will gradually outcompete local plants. However, field observations of phenological rhythms in adult trees at mid-elevations evidence the presence of early leafing trees (P. M. and A. C. P., personal observation), which may indicate that upslope migration of low-elevation plants is already ongoing at Challhuaco Valley. Studies developed on several species have demonstrated that plants that possess greater phenotypic plasticity outperform native species (Porte et al. 2011), and frequently invade new habitats by competitive advantage (Matesanz et al. 2012). Therefore, better performer neighboring lower-elevation genotypes may gradually replace the high-elevation ones, which in turn have a relatively reduced potential for evolutionary adaptation (Fig. 6).
Under the assumption that all species are in equilibrium with the climate, their range limits should be determined by climatic factors, more specifically temperature-driven factors (Randin et al. 2013). In spite of its wide latitudinal range, N. pumilio has a narrow climatic niche, as it only grows along the upper tree line of the Chilean and Argentinean Andes (Lara et al. 2005), and is the dominant and almost unique tree species of such montane habitats. The low-elevation limit of N. pumilio forests is represented by a sharp line below which it is never found, particularly at low latitudes (i.e., northern range limit). Abiotic stress will be the limiting factor of range limits in abiotically stressful places (Louthan et al. 2015). The significant negative relationship between the mean temperature and precipitation of the warmest months and the lower altitudinal limit along the entire latitudinal range of N. pumilio (data not shown), suggests that it poorly performs under hot and dry conditions (Fig. 6). As a typical cold-tolerant species, N. pumilio better performs in cooler habitats and hardly survives in warmer ones. N. pumilio have drought-induced facultative leaf abscission (A. C. P., personal observation) to prevent plant death, and may also suffer partial death of their peripheral branches (dieback) under intense drought conditions (Veblen et al. 2003). Therefore, we may conclude that the lower altitudinal limit of N. pumilio forests is mainly controlled by abiotic factors, particularly under high stress conditions, i.e., drought. In addition, increased folivory in dry forests may negatively affect N. pumilio performance due to serious leaf damage by insects inhabiting warmer zones (Mazía et al. 2012). We would expect that herbivory will have larger effects under stressful conditions because of a plant’s decreased ability to recover.
Therefore, under scenarios of global warming N. pumilio will most probably retract its range from current low-elevation (i.e., warmer) locations, tracking less harsh environments towards higher elevations (Fig. 7). Nonetheless, the use of complex modeling approaches are needed to improve predictions of climate change impacts on pure N. pumilio stands, as well as on other high-elevation forests.
Illustration showing the general global trend of upward movement of the lower elevation treeline in mountains. Current distribution (a) and potential future migration (b) of N. pumilio forests along the altitudinal gradient. Low-elevation populations are indicated by squares as the leading edge of the migration front (range retraction)
References
Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S (2008) Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl 1:95–111. doi:10.1111/j.1752-4571.2007.00013.x
Armesto JJ, Rozzi R, Smith-Ramirez C, Arroyo MTK (1998) Conservation targets in South American temperate forests. Science 282:1271–1272. doi:10.1126/science.282.5392.1271
Barrera MD, Frangi JL, Richter LL, Perdomo MH, Pinedo LB (2000) Structural and functional changes in Nothofagus pumilio forests along an altitudinal gradient in Tierra del Fuego, Argentina. J Veg Sci 11:179–188. doi:10.2307/3236797
Barrett RD, Schluter D (2008) Adaptation from standing genetic variation. Trends Ecol Evol 23:38–44. doi:10.1016/j.tree.2007.09.008
Bresson CC, Vitasse Y, Kremer A, Delzon S (2011) To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiol 31:1164–1174. doi:10.1093/treephys/tpr084
Chevin L-M, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8(4):e1000357. doi:10.1371/journal.pbio.1000357
Clausen J, Keck DD, Hiesey WM (1940) Experimental studies on the nature of species. I. Effect of varied environment on Western North American plants. , Publications no. 520. Carnegie Institution of Washington, Washington, DC
Cohen J (1988) Statistical power analysis for the behavioral sciences. Erlbaum, New Jersey
Cuevas JG (2000) Tree recruitment at the Nothofagus pumilio alpine timberline in Tierra del Fuego, Chile. J Ecol 88:840–855. doi:10.1046/j.1365-2745.2000.00497.x
Daniels LD, Veblen TT (2003) Regional and local effects of disturbance and climate on altitudinal treelines in northern Patagonia. J Veg Sci 14:733–742. doi:10.1111/j.1654-1103.2003.tb02205.x
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations, modeling, and impacts. Science 289:2068–2074. doi:10.1126/science.289.5487.2068
Givnish TJ (1987) Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol 106:131–160. doi:10.1111/j.1469-8137.1987.tb04687.x
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467. doi:10.1111/j.1461-0248.2005.00739.x
Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic Press, New York
Heinemann K, Kitzberger T, Veblen TT (2000) Influences of gap microheterogeneity on the regeneration of Nothofagus pumilio in a xeric old-growth forest of northwestern Patagonia, Argentina. Can J For Res 30:25–31. doi:10.1139/x99-181
Hill JK, Griffiths HM, Thomas CD (2011) Climate change and evolutionary adaptations at species’ range margins. Annu Rev Entomol 56:143–159. doi:10.1146/annurev-ento-120709-144746
Hoffmann AA, Sgro CM (2011) Climate change and evolutionary adaptation. Nature 470:479–485. doi:10.1038/nature09670
Huenneke LF (1991) Ecological implication of genetic variation in plant populations. In: Falk DA, Holsinger KE (eds) Genetic and conservation of rare plants. Oxford University Press, New York, pp 31–44
Hulme M, Jenkins GJ, Lu X Turnpenny JR, Mitchell TD, Jones RG, Lowe J, Murphy JM, Hassell D, Boorman P et al (2002) Climate change scenarios for the United Kingdom: the UKCIP02 scientific report. Tyndall Centre for Climate Change Research, School of Environmental Sciences, University of East Anglia, Norwich, UK
IPCC (2013) Summary for policymakers. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, New York
Ishizuka W, Goto S (2012) Modeling intraspecific adaptation of Abies sachalinensis to local altitude and responses to global warming, based on a 36-year reciprocal transplant experiment. Evol Appl 5:229–244. doi:10.1111/j.1752-4571.2011.00216.x
Jones PD, Osborn TJ, Briffa KR, Folland CK, Horton B, Alexander LV, Parker DE, Rayner N (2001) Adjusting for sampling density in grid box land and ocean surface temperature time series. J Geophys Res 106:3371–3380. doi:10.1029/2000JD900564
Joshi J, Schmid B, Caldeira MC, Dimitrakopoulos PG, Good J, Harris R, Hector A, Huss-Danell K, Jumpponen A, Minns A et al (2001) Local adaptation enhances performance of common plant species. Ecol Lett 4:536–544. doi:10.1046/j.1461-0248.2001.00262.x
Jump AS, Peñuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8:1010–1020. doi:10.1111/j.1461-0248.2005.00796.x
Jump AS, Mátyás C, Peñuelas J (2009) The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol Evol 24:694–701. doi:10.1016/j.tree.2009.06.007
Kitzberger T, Raffaele E, Heinemann K, Mazzarino MJ (2005) Effects of fire severity in a north Patagonian subalpine forest. J Veg Sci 16:5–12. doi:10.1111/j.1654-1103.2005.tb02333.x
Körner C (2007) The use of altitude in ecological research. Trends Ecol Evol 22:569–574. doi:10.1016/j.tree.2007.09.006
Lande R, Shannon S (1996) The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50:434–437
Lara A, Villalba R, Wolodarsky-Franke A, Aravena JC, Luckman BH, Cuq E (2005) Spatial and temporal variation in Nothofagus pumilio growth at tree line along its latitudinal range (35°40′–55°S) in the Chilean Andes. J Biogeogr 32:879–893. doi:10.1111/j.1365-2699.2005.01191.x
Leimu R, Fischer M (2008) A meta-analysis of local adaptation in plants. PLoS One 3(12):e4010. doi:10.1371/journal.pone.0004010
Lenoir J, Gegout JC, Marquet PA, De Ruffray P, Brisse H (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771. doi:10.1126/science.1156831
Linhart YB, Grant MC (1996) Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Syst 27:237–277. doi:10.1146/annurev.ecolsys.27.1.237
Lopez OR, Farris-Lopez K, Montgomery RA, Givnish TJ (2008) Leaf phenology in relation to canopy closure in southern Appalachian trees. Am J Bot 95:1395–1407. doi:10.3732/ajb.0800104
Louthan AM, Doak DF, Angert AL (2015) Where and when do species interactions set range limits? Trends Ecol Evol 30:780–792. doi:10.1016/j.tree.2015.09.011
Martin M, Gavazov K, Korner C, Hattenschwiler S, Rixen C (2010) Reduced early growing season freezing resistance in alpine treeline plants under elevated atmospheric CO2. Glob Chang Biol 16:1057–1070. doi:10.1111/j.1365-2486.2009.01987.x
Matesanz S, Horgan-Kobelski T, Sultan SE (2012) Phenotypic plasticity and population differentiation in an ongoing species invasion. PLoS One 7(9):e44955. doi:10.1371/journal.pone.0044955
Mathiasen P, Premoli AC (2010) Out in the cold: genetic variation of Nothofagus pumilio (Nothofagaceae) provides evidence for latitudinally distinct evolutionary histories in austral South America. Mol Ecol 19:371–385. doi:10.1111/j.1365-294X.2009.04456.x
Mathiasen P, Premoli AC (2013) Fine-scale genetic structure of Nothofagus pumilio (lenga) at contrasting elevations of the altitudinal gradient. Genetica 141:95–105. doi:10.1007/s10709-013-9709-6
Mazía N, Chaneton EJ, Dellacanonica C, Dipaolo L, Kitzberger T (2012) Seasonal patterns of herbivory, leaf traits and productivity consumption in dry and wet Patagonian forests. Ecol Entomol 37:193–203. doi:10.1111/j.1365-2311.2012.01356.x
Mazzarino MJ, Bertiller M, Schlichter T, Gobbi M (1998) Nutrient cycling in Patagonian ecosystems. Ecol Aust 8:167–181
McLachlan JS, Clark JS, Manos PS (2005) Molecular indicators of tree migration capacity under rapid climate change. Ecology 86:2088–2098. doi:10.1890/04-1036
Mitton JB (1995) Genetics and the physiological ecology of conifers. In: Smith WK, Hinckley TM (eds) Ecophysiology of coniferous forests. Academic Press, San Diego, pp 2–27
Møller A, Jennions MD (2002) How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132:492–500. doi:10.1007/s00442-002-0952-2
Morin X, Chuine I (2014) Will tree species experience increased frost damage due to climate change because of changes in leaf phenology? Can J For Res 44:1555–1565. doi:10.1139/cjfr-2014-0282
Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, van Kleunen M (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692. doi:10.1016/j.tplants.2010.09.00
Ohsawa T, Ide Y (2008) Global patterns of genetic variation in plant species along vertical and horizontal gradients on mountains. Global Ecol Biogeogr 17:152–163. doi:10.1111/j.1466-8238.2007.00357.x
Parisod C, Joost S (2010) Divergent selection in trailing-versus leading-edge populations of Biscutella laevigata. Ann Bot 105:655–660. doi:10.1093/aob/mcq014
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. doi:10.1038/nature01286
Pearson RG (2006) Climate change and the migration capacity of species. Trends Ecol Evol 21:111–113. doi:10.1016/j.tree.2005.11.022
Porte AJ, Lamarque LJ, Lortie CJ, Michalet R, Delzon S (2011) Invasive Acer negundo outperforms native species in non-limiting resource environments due to its higher phenotypic plasticity. BMC Ecol 11(1):28. doi:10.1186/1472-6785-11-28
Premoli AC (2003) Isozyme polymorphisms provide evidence of clinal variation with elevation in Nothofagus pumilio. J Hered 94:218–226. doi:10.1093/jhered/esg052
Premoli AC (2004) Variación en Nothofagus pumilio (Poepp. et Endl.) Krasser (lenga). In: Donoso C, Premoli AC, Gallo L, Ipinza R (eds) Variación intraespecífica en las especies arbóreas de los bosques templados de Chile y Argentina. Editorial Universitaria, Santiago de Chile, Chile, pp 145–166
Premoli AC, Brewer CA (2007) Environmental v. genetically driven variation in ecophysiological traits of Nothofagus pumilio from contrasting elevations. Aust J Bot 55:585–591. doi:10.1071/BT06026
Premoli AC, Mathiasen P (2011) Respuestas ecofisiológicas adaptativas y plásticas en ambientes secos de montaña: Nothofagus pumilio, el árbol que acaparó los Andes australes. Ecol Aust 21:251–269
Premoli AC, Raffaele E, Mathiasen P (2007) Morphological and phenological differences in Nothofagus pumilio from contrasting elevations. Aust Ecol 32:515–523. doi:10.1111/j.1442-9993.2007.01720.x
Randin CF, Paulsen J, Vitasse Y, Kollas C, Wohlgemuth T, Zimmermann NE, Körner C (2013) Do the elevational limits of deciduous tree species match their termal latitudinal limits? Global Ecol Biogeogr 22:913–923. doi:10.1111/geb.12040
Rehfeldt GE (1983) Adaptation of Pinus contorta populations to heterogeneous environments in northern Idaho. Can J For Res 13:405–411. doi:10.1139/x83-061
Rehfeldt GE (1989) Ecological adaptations in Douglas-fir (Pseudotsuga menziesii var glauca)—a synthesis. For Ecol Manage 28:203–215. doi:10.1016/0378-1127(89)90004-2
Rehfeldt GE (1995) Genetic-variation, climate models and the ecological genetics of Larix occidentalis. For Ecol Manage 78:21–37. doi:10.1016/0378-1127(95)03602-4
Rehfeldt GE, Ying CC, Spittlehouse DN, Hamilton DA (1999) Genetic responses to climate in Pinus contorta: niche breadth, climate change, and reforestation. Ecol Monogr 69:375–407. doi:10.1890/0012-9615(1999)069[0375:GRTCIP]2.0.CO;2
Rehfeldt GE, Tchebakova NM, Parfenova YI, Wykoff WR, Kuzmina NA, Milyutin LI (2002) Intraspecific responses to climate in Pinus sylvestris. Glob Chang Biol 8:912–929. doi:10.1046/j.1365-2486.2002.00516.x
Rusch VE (1993) Altitudinal variation in the phenology of Nothofagus pumilio in Argentina. Rev Chil Hist Nat 66:131–141
Salmela MJ (2014) Rethinking local adaptation: mind the environment! For Ecol Manage 312:271–281. doi:10.1016/j.foreco.2013.10.013
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol S 40:415–436. doi:10.1146/annurev.ecolsys.110308.120317
Singer R, Morello JH (1960) Ectotrophic forest tree mycorrhizae and forest communities. Ecology 41:549–551. doi:10.2307/1933331
Smith WK, Germino MJ, Hancock TE, Johnson DM (2003) Another perspective on altitudinal limits of alpine timberlines. Tree Physiol 23:1101–1112. doi:10.1093/treephys/23.16.1101
Veblen TT, Ashton DH (1979) Successional pattern above timberline in South-Central Chile. Vegetatio 40:39–47. doi:10.1007/BF00052013
Veblen TT, Kitzberger T, Raffaele E, Lorenz DC (2003) Fire history and vegetation changes in Northern Patagonia, Argentina. In: Veblen TT, Baker WL, Montenegro G, Swetnam TW (eds) Fire and climatic change in temperate ecosystems of the Western Americas. Springer, New York, pp 265–295
Vitasse Y, Porté AJ, Kremer A, Michalet R, Delzon S (2009) Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecologia 161:187–198. doi:10.1007/s00442-009-1363-4
Vitasse Y, Bresson CC, Kremer A, Michalet R, Delzon S (2010) Quantifying phenological plasticity to temperature in two temperate tree species. Funct Ecol 24:1211–1218. doi:10.1111/j.1365-2435.2010.01748.x
Vitasse Y, Hoch G, Randin CF, Lenz A, Kollas C, Scheepens JF, Körner C (2013) Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171:663–678. doi:10.1007/s00442-012-2580-9
Vitasse Y, Lenz A, Kollas C, Randin CF, Hoch G, Körner C (2014) Genetic vs. non-genetic responses of leaf morphology and growth to elevation in temperate tree species. Funct Ecol 28:243–252. doi:10.1111/1365-2435.12161
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoeg-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395. doi:10.1038/416389a
Woodall CW, Oswalt CM, Westfall JA, Perry CH, Nelson MD, Finley AO (2009) An indicator of tree migration in forests of the eastern United States. For Ecol Manage 257:1434–1444. doi:10.1016/j.foreco.2008.12.013
Acknowledgments
We are most grateful to M. C. Acosta, G. A. Carrizo, R. Carrizo, S. Diaz, P. Edwards, M. Fasanella, J. Gutierrez, G. Ignazi, and A. E. Rovere, for assistance during fieldwork. We thank various anonymous reviewers for their thoughtful comments that helped to improve the science and presentation of our manuscript. Administración de Parques Nacionales allowed establishment of the reciprocal transplant experiment within Nahuel Huapi National Park. This work was supported by Agencia de Promoción Científica y Tecnológica PICT 2012-0688, PICT 2013-2404; Consejo Nacional de Investigaciones Científicas y Técnicas CONICET PIP 2013-646; and Universidad Nacional del Comahue (04/B157). P. M. and A. C. P. are members of the National Research Council of Argentina (CONICET).
Author contribution statement
P. M. and A. C. P. contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Kouki Hikosaka.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mathiasen, P., Premoli, A.C. Living on the edge: adaptive and plastic responses of the tree Nothofagus pumilio to a long-term transplant experiment predict rear-edge upward expansion. Oecologia 181, 607–619 (2016). https://doi.org/10.1007/s00442-016-3568-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3568-7