Abstract
Conditions experienced in early developmental stages can have long-term consequences for individual fitness. High intraspecific density during the natal period can affect juvenile and eventually adult growth rates, metabolism, immune function, survival, and fecundity. Despite the important ecological and evolutionary effects of early developmental density, the form of the relationship between natal density and resulting juvenile phenotype is poorly understood. To test competing hypotheses explaining responses to intraspecific density, we experimentally manipulated the initial larval density of ringed salamanders (Ambystoma annulatum), a pond-breeding amphibian, over 11 densities. We modeled the functional form of the relationship between natal density and juvenile traits, and compared the relative support for the various hypotheses based on their goodness of fit. These functional form models were then used to parameterize a simple simulation model of population growth. Our data support non-additive density dependence and presents an alternate hypothesis to additive density dependence, self-thinning and Allee effects in larval amphibians. We posit that ringed salamander larvae may be under selective pressure for tolerance to high density and increased efficiency in resource utilization. Additionally, we demonstrate that models of population dynamics are sensitive to assumptions of the functional form of density dependence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A central goal of ecology is to determine the factors influencing population dynamics (Cole 1954). The size of a population is determined by both non-feedback mechanisms, such as external perturbations (e.g., disease, catastrophes, and boom years), and by density-dependent factors, which can stabilize population size around a mean. Negative density dependence regulates populations through intra- and interspecific competition, whereby per capita resources are reduced, resulting in decreased growth, fecundity, or survival of individuals, and ultimately lower population growth (reviewed in Herrando-Perez et al. 2012). Population regulation through negative density dependence is broadly observed in natural and experimental systems across a range of organisms. In a meta-analysis of 1198 species, Brook and Bradshaw (2006) found that density dependence is a pervasive feature of population dynamics. Density dependence is central not only to theories of population regulation, but also to community structure and species interactions (Wright 2002).
When experienced during early developmental stages (i.e. before sexual maturation), conditions such as high intraspecific density can have immediate and long-term consequences for individual development and fitness. Effects can include altered growth rate, metabolism, immune function, and decreased fecundity, and there is substantial evidence for these patterns in many taxa (Lindström 1999; Monaghan 2008; Herrando-Perez et al. 2012). For example, zebra finches (Taeniopygia guttata) reared in experimentally larger broods had greater mortality before and after fledging, weighed less, had higher metabolism, poorer immune responses, and bred later than those raised in experimentally smaller broods (De Kogel 1997; Monaghan 2008). Similar patterns occur in explosive breeders, such as salmonids. Fry from stream reaches with high intraspecific density have lower survival, depressed growth and thus delayed maturity, poorer immune responses, select different habitat, and are more likely to disperse (Post et al. 1999; Bult et al. 1999; Einum et al. 2006).
The effects of increased intraspecific density have also been broadly documented in pond-breeding amphibians. Amphibian larvae reared at high densities have lower survival to metamorphosis, slower growth, later metamorphosis, and emerge from ponds as smaller juveniles (Wilbur 1976; Scott 1994; Loman 2004). While amphibian populations are thought to be regulated during terrestrial life-history stages (Wilbur 1980; Pechmann 1995; Biek et al. 2002), the phenotype resulting from larval environments have fitness implications that carry over into later life-history stages. Smaller juveniles have an increased risk of desiccation, depressed immune function, lower lipid levels, decreased survival, later age of first reproduction, and lower fecundity (Scott 1994; Davis and Maerz 2009; Peterman et al. 2013).
Although the relationship between natal intraspecific density and adult phenotype is broadly understood in many taxonomic groups, there is not a consensus concerning the form of functional responses because most studies are limited by testing five or fewer densities (Wilbur 1977; Semlitsch and Caldwell 1982; Vonesh 2005; Anderson and Semlitsch 2014). We sought to test four competing hypotheses concerning the relationship between density and phenotype: (1) density dependence is additive (e.g., Anderson and Semlitsch 2014), (2) density dependence is non-additive (e.g., Morin 1988), (3) self-thinning occurs (Semlitsch and Caldwell 1982), and (4) Allee effects occur (Wilbur 1977; Smith-Gill and Gill 1978). Additive density dependence occurs when there is a linear response to increased density, with each additional individual, resulting in an equal change in the response of interest (Fig. 1). Additive effects would be expected if each additional individual resulted in a proportional decrease in resources. Alternatively, if the response is less than expected by an additive model, non-additive density dependence may be occurring (Fig. 1). Self-thinning occurs when competition is reduced as individuals metamorphose, allowing the remaining larvae to develop in lower densities. This results in individuals at high densities performing similarly or better than individuals at medium densities (Fig. 1). Allee effects occur when populations with a density smaller than a critical mass perform more poorly than those with a density slightly greater than the critical mass (Fig. 1). Such positive density dependence can result from difficulty in finding mates, or modification of the environment (e.g., toad larvae stirring sediment to release food; Wilbur 1977).
The functional form, models, and parameter space that would support each hypothesis. Responses include survival, size at metamorphosis, body condition, or percent crude lipids. For the response of length of larval period, we would expect each curve to be reflected over the x-axis (e.g., additive density dependence would exhibit a positive slope and non-additive would be supported by an increasing function at a decelerating rate rather than a decreasing function). A linear model is used to test for no density dependence and additive density dependence. A non-additive response to natal density is tested with a Shepherd function and exponential model. A Ricker equation is used to test for self-thinning and Allee effects
To test these contrasting hypotheses, we experimentally manipulated the initial larval density of a pond-breeding amphibian, the ringed salamander (Ambystoma annulatum), and compared the functional form of eventual juvenile phenotype to previously proposed models. To better fit density dependence, we tested 11 densities (range: 0.001 to 0.036 larvae/L), which encompassed the range of larval salamander densities found in natural ponds (Semlitsch 1987; Scott 1990; Ousterhout et al. 2015). As individuals metamorphosed, we measured size, body condition, and length of larval period. We also assayed energy stores and survival. To test whether models of population growth are sensitive to the functional form of density dependence, we built a series of simple population growth models differing only in the shape of density dependence. Here, we report evidence of non-additive density dependence. Additionally, we demonstrate that population models are sensitive to the functional form of density dependent size, highlighting the importance of our findings for modeling population dynamics.
Materials and Methods
Study system
The ringed salamander is a pond-breeding amphibian endemic to the Ozark Plateau (southern Missouri, western Arkansas, and eastern Oklahoma; Petranka 1998). Fossorial adults migrate to breeding ponds late August–October (Hocking et al. 2008). Following oviposition, eggs hatch, and larvae overwinter in ponds. While most surviving offspring metamorphose in May, individuals emerge from ponds as late as July (Hocking et al. 2008).
Mesocosm experiment
To test potential mechanisms of conspecific density affecting larval ringed salamanders, we conducted a pond mesocosm experiment. We tested the following densities (larvae/1000 L mesocosm): 9, 12, 16, 21, 24, 27, 30, and 33 (n = 4); 6 and 18 (n = 9); and 36 (n = 7). We increased the replication of three treatments (6, 18 and 36 larvae/mesocosm) as those animals where later used for another experiment. We established experimental pond mesocosms (1000 L volume, 1.52 m diameter polyethylene cattle tanks) in the same manner as similar studies (Earl et al. 2011). We arranged mesocosms in a rectangular array in a fenced research facility at the University of Missouri, Columbia, Missouri. On 5 September 2011, each mesocosm was filled with tap water, and left to dechlorinate for 14 days. We added 2 kg air-dried leaves (primarily Quercus spp. and Acer spp.) to each mesocosm on 19 September 2011 to create a nutrient base. We inoculated each mesocosm with aliquots of zooplankton (4 L added in total; 22 September to 11 October 2011) to establish natural plankton and periphyton communities. Mesocosms were left uncovered to allow colonization by flying insects (e.g., dipterans) as an additional food source for larvae. Water levels were maintained at a constant depth of approximately 50 cm during the experiment.
We collected early stage salamander embryos at similar developmental stages on 28 September 2011 from four ponds at Fort Leonard Wood, Missouri, USA. Egg masses were stored in the laboratory at 10 °C, approximately the water temperature of natural ponds (Anderson et al., unpublished data), until they hatched. Following hatching but prior to feeding, we combined all salamander larvae from the four ponds, and then randomly assigned each larva to an initial density treatment (hereafter, treatment) following a randomized block design (larvae added on 15, 16, and 21 October 2011). Each treatment was then randomly assigned to a mesocosm. As larvae were assigned to treatments, we inspected them for damage and irregularities, and replaced individuals if one of those conditions existed.
Beginning in April 2012, we searched for metamorphosed salamanders by checking mesocosms after sunset with a light at least every other night. Individuals were considered to have metamorphosed if their gills were <1 mm and their tailfin was reabsorbed (Mott and Maret 2011; Ousterhout et al. 2014). Mesocosms were searched until 1 June 2012, when we drained all mesocosms and thoroughly searched the leaf litter for remaining salamanders to calculate survival. At metamorphosis, we recorded date of metamorphosis, wet mass (±0.001 g; Mettler AT-100 electronic balance; Mettler Toledo, Columbus, OH, USA) and snout-vent length (SVL; ±1 mm) Animals that failed to metamorphose prior to the end of the experiment were only included in the survival modeling.
To assay energy stores, we immediately euthanized animals by immersion in tricaine methanesulfonate (MS-222) from eight treatments levels (all except 6, 18, and 36 individuals/mesocosm). Individuals were stored at −20 °C until the conclusion of the mesocosm experiment. To test for effects of density on energy stores, we homogenized samples by mesocosm and then determined the amount of crude lipids using a modified Folch extraction followed by gravimetric lipid determination (Fisheries and Illinois Aquaculture Center, Southern Illinois University).
Statistical analysis
We were interested in six responses to natal density: length of larval period, body size (SVL), body condition, percent crude lipids, and survival to metamorphosis. Length of larval period was defined as the elapsed time from the addition of hatchlings to a mesocosm to an individual’s metamorphosis. We defined body condition as the size independent mass. We calculated body condition by mean scaling the mass to decouple variance from measurement scale and means. We then regressed mass against SVL, and used the residuals as the response in the body condition models (Berner 2011).
We conducted nonlinear regressions to compare our data to five possible mechanisms underlying density dependence (Fig. 1; Harper and Semlitsch 2007; Rollinson and Hutchings 2013). Nonlinear least-squares models relax the assumption of linearity, but still require independence, equal variance, and normally distributed errors (Bates and Watts 1988). If additive density dependence was occurring such that each additional individual decreased the per capita resources equally, then a linear model with a negative slope would be best supported (Anderson and Semlitsch 2014). If responses were less than predicted by the additive model, non-additive density dependence may be occurring, and a model of a decelerating curve would be best supported. A convex curve would be best supported by the data if self-thinning was occurring (Semlitsch and Caldwell 1982). Finally, if an Allee effect was occurring, then a concave curve would best fit the data (Wilbur 1977). In all cases, the strength of density dependence would increase with steeper slopes.
We compared our data to these proposed mechanisms using four functional equation models. We tested for an additive response using a linear model (y = ax + b). We used a Shepherd model (y = ax/(b + x c )) and an exponential function (y = ae−bx) to test for non-additive density dependence. Finally, we used a Ricker equation (y = axe−bx) to test for Allee effects and self-thinning, depending on the parameter space (Fig. 1). Because nonlinear regression does not use least-squares, non-nested models cannot be compared using Akaike information criterion. We instead compared candidate nonlinear regression models for each response variable by calculating an adjusted r 2 as a measure of goodness of fit (Crawley 2013). Models that account for the same amount of variation as the null intercept model had an adjusted r 2 of zero. For all analyses, mesocosm was the experimental unit, and as such, mesocosm means were used. Preliminary analysis revealed block accounted for very little variation (P > 0.60), so we did not consider it in our models. All analyses were performed using nls in R 3.0.2 (R Core Team 2015).
Results
Length of Larval Period
Salamanders began metamorphosing 14 April, 2 weeks after we began checking mesocosms. Ninety-nine percent of all individuals surviving to metamorphosis had left the mesocosms by 1 June, the day we concluded the experiment. Initial larval density affected length of larval period (ANOVA: F 10,44 = 13.87, P < 0.0001). The mean length of the larval period was 18 days longer for individuals from the highest density treatment (mean ± 1 SD: 205 days ± 8.7) than those from the lowest density treatment (mean 187 days ± 6.4). Two non-additive models best fit our data, and the linear model also received support (non-additive: adjusted r 2 = 0.72 and 0.69, linear: adjusted r 2 = 0.69; Table 1; Fig. 2a).
Predicted model values (solid black lines) with 95 % prediction intervals (dashed lines) for initial larval density and a days to metamorphosis (n = 55), b body size (SVL; n = 55), c body condition (n = 55), d survival to metamorphosis (n = 55), and e percent crude fat (n = 30) as a function of initial larval density. A Shepherd equation was used to fit all responses except for survival, which was fit with a linear model. Dots represent mesocosm means
Body size
Body size was negatively affected by initial density (ANOVA: F 10,44 = 25.35, P < 0.0001). Juveniles metamorphosing from high density mesocosms were 22 % smaller than animals from low density mesocosms (low: 45.9 mm ± 2.5, high: 35.8 mm ± 3.0). A non-additive model best described the relationship between initial density and SVL (adjusted r 2 = 0.83; Table 1; Fig. 2b).
Body condition
Body condition did not vary with initial larval density (ANOVA: F 10,44 = 1.47, P = 0.18) and no model received appreciably more support than the null model (adjusted r 2 of best fit model = 0.11; Table 1; Fig. 2c). Body condition was 0 ± 0.08 (minimum: −0.14, maximum: 0.35), where a body condition of zero indicated an animal’s mass corresponded to its SVL, a negative body condition indicated animals weighed less than their SVL would predict, and a positive body condition signaled that animals weighed more than their SVL would indicate.
Survival
There was no relationship between initial larval density and survival to metamorphosis (ANOVA: F 10,44 = 1.5, P = 0.17). Survival was 91.3 % ± 17 (minimum: 42.9 %; maximum 100 %). No model received substantially more support than the null model. The best fitting model explained little of the variance (adjusted r 2 = 0.07; Table 1; Fig. 2d)
Energy stores
As density increased, percent crude lipids decreased nonlinearly (ANOVA: F 7,23 = 29.927, P < 0.0001). Individuals reared in the highest density treatments had 34 % lower lipid content than those reared in the lowest density treatment (low: 12.90 % crude lipids ± 0.65; high: 8.52 % crude lipids ± 0.80). The relationship between lipids and initial density of larvae was best described by the non-additive model (adjusted r 2 = 0.63; Table 1; Fig. 2e).
Discussion
Here, we show that intraspecific natal density exerts clear non-additive effects on juvenile phenotype. The non-additive model accounted for at least 8 % more variation than the next most supported hypothesis for all responses affected by density, except for length of larval period (3 %). Individuals metamorphosing from low density ponds were larger and metamorphosed earlier than juveniles from higher density treatments, but the differences between treatments diminished as density increased. We did not detect an effect of density on survival. As most amphibians occur at high larval densities in natural ponds, the diminishing difference between treatments at high densities may help account for the limited explanatory power of intraspecific density in field studies (Van Buskirk 2005; Ousterhout et al. 2015).
Two non-exclusive mechanisms could result in the non-additive density dependence observed in this study: resource limitation and increased stress hormones. The lipid assay in this study indicates that food resources limited energy assimilation. Percent crude lipids decreased nonlinearly; the percent crude lipids of animals in the lowest density mesocosms was 51 % greater than animals reared in the highest density pond. If resource limitation was the mechanism resulting in non-additive density dependence, we would expect there to be lower quantities of zooplankton in ponds with a higher density of larvae. This would result in non-additive density dependence if beyond a threshold density of larvae zooplankton density was uniformly low. However, with one exception (Scott 1990), previous studies have found no effect of the density of salamander larvae on the density, biomass or composition of zooplankton (Figiel and Semlitsch 1990; Van Buskirk and Smith 1991; Davis 2012), including one study with ringed salamanders (Anderson et al., unpublished data). We suggest that although resource limitation is certainly occurring, further experiments that frequently sample zooplankton will be required to capture the highly cyclic dynamics of food resources and test the resource limitation hypothesis.
Alternatively, the non-additive density dependence observed in this study may be caused by increased stress hormones as a result of more frequent encounters with conspecifics in higher density ponds. Elevated stress in response to conspecifics has been well documented in amphibian larvae (Glennemeier and Denver 2002; Crespi and Denver 2004; Rot-Nikcevic et al. 2005; Davis and Maerz 2009; Davis 2012), and is directly linked to reduced foraging (Crespi and Denver 2004) and size at metamorphosis (Davis and Maerz 2009). Davis (2012) observed a strong effect of intraspecific density on the stress hormones of larval spotted salamanders (A. maculatum). However, there was no effect of food additions on stress hormones in that study, indicating that stress rather than resource limitation affected metamorph traits.
Additionally, it seems unlikely that aggressive interactions alone could account for the patterns we observed. While larvae of Ambystoma can be agonistic in their interactions, levels of aggression vary greatly between species (Walls and Jaeger 1987; Mott and Maret 2011). Cannibalism does occur among larvae of ringed salamanders (Nyman et al. 1993). If cannibalism happened in our experiment, the high survival we observed indicates that it occurred infrequently. Furthermore, we did not observe any limb, body, or tail injuries suggestive of overt aggression when salamanders were recaptured at metamorphosis. However, previous studies have found that the perception of density, rather than direct aggression, may be sufficient to alter behavior. When high intraspecific density was mimicked through exposure to clay models of wood frog (Rana sylvatica) larvae, Rot-Nikcevic et al. (2005) found that individuals were smaller and had elevated stress hormones at metamorphosis. In low density treatments, encounters between larvae by chance should occur less frequently than in high-density mesocosms. This would correspond to fewer stress responses interrupting activities such as foraging. Following an encounter with another larva, stress hormones do not immediately decrease, but rather do so over time. In medium and high density treatments subsequent encounters may occur before normal behavior is resumed, resulting in the diminishing difference between treatments as density increases. Experiments quantifying encounter rates in different densities and stress hormones corresponding to these encounters are required to test this hypothesis.
We found no effect of initial larval density on survival to metamorphosis. This supports our speculation that resource levels were not limiting, at least not to the degree of causing starvation. Additional experiments with greater densities would be required to test this. Alternatively, the uniformly high survival to metamorphosis may indicate non-sequential density dependence, whereby the effects of high larval density are not experienced immediately (i.e. mortality), but carried over as costs into the juvenile stage through smaller body size at metamorphosis. Such non-sequential density dependence through decreased fitness in later life-history stages is characteristic of species that breed in variable environments (Berven and Gill 1983; Smith 1987; Semlitsch et al. 1988).
The non-additive response of larval ringed salamanders to initial intraspecific density presents evidence for an alternate hypothesis to additive density dependence, self-thinning (Semlitsch and Caldwell 1982), or an Allee effect in larval amphibians (Wilbur 1977; Smith-Gill and Gill 1978). As suggested by Semlitsch and Caldwell (1982), the different phenotypic patterns observed in response to high natal density may be due to alternative evolutionary pathways: dispersal ability and tolerance (Gill 1978). Gill (1978) suggested that in situations where the environment is deteriorating and unlikely to improve, there will be selection for rapid growth and a short natal period (i.e. dispersal ability). Semlitsch and Caldwell (1982) observed this strategy in spadefoot toads (Scaphiopus holbrooki), which are explosive breeders that utilize ephemeral wetlands. As the habitat this species uses is unlikely to improve (e.g., longer hydroperiod), selection favors rapid larval growth. While species with short larval periods, such as spadefoot toads, are predicted to undergo selection for dispersal ability, Gill (1978) predicted species with longer larval periods may evolve to be more tolerant of higher densities, and thus selection would increase efficiency of resources utilization. We would expect ringed salamanders to fall under the tolerance model. Ringed salamanders breed in the fall and overwinter in relatively permanent ponds before metamorphosing in the late spring–early summer. Cold temperatures during the early larval period would prevent individuals from attaining a minimum size or surviving dispersal if they metamorphosed early (i.e. low selection for dispersal ability). Thus, we expect selection to favor improved resource utilization efficiency (i.e. tolerance) in ringed salamanders. This evolutionary pathway would account for the diminishing effect of increased natal density; while individuals at low densities grow faster than those at high densities, the effect of increased density may be dampened by selection for efficiency of resource use, resulting in non-additive density dependence.
The effects of early developmental environment on juvenile and adult phenotype have far-reaching individual survival and fecundity consequences. By elucidating the non-additive relationship between initial intraspecific density and individual traits, this study may assist future models in using empirical data rather than expert opinion (Cosentino et al. 2011; Richardson 2012). The equations presented in this study can aid in simulating the characteristics of salamanders metamorphosing from ponds which differ in intraspecific density. Resulting phenotypic differences could be used to select survival probabilities, movement behavior, age of first reproduction, or fecundity (Scott 1994; Chelgren et al. 2006). Additionally, models of discrete population dynamics are sensitive to the functional form of density dependence. For example, population models with an exponential decline in survival as a function of density have larger regions of stability than models based on linear declines (May and Oster 1976; Bellows 1981). To demonstrate the sensitivity of population models to the functional form of density dependence, we simulated population dynamics using different equations for the shape of density dependence. In this model, body size was a function of the population size, and the probability of reproduction and fecundity were a function of body size. The functional form of the density-dependent size model was parameterized with a linear, exponential, Ricker, or Shephard equation (from Table 1; for more details, see Online Resource 1). Both the stable population size and the amplitude of population oscillations were sensitive to functional form of density dependence (Fig. 3). While this was a very simple modeling exercise, it highlights the importance of considering nonlinearities in density dependence.
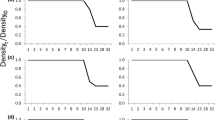
Predicted dynamics of a population over 20 years differed based on assumptions of the functional form of density-dependent size. Solid lines represents a mean population estimate from the bootstrap analyses parameterized with an a exponential, b linear, c Ricker, or d Shepherd equation. The 95 % confidence interval for each parameterization is represented with a dashed line. Population estimates are based on 10,000 bootstrapped simulations
In conclusion, our study suggests that non-additive density dependence can occur among larval amphibians. By testing four-times more densities than previous studies, we were able to better fit nonlinearities in the response of salamanders to density dependence. We suggest that the proximate mechanism for nonlinearities are stress-mediated behaviors, and that the ultimate mechanism is tolerance to high-density conditions through selection on resource utilization. Experiments are required to test these proposed mechanisms. Intraspecific density affected juvenile phenotype in a nonlinear manner, with the strength of effects decreasing as initial density increased. By modeling the effects of density on phenotype, our experiment contributes to a larger body of work focused on how organisms adapt to negative density dependence. Such studies are important for developing population models that can inform theory and ultimately predict dynamics in the face of anthropogenic change.
References
Anderson TL, Semlitsch RD (2014) High intraguild predator density induces thinning effects on and increases temporal overlap with prey populations. Popul Ecol 56:265–273. doi:10.1007/s10144-013-0419-9
Bates DM, Watts DG (1988) Nonlinear regression analysis and its applications. Wiley, New York
Bellows TS (1981) The descriptive properties of some models for density dependence. J Anim Ecol 50:139. doi:10.2307/4037
Berner D (2011) Size correction in biology: how reliable are approaches based on (common) principal component analysis? Oecologia 166:961–971. doi:10.1007/s00442-011-1934-z
Berven KA, Gill DE (1983) Interpreting geographic variation in life-history traits. Am Zool 23:85–97
Biek R, Funk WC, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv Biol 16:728–734. doi:10.1046/j.1523-1739.2002.00433.x
Brook BW, Bradshaw CJA (2006) Strength of evidence for density dependence in abundance time series of 1198 species. Ecology 87:1445–1451
Bult TP, Riley SC, Haedrich RL et al (1999) Density-dependent habitat selection by juvenile Atlantic salmon (Salmo salar) in experimental riverine habitats. Can J Fish Aquat Sci 56:1298–1306. doi:10.1139/f99-074
Chelgren ND, Rosenberg DK, Heppell SS, Gitelman AI (2006) Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecol Appl 16:250–261
Cole LC (1954) The population consequences of life history phenomena. Q Rev Biol 29:103–137
Cosentino BJ, Schooley RL, Phillips CA (2011) Spatial connectivity moderates the effect of predatory fish on salamander metapopulation dynamics. Ecosphere 2:art95. doi:10.1890/ES11-00111.1
Crawley MJ (2013) The R book, 2nd edn. Wiley, West Sussex
Crespi EJ, Denver RJ (2004) Ontogeny of corticotropin-releasing factor effects on locomotion and foraging in the Western spadefoot toad (Spea hammondii). Horm Behav 46:399–410. doi:10.1016/j.yhbeh.2004.03.011
Davis AK (2012) Investigating the optimal rearing strategy for Ambystoma salamanders using a hematological stress index. Herpetol Conserv Biol 7:95–100
Davis AK, Maerz JC (2009) Effects of larval density on hematological stress indices in salamanders. J Exp Zool 311A:697–704. doi:10.1002/jez.557
De Kogel CH (1997) Long-term effects of brood size manipulation on and sex-specific mortality of morphological development offspring. J Anim Ecol 66:167–178
Earl JE, Luhring TM, Williams BK, Semlitsch RD (2011) Biomass export of salamanders and anurans from ponds is affected differentially by changes in canopy cover. Freshw Biol 56:2473–2482. doi:10.1111/j.1365-2427.2011.02672.x
Einum S, Sundt-Hansen L, Nislow KH (2006) The partitioning of density-dependent dispersal, growth and survival throughout ontogeny in a highly fecund organism. Oikos 113:489–496. doi:10.1111/j.2006.0030-1299.14806.x
Figiel CR, Semlitsch RD (1990) Population variation in survival and metamorphosis of larval salamanders (Ambystoma maculatum) in the presence and absence of fish predation. Copeia 1990:818–826
Gill DE (1978) On selection at high population density. Ecology 59:1289–1291
Glennemeier KA, Denver RJ (2002) Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. J Exp Zool 292:32–40. doi:10.1002/jez.1140
Harper EB, Semlitsch RD (2007) Density dependence in the terrestrial life history stage of two anurans. Oecologia 153:879–889. doi:10.1007/s00442-007-0796-x
Herrando-Perez S, Delean S, Brook BW, Bradshaw CJA (2012) Density dependence: an ecological Tower of Babel. Oecologia 170:585–603. doi:10.1007/s00442-012-2347-3
Hocking DJ, Rittenhouse TAG, Rothermel BB et al (2008) Breeding and recruitment phenology of amphibians in Missouri oak-hickory forests. Am Midl Nat 160:41–60. doi:10.1674/0003-0031(2008)160
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Loman J (2004) Density regulation in tadpoles of Rana temporaria: a full pond field experiment. Ecology 85:1611–1618. doi:10.1890/03-0179
May RM, Oster GF (1976) Bifurcations and dynamic complexity in simple ecological models. Am Nat 110:573–599
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans Biol Sci 363:1635–1645. doi:10.1098/rstb.2007.0011
Morin PJ (1988) Functional redundancy, non-additive interactions, and supply-side dynamics in experimental pond communities. Ecology 76:133–149
Mott CL, Maret TJ (2011) Species-specific patterns of agonistic behavior among larvae of three syntopic species of Ambystomatid salamanders. Copeia 2011:9–17. doi:10.1643/CE-09-065
Nyman S, Wilkinson RF, Hutcherson JE (1993) Cannibalism and size relations in a cohort of larval ringed salamanders (Ambystoma annulatum). J Herpetol 27:78–84
Ousterhout BH, Luhring TM, Semlitsch RD (2014) No evidence of natal habitat preference induction in juveniles with complex life histories. Anim Behav 93:237–242. doi:10.1016/j.anbehav.2014.04.035
Ousterhout BH, Anderson TL, Drake DL et al (2015) Habitat traits and species interactions differentially affect abundance and body size in pond-breeding amphibians. J Anim Ecol 84:914–924
Pechmann JHK (1995) Use of large field enclosures to study the terrestrial ecology of pond-breeding amphibians. Herpetologica 51:434–450
Peterman WE, Locke JL, Semlitsch RD (2013) Spatial and temporal patterns of water loss in heterogeneous landscapes: using plaster models as amphibian analogues. Can J Zool 140:135–140
Petranka JW (1998) Salamanders of the United States and Canada. Smithsonian Institution Press, Wasington, DC
Post JR, Parkinson EA, Johnston NT (1999) Density-dependent processes in strucutred fish populations: interaction strengths in whole-lake experiments. Ecol Monogr 69:155–175
R Core Team (2015) R: A language and environment for statistical computing
Richardson JL (2012) Divergent landscape effects on population connectivity in two co-occurring amphibian species. Mol Ecol 21:4437–4451. doi:10.1111/j.1365-294X.2012.05708.x
Rollinson N, Hutchings JA (2013) The relationship between offspring size and fitness: integrating theory and empiricism. Ecology 94:315–324
Rot-Nikcevic I, Denver RJ, Wassersug RJ (2005) The influence of visual and tactile stimulation on growth and metamorphosis in anuran larvae. Funct Ecol 19:1008–1016. doi:10.1111/j.1365-2435.2005.01051.x
Scott DE (1990) Effects of larval density in Ambystoma opacum: an experiment in large-scale field enclosures. Ecology 71:296–306
Scott DE (1994) The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 75:1383–1396
Semlitsch RD (1987) Density-dependent growth and fecundity in the paedomorphic salamander Ambystoma talpoideum. Ecology 68:1003–1008
Semlitsch RD, Caldwell JP (1982) Effects of density on growth, metamorphosis, and survivorship in tadpoles of Scaphiopus holbrooki. Ecology 63:905–911
Semlitsch RD, Scott DE, Pechmann JHK (1988) Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69:184–192
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68:344–350
Smith-Gill SJ, Gill DE (1978) Curvilinearities in the competition equations: an experiment with ranid tadpoles. Am Nat 112:557–570
Van Buskirk J (2005) Local and landscape influence on amphibian occurance and abundance. Ecology 86:1936–1947
Van Buskirk J, Smith DC (1991) Density-dependent population regulation in a salamander. Ecology 72:1747–1756
Vonesh JR (2005) Sequential predator effects across three life stages of the African tree frog, Hyperolius spinigularis. Oecologia 143:280–290. doi:10.1007/s00442-004-1806-x
Walls SC, Jaeger RG (1987) Aggression and exploitation as mechanisms of competition in larval salamanders. Can J Zool 65:2938–2944
Wilbur HM (1976) Density-dependent aspects of metamorphosis in Ambystoma and Rana sylvatica. Ecology 57:1289–1296
Wilbur HM (1977) Density-dependent aspects of growth and metamorphosis in Bufo americanus. Ecology 58:196–200
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Evol Syst 11:67–93
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14. doi:10.1007/s004420100809
Acknowledgments
We thank T. Anderson, D. Drake, C. Farmer, A. Milo, and K. Proffit for help collecting data. T. Anderson, R. Holdo, D. Kesler, and H. Wilbur provided comments on earlier drafts of this manuscript. We also thank R. Holdo for his assistance with the population model. This work was supported by a National Science Foundation Graduate Research Fellowship to B. H. Ousterhout and the Department of Defense (SERDP RC-2155). Animals were collected and maintained under Missouri Department of Conservation permit 14922 and ACUC Protocol 7403. All experiments comply with the current laws of the United States of America. The authors declare no conflict of interest.
Author contribution statement
BHO and RDS conceived and designed the experiments. BHO performed the experiments, analyzed the data, and wrote the manuscript. RDS provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Steven Kohler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ousterhout, B.H., Semlitsch, R.D. Non-additive response of larval ringed salamanders to intraspecific density. Oecologia 180, 1137–1145 (2016). https://doi.org/10.1007/s00442-015-3516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3516-y