Abstract
Herbivorous insects specialized on a narrow set of plants are believed to be better adapted to their specific hosts. This hypothesis is supported by observations of herbivorous insect species with a broader diet breadth which seemingly pay a cost through decreased oviposition accuracy. Despite many studies investigating female oviposition behavior, there is a lack of knowledge on how larvae cope behaviorally with their mothers’ egg-laying strategies. We have examined a unique system of five nymphalid butterfly species with different host plant ranges that all feed on the same host plant. The study of this system allowed us to compare at the species level how oviposition preference is related to neonate larval responses in several disadvantageous situations. We found a general co-adaptation between female and larval abilities, where species with more discriminating females had larvae that were less able to deal with a suboptimal initial feeding site. Conversely, relatively indiscriminate females had more precocious larvae with better abilities to cope with suboptimal sites. Despite similarities between the tested species with similar host ranges, there were also striking differences. Generalist and specialist species can be found side by side in many clades, with each clade having a specific evolutionary history. Such clade-specific, phylogenetically determined preconditions apparently have affected how precisely a broad or narrow diet breadth can be realized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most insect species are resource specialists (Futuyma and Moreno 1988), and there are currently many indications of a general trend that drives herbivorous insects towards host plant specialization. Such drivers of specialization can, for example, be trade-offs in performance due to variation in host plant chemistry (Joshi and Thompson 1997; Agrawal 2000), predator avoidance (Bernays and Graham 1988; Murphy 2004; Wiklund and Friberg 2008), neural limitations in information processing (Bernays and Wcislo 1994; Janz and Nylin 1997) or differential local selection pressures (Thompson 2005).
Despite this ubiquitous trend towards specialization, however, a considerable number of species still utilize a broader range of host plants. The frequency and extent of relative generalists vary between groups, but it is interesting to note that even otherwise specialized groups of insects appear to be occasionally interspersed by periods of polyphagy (Nylin et al. 2014). The apparent ephemeral nature of these events suggests that the benefits of specialization are occasionally reversed or relaxed. The relative benefits of generalization may be temporary, but they could play an important role in evolution, both for the formation of new species interactions and for host-driven diversification (Janz and Nylin 2008; Janz 2011). Hence, even though the trend towards specialization is important to explain, we also need to understand how polyphagy can evolve and be maintained in spite of its apparent costs.
As a general explanation for the predominance of specialists over generalists, the information processing hypothesis is particularly promising because of its wide applicability across a broad range of situations and contexts (Bernays and Wcislo 1994; Dukas 1998; Bernays 2001). Studies testing this hypothesis during the last two decades show strong evidence for a cost of generalization in the behavior leading to host plant selection (Janz and Nylin 1997; Janz 2003; Egan and Funk 2006). Empirical comparative studies have demonstrated that relative generalists pay a cost, such as in the speed or accuracy of the decision-making process. For example, feeding generalist larvae are found to be less efficient and to require more time to start feeding on a host than their specialized counterparts (Bernays et al. 2004). Similarly, for an ovipositing adult, having a broader host range is correlated with both the detection of an inferior host plant and a longer host evaluation time (Janz and Nylin 1997; Janz 2003; Egan and Funk 2006).
With a broader diet, these constraints are believed to limit the possibility of generalists to differentiate between individuals of the same species, since many of their neural resources have to be directed towards the differentiation between and recognition of varying host plant species (Bernays 2001). Recent morphological and physiological studies have also corroborated that generalist and specialist butterflies do indeed differ in the number of antennal sensilla and in their physiological responses towards host plant odors (Carlsson et al. 2011, 2013).
The larval stage of Lepidoptera is usually considered to be rather immobile, which is why much attention has been directed towards adult oviposition preferences as the determinant of the larval host plant. The female’s careful search for a suitable host plant has even be interpreted by some authors as a form of parental care (Wiklund and Persson 1983; Janz and Nylin 1997). Although females do not actively tend or protect their offspring, they may reject seemingly suitable host plants to invest additional time to find even better oviposition sites to increase the fitness of individual offspring (Doak et al. 2006; Friberg et al. 2008). The more indiscriminate oviposition strategy seen in relative generalists (Janz and Nylin 1997) could have the potential benefit of increased realized fecundity, but at the rather serious expense of mean offspring fitness. However, this negative impact could potentially be lowered if larval capabilities were co-adapted; i.e. if larvae of indiscriminate females were more precocious and able to search for and locate suitable resources on their own (Wiklund 1984; Zalucki et al. 2002; Gamberale-Stille et al. 2014). Conversely, in species with a more ‟caring” oviposition strategy, there would be no need for the newly hatched larvae to cover longer distances or to evaluate potential food resources themselves. As a consequence, these species would not have to allocate as many resources to each egg.
This general idea of co-adaptation between adult oviposition strategy and larval ability has been poorly explored and can best be tested experimentally in a comparative framework. We took advantage of a unique system of several closely related butterfly species with different degrees of host plant specialization that all include the same species in their host plant repertoire. Thus, we were able to assess female oviposition preference of herbivore species with different life-histories and relate this to neonate larval behavior and performance in a variety of situations, without any inter-species effects that may arise from comparing different host plants.
We expected those butterfly species that are host plant specialists to have discriminating females and larvae with poor host location and evaluation abilities; alternatively, we expected those butterfly species that are relative host plant generalists to have less discriminating females but larvae that are more capable of coping with unfavorable oviposition sites. In summary, we hypothesized that (1) larval precociousness and female care during oviposition are generally negatively associated and (2) these differences should correspond to the specialist–generalist axis. More specifically, we first established female oviposition preference between host plant individuals of varying quality and then we related larval behavior and performance to female preference in a series of experiments aimed at investigating (1) larval capability to survive in unfavorable conditions and (2) larval ability to leave an unfavorable site and to find another feeding site.
Materials and methods
Study organisms and rearing
We compared five species of nymphalid butterflies, two generalists (Polygonia c-album and Vanessa cardui) and three specialists (Aglais urticae, A. io and Vanessa atalanta) that all feed and oviposit on the host plant Urtica dioica (Urticaceae). P. c-album feeds on a number of species from several plant orders but prefers U. dioica (Nylin 1988). V. cardui is a generalist reported to feed on more than 100 species from many plant orders, but they seem to prefer plants in Asteraceae and Malvaceae (Ebert 1993; Stefanescu 1997). U. dioica is among the host plants of V. cardui and can at times be a preferred host (Seppänen 1970; Ebert 1993). A. urticae and A. io are specialists on U. dioica (Eliasson et al. 2005). In Europe, V. atalanta is a specialist on Urtica and Parietaria (both Urticaceae) (Stefanescu 2001). The stinging nettle, U. dioica (Urticaceae), is a common, perennial herb that prefers nitrogen-rich soils. Plant quality declines with advancing season, but re-growth foliage can provide “high”-quality leaves throughout the growing season (Pullin 1987).
P. c-album, V. cardui, A. urticae and A. io individuals were caught in the Stockholm area in May of 2013 and 2014. In April 2014, V. atalanta individuals were collected in Catalonia, Spain. Additional stock of V. cardui was obtained in 2013 from a commercial butterfly provider (Worldwide Butterflies, www.wwb.co.uk). All applicable institutional and/or national guidelines for the care and use of animals were followed. Experiments were conducted from May to July in both 2013 and 2014. Offspring of all species were reared on U. dioica under long daylength conditions to pupation as described by Janz et al. (2009). Eclosed individuals were placed in mating cages (approx. 70 × 70 × 50 cm) with a light regime of 18/6 h light/dark.
Oviposition experiment, egg weights and leaf quality
Mating pairs of P. c-album, V. atalanta and V. cardui were separated and the females placed individually in oviposition cages (approx. 50 × 50× 50 cm). Mating pairs are not easily observed in A. io and A. urticae, so individuals were allowed to mate freely over several days, after which time they were given a host plant choice following the protocol described below, and mated females were identified through continuous observation. Once a day host plants were placed in the cages of the single egg-layers P. c-album, V. cardui and V. atalanta for periods of 3 h until they had laid at least ten eggs on 3 different days; if they did not lay this minimum number of eggs, they were not included in the analyses. The protocol of the oviposition experiment was similar to that used by Janz and Nylin (1997) where a simultaneous choice between two host plant shoots of differing quality was given: (1) a “high”-quality leaf pair of U. dioica, i.e. fresh, green leaves, and (2) a “poor”-quality leaf pair, i.e. partly yellow or brown leaves but with no signs of wilting. Measurements were made of leaf quality, such as leaf toughness, water content and nitrogen levels. Leaves were collected during the same time window as when the experiments were conducted and from the same areas on the Stockholm University Campus, Stockholm, Sweden, as the leaves used in the experiment. On 15 July 2013 the leaf toughness of “high”- and “poor”-quality U. dioica leaves was determined by measuring the weight (force) required to punch the leaf with a device constructed following the description of Feeny (1970). Water content was determined in “high”- and “poor”-quality leaves by weighing the leaves before and after drying at 50 °C in an oven and measuring the difference. Nitrogen content was obtained from “high”- and “poor”-quality U. dioica leaves collected on several sites of the Campus of Stockholm University on 24/25 June 2014.
Eggs were counted and collected daily, and egg-clutches were separated from the leaves. At least 30 eggs from five or more females of each species were weighed within 24 h of oviposition.
Experiments: test of neonate larval performance
After oviposition the eggs were transferred into small plastic jars and stored at room temperature. When larvae hatched, care was taken to test them as soon as possible but no more than 12 h after hatching.
Survival experiment
To investigate whether there is a general relationship between female oviposition preference and larval ability to feed on “high”- or “poor”-quality leaves, we placed newly hatched larvae in petri dishes on either a “high”- or “poor”-quality leaf. Survival and larval instar were checked every 24 h for 8 days. At the end of the period the surviving larvae were weighed. All larvae were tested singly; however, to test for an effect of gregariousness in the clutch-laying species A. urticae and A. io, ten larvae of these species were also tested together in the same treatment as described above.
Mobility
To obtain a measure reflecting the ability of neonate larvae to cover a distance, we recorded the distance and direction that neonate larvae covered within 30 min. Using a soft brush we placed a single larva in the middle of a vertical, 1-m-long wooden rod and recorded its position at 5-min intervals. The minimum distance covered was calculated.
Nettle stalk experiment
This experiment combined the survival and mobility test in a more realistic setup. Using this setup we were able to investigate the extent to which neonate larvae had the ability to (1) move from an inferior position, which may have resulted from an inaccurate female oviposition on a host plant stalk, and (2) locate a leaf and (3) whether they were able to establish on that leaf and start feeding. With these aims, we placed one cut shoot of nettle in a flask with water and removed all edible parts (leaves, reproductive parts, shoots) between an upper and lower leaf pair so that about 12–20 cm of bare stalk remained between the leaf pairs. Newly hatched larvae were placed on the stem at an equal distance from the upper and lower leaf pair. Larval position on the shoot and survival was checked after 1, 2, 3, 6, 24 and 48 h, although only results from the final observation are reported here. Plants that showed signs of wilting during the experiment were excluded from the data analysis.
Statistical analyses
Statistical analyses were conducted in R (R Core Team 2014). Leaf water content and the inverted data of leaf toughness were analyzed with Students t test. To compare levels of leaf nitrogen between the “high”- and “poor”-quality host plants we used a linear mixed model with site as random factor. Since Aglais urticae and A. io deposit their eggs in clutches, their oviposition choice was analyzed with a binomial test. The preference of the other species was tested with a paired Wilcoxon signed-rank test. Egg weights were compared between species with a one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. Survival on the leaves of different quality was tested with a generalized linear model (GLM) (logistic regression). To test for differences in larval weight after 8 days on the leaves of different quality we used Wilcoxon signed-rank tests. When analyzing survival and weight after 8 days on a leaf of specific quality, the two Aglais species were tested in several experiments both in the “single” and “gregarious” treatment, as well as compared between treatments; in these cases we conducted Bonferroni correction where multiple testing of the same data required it. The distances covered on the wooden rod were square-root transformed to obtain normality of the data and then tested with a one-way ANOVA, followed by Tukey’s post hoc test to detect where differences in mobility occurred. Survival on the nettle stalk and the percentage of larvae that reached a leaf after 48 h were also tested with a GLM (logistic regression).
Results
The water content of “high”-quality and “poor”-quality leaves was 75.4 % ± 1.1 [mean ± standard deviation (SD); n = 10] and 69.6 % ± 1.9 (n = 10), respectively; this difference was statistically significant between the categories (t test: t = 8.47, df = 14.5, p < 0.001). In addition, it required 133.3 g (± 11.1; n = 10) of weight to punch a hole through “high”-quality leaves and 186.9 g (±29.2; n = 10) of weight to punch a hole through “poor”-quality leaves, which was a significant difference in toughness between the two categories (t test; t = 5.95, df = 16.5, p < 0.001). Lastly, the nitrogen level of the “high”-quality and “poor”-quality leaves was 3.62 % ± 0.72 and 2.25 ± 0.38, respectively, which was a significant difference (both categories n = 15; linear mixed model: χ2 = 74.4, df = 1, p < 0.001).
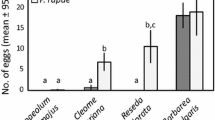
Before investigating larval behavior, we assessed female oviposition preference in the five species of butterflies with a choice between a “poor”- and “high”-quality host plant (Fig. 1). Both A. urticae (n = 22) and A. io (n = 11) showed a significant preference for the “high”-quality host plant (binomial test: both p < 0.001), whereas the remaining three species did not show a preference for either alternative (paired Wilcoxon signed-rank test; V. atalanta: n = 6, V = 1, p = 0.063; V. cardui: n = 16; V = 87, p = 0.35; P. c-album: n = 19, V = 46, p = 0.16). Egg weight was significantly different between but not within genera (ANOVA: F (4,32) = 579.1, p < 0.001; followed by a post hoc Tukey test); data for the five species is shown in Fig. 2.
Oviposition preference between a “poor”- and a “high”-quality host plant. On the left Mean proportion of preference for the clutch-laying species Aglais urticae (n = 26) and A. io, (n = 14) (binomial test: ***p < 0.001). It should be noted that the data are binomial and that no A. io females laid eggs on “poor”-quality leaves. For the remaining three species box plots show the median proportion of preference (horizontal line), the 25th and 75th percentile (lower and upper margins of the box, respectively) together with the minimum and maximum values (whiskers), using the paired Wilcoxon signed-rank test. ns Not significantly different: Vanessa atalanta (p = 0.063; n = 6), V. cardui (p = 0.348; n = 19) and Polygonia c-album (p = 0.156; n = 16)
Egg weights of the five species. The box plots show the median egg weight (horizontal line), the 25th and 75th percentile (lower and upper margins of the box, respectively) together with the minimum and maximum weights (whiskers). Box plots capped by different lowercase letters differ significantly (ANOVA: F (4,32) = 579.1, followed by post hoc Tukey test, p < 0.01). A. urticae: n = 30; A. io: n = 44; V. atalanta: n = 41; V. cardui: n = 42 and P. c-album: n = 50
Next we observed survival of neonate larvae manually distributed on either “poor”- or “high”-quality host plants (Fig. 3). After 8 days we found that only A. urticae larvae had survived significantly better on a “high”-quality compared to “poor”-quality host plant (logistic regression: χ2 = 27.87, df = 1, p < 0.001). We did not detect any difference in survival after 8 days between the two leaf categories for the other species (logistic regression: p > 0.05; range 0.50–0.89). We further tested differences in survival on the two plant qualities when larvae were allowed to feed in groups (gregarious), which was considered to be a more realistic environment for species laying eggs in clutches (A. urticae and A. io). For A. urticae, the result was similar to that found for solitary larvae, with gregarious larvae surviving better on “high”-quality than on “low”-quality leaves (χ2 = 55.19, df = 1, p < 0.001). For A. io, however, there was no difference in survival on the two different categories of leaf within the gregarious treatment (χ2 = 0.45, df = 1, p = 0.76), but leaf quality did influence larval survival in the case of solitary versus gregarious, with A. io larvae surviving better in the gregarious treatment independently of leaf quality (χ2 = 87.44, df = 1, p < 0.001).
Larval survival after 8 days of feeding on a “poor”- or “high”-quality host plant. Bars proportion of larvae alive after 8 days, number in bars number of larvae surviving. Logistic regression was applied to detect differences in survival on the two leaf categories (***p < 0.001, ns = not significant). For Aglais urticae and A. io, data are shown for both single and gregarious treatment. Significant p values of the multiply tested Aglais species were still significant after Bonferroni correction. The line above the bars for A. io larvae tested singly and gregariously refers to differences in survival of gregarious vs. single larvae independent of leaf quality
The weight after 8 days was significantly higher for larvae feeding on “high”-quality host plants than for those feeding on “poor”-quality hosts (Wilcoxon signed-rank test; A. urticae: W = 18, p < 0.001; A. io: W = 11, p = 0.0049; V. atalanta: W = 378.5, p < 0.001, P. c-album: W = 25.5, p < 0.001), with the exception of V. cardui (W = 135, p = 0.20) for which larval weight was similar on both qualities of plant (Fig. 4). Likewise, the weight of gregariously feeding larvae was significantly higher on “high”-quality leaves than on “poor”-quality ones (A. urticae: W = 23.5, p < 0.001; A. io: W = 145, p < 0.001). In addition, when we compared weights between single and gregarious larvae within each leaf quality category we found that gregariously feeding larvae were significantly heavier on “high”-quality leaves for both A. urticae (W = 1371, p < 0.001) and A. io (W = 748, p < 0.001). On “poor”-quality leaves, gregariously feeding larvae of A. io were heavier than their singly feeding counterparts (W = 544, p < 0.001), but single and gregarious larvae of A. urticae larvae showed no difference in weight (W = 92, p = 0.10).
Analysis of larval weight after 8 days of feeding on a “poor”- or “high”-quality host plant using Wilcoxon’s signed-rank test. The box plots show the median weight (horizontal line), the 25th and 75th percentile (lower and upper margins of the box, respectively) together with the minimum and maximum weights (whiskers). ***p < 0.001, **p < 0.01, ns not significant. Numbers below each bar n values. Significant p values of the multiply tested Aglais species were still significant after Bonferroni correction
Finally, we investigated neonate larval mobility and performance when the larva was placed some distance from a feeding site (a vertical rod or a nettle stalk). There were significant differences in the distance covered on the rod between the tested species (ANOVA: F (4115) = 105, p < 0.001; Fig. 5). The most mobile species was V. atalanta, followed by P. c-album and V. cardui; the least mobile species were A. urticae and A. io. The mobility of the Aglais species did not differ from each other (p = 0.92), however, Tukey’s post hoc test revealed that all other comparisons were significantly different from each other (p < 0.05). In the second part of this experiment we placed neonate larvae on a naked nettle stalk with only the upper and lower leaf pairs left intact and subsequently recorded the percentage of larvae that reached a leaf within 48 h and the percentage survival after 48 h. We found significant differences between species in terms of the likelihood to reach a leaf (logistic regression: χ2 = 51.4, df = 4, p < 0.001) and to survive on the nettle stalk for 48 h (χ2 = 92.8, df = 4, p < 0.001). In general, the two Aglais species performed worse than the other three species in that fewer larvae survived and reached a leaf. V. cardui seemed to perform somewhat intermediately, and V. atalanta and P. c-album performed best. Comparisons of these five species are given in detail in Tables 1 and 2 (where multiple testing of the data was required, Bonferroni correction was performed). The two Vanessa species did occasionally feed on the stalk (25 % of V. atalanta and 2.5 % of V. cardui), which may partly explain why more larvae of V. atalanta survived than actually reached the leaves.
Larval mobility on wooden rod. The box plots show the median distance (cm) covered per hour (horizontal line), the 25th and 75th percentile (lower and upper margins of the box, respectively) together with the minimum and maximum speeds (whiskers). Box plots capped by different lowercase letters differ significantly [ANOVA (F (4115) = 105) followed by Tukey post hoc test, p < 0.05]. n = 20, 28, 33, 19 and 20, respectively
Discussion
As we predicted, there was a general negative association between female and larval abilities. Species with relatively indiscriminate females had more precocious larvae with a higher ability to cope with suboptimal oviposition sites, either by an increased ability to feed on “poor”-quality leaves or to relocate to a better feeding site (or both). Conversely, species with more discriminating females had larvae that were less able to deal with a suboptimal initial feeding site (Figs. 3, 4; Tables 1, 2).
Although there was a general correspondence between our results and the previous findings of Janz and Nylin (1997), there were also some differences. Most of these can be summarized by the observation that the observed differences did not correspond very well to the “specialist” and “generalist” categorization. To some extent, clutch size appears to describe host specificity better than host range in our study system: females of the clutch-laying species A. urticae and A. io had strong preferences for “high”-quality leaves, whereas the females of single egg-laying species accepted “poor”-quality leaves for oviposition to a relatively high extent (Fig. 1). However, there were also other, more individual differences between the tested species, as demonstrated in the larval assays. Larval mobility varied greatly between the species, with V. atalanta being the most mobile, followed by the two generalists P. c-album and V. cardui, with A. urticae and A. io being substantially least mobile (Fig. 5). The measured mobility levels of V. atalanta and P. c-album matched those observed by Reavey (1992).
In the larval feeding assays, rates of neonate survival on “high”- and “poor”-quality leaves were comparable within species (except for A. urticae). Larvae feeding on “poor”-quality leaves however paid a significant cost in terms of growth during the 8 days that this experiment lasted––with the exception of the generalist V. cardui (Fig. 4). This fitness cost would likely be severe if extrapolated to the whole period of larval growth (compare Feeny 1970; Kelber 1999; De Bruyn et al. 2002). Indeed, in comparison to “high”-quality leaves, those of the “poor”-quality category were found to contain less water and nitrogen and to be tougher. Thus, as expected, most species gained more weight on “high”-quality plants, with V. atalanta in particular showing a remarkable weight gain after 8 days both in comparison to its relative V. cardui and after taking into account that the weights of its eggs and neonates are at best only one-half that of those of P. c-album (this study and Reavey 1992). The outcome of the mobility and survival experiments could be confirmed in the more natural setup of the nettle stalk experiment where we investigated larval ability to move from an inferior position in the search for a new feeding site and to establish at this site. Taken together, these findings indicate that in parallel to the general co-adaptation between female preference and larval abilities, species-specific variation in the measured traits does exist across all species.
The clutch-laying species fit well with the “hypothesized specialist”, exhibiting parental care via high female oviposition accuracy, but paired with low larval mobility and rather poor larval performance in unfavorable situations. However, even in the closely related A. urticae and A. io, which share a similar ecological niche (Bryant et al. 2000), species’ larval performance differed in the respect that larval A. urticae were most affected by host plant quality, whereas social context appeared to be an additional factor important for neonate A. io survival and weight gain. On the other hand, the “hypothesized generalist” with indiscriminate female oviposition behavior and precocious larvae was represented by the generalist species V. cardui and P. c-album, but—unexpectedly—also to some extent by the specialist V. atalanta. In our experiments the larvae of these latter three species showed a relatively high probability to survive in disadvantageous situations and to find resources of better quality, which suggests a potential to counterbalance inaccurate or indiscriminate female oviposition behavior—as also suggested by Nylin and Janz (1996) and Gamberale-Stille et al. (2014). The specialist V. atalanta exhibits a marginally significant preference for “high”-quality leaves, but, intriguingly, also accepts to a relatively high extent “poor”-quality host leaves. With their precocious larvae they seem to behave like a “hypothesized generalist”, which is puzzling since their host range is narrow. One should note, however, that V. atalanta larvae typically weave characteristic tents from the leaves of its host plant (Thomas and Lewington 2010), which likely requires that the larvae be mobile both to find a suitable place for the tent and to actually build it. In addition, a comparably high growth rate on both the “poor”- and “high”-quality leaves suggest a good ability of V. atalanta larvae to exploit its plant resource rather effectively.
Even though both of the generalist species tested here fit relatively well into the “generalist category” based on their indiscriminate oviposition behavior and precocious larvae, they were surprisingly different from each other in almost every aspect measured. Whereas P. c-album females laid an average of 25 comparably large eggs during a 3-h interval, V. cardui averaged about 120 eggs during the same length of time, but then with relatively high larval mortality rates on their host plants (see also Janz 2005) when compared to P. c-album. Moreover, these two species also differed in terms of egg weight, larval mobility and survival in the nettle stalk setup, indicating that they are realizing their broad host ranges quite differently.
Egg weight is likely to be an important cause for these differences, as it seems to be a relatively phylogenetically constrained trait in the tribe Nymphalini. The relatively high egg weight in the closely related genera Polygonia and Nymphalis (S. Nylin and N. Janz, unpublished data) may be an adaptation to the colonization of trees as host plants at the time when these genera diverged (Janz et al. 2001; Weingartner et al. 2006), since Lepidoptera feeding on woody plants have been found to have larger eggs than those feeding on herbaceous plants (Reavey 1992). Ancestors of the genera Vanessa and Aglais did not colonize trees (Janz et al. 2001), and thus have retained the ancestral small egg size. We suggest that phylogenetically constrained egg sizes provided different preconditions for how host plant generalization could be achieved in the two generalist species studied here. This implies that the “generalist” and “specialist” categories should not be treated as fixed life-history syndromes and that the pathways to generalization (and perhaps specialization) could be varied and highly context dependent.
Another example of such varied pathways to evolution of host range and co-adapted traits is the pattern observed for clutch size. Batch-laying was well correlated to a “hypothesized specialist” in our study and can be understood as the outcome of a choosy specialist which lays many eggs once a suitable oviposition site is found. Still, broader host ranges are known from clutch-laying species such as some Nymphalis butterflies (Tolman and Lewington 1997), Spodoptera moths (Salama et al. 1971) or indeed species with wingless females (Hunter 1995). Hence, species on both sides of the specialist–generalist axis can have such specific traits in common, and the evolutionary outcome would depend on many different ecological factors (Stamp 1980).
In conclusion, we found female oviposition strategy and degree of larval precociousness to be co-adapted. Contrary to our expectations, we did not find that these traits fully match the generalist–specialist gradient. Hence, traits such as discriminate/indiscriminate oviposition strategy with respect to the quality of the oviposition site within host species, or degree of larval precociousness, can be assumed to be found both among generalist and specialist species. Specific traits that arose in the evolutionary history of a species can clearly alter the conditions for precisely how a wider or narrower diet niche can be accomplished.
Author contribution statement
AS, SN and NJ conceived and designed the experiment. AS performed the experiments. AS and MAC analyzed the data. AS wrote the first draft of the manuscript, and all authors contributed to the final version of the manuscript.
References
Agrawal AA (2000) Host-range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81:500–508
Bernays EA (2001) Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Annu Rev Entomol 46:703–727
Bernays EA, Graham M (1988) On the evolution of host specificity in phytophagous arthropods. Ecology 69:886–892
Bernays EA, Wcislo WT (1994) Sensory capabilities, information processing, and resource specialization. Q Rev Biol 69:187–204
Bernays EA, Singer MS, Rodrigues D (2004) Foraging in nature: foraging efficiency and attentiveness in caterpillars with different diet breadths. Ecol Entomol 29:389–397
Bryant SR, Thomas CD, Bale JS (2000) Thermal ecology of gregarious and solitary nettle-feeding nymphalid butterfly larvae. Oecologia 122:1–10
Carlsson MA, Bisch-Knaden S, Schäpers A, Mozuraitis R, Hansson BS, Janz N (2011) Odour maps in the brain of butterflies with divergent host-plant preferences. PLoS ONE 6:e24025
Carlsson MA, Schäpers A, Nässel DR, Janz N (2013) Organization of the olfactory system of nymphalidae butterflies. Chem Senses 38:355–367
Core Team R (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
De Bruyn L, Scheirs J, Verhagen R (2002) Nutrient stress, host plant quality and herbivore performance of a leaf-mining fly on grass. Oecologia 130:594–599
Doak P, Kareiva P, Kingsolver J (2006) Fitness consequences of choosy oviposition for a time-limited butterfly. Ecology 87:395–408
Dukas R (1998) Cognitive ecology––the evolutionary ecology of information processing and decision making. The University of Chicago Press, Chicago
Ebert G (1993) Die Schmetterlinge Baden-Württembergs, Bd. 1, Tagfalter 1. Ulmer, Stuttgart
Egan SP, Funk DJ (2006) Individual advantages to ecological specialization: insights on cognitive constraints from three conspecific taxa. Proc R Soc B Biol Sci 273:843–848
Eliasson CU, Ryrholm N, Gärdenfors U (2005) Nationalnyckeln till Sveriges flora och fauna. Dagfjärilar: Hesperiidae-Nymphalidae. ArtDatabanken, Sveriges lantbruksuniversitet, Uppsala
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565
Friberg M, Olofsson M, Berger D, Karlsson B, Wiklund C (2008) Habitat choice precedes host plant choice––niche separation in a species pair of a generalist and a specialist butterfly. Oikos 117:1337–1344
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Gamberale-Stille G, Söderlind L, Janz N, Nylin S (2014) Host plant choice in the comma butterfly–larval choosiness may ameliorate effects of indiscriminate oviposition. Insect Sci 21:499–506
Hunter AF (1995) The ecology and evolution of reduced wings in forest macrolepidoptera. Evol Ecol 9:275–287
Janz N (2003) The cost of polyphagy: oviposition decision time vs error rate in a butterfly. Oikos 100:493–496
Janz N (2005) The relationship between habitat selection and preference for adult and larval food resources in the polyphagous butterfly Vanessa cardui (Lepidoptera : Nymphalidae). J Insect Behav 18:767–780
Janz N (2011) Ehrlich and Raven revisited: mechanisms underlying codiversification of plants and enemies. Annu Rev Ecol Evol Syst 42:71–89
Janz N, Nylin S (1997) The role of female search behaviour in determining host plant range in plant feeding insects: a test of the information processing hypothesis. Proc R Soc B Biol Sci 264:701–707
Janz N, Nylin S (2008) The oscillation hypothesis of host-plant range and speciation. In: Tilmon KJ (ed) Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects. University of California Press, Berkeley, California, pp 203–215
Janz N, Nyblom K, Nylin S (2001) Evolutionary dynamics of host-plant specialization: a case study of the tribe Nymphalini. Evolution 55:783–796
Janz N, Söderlind L, Nylin S (2009) No effect of larval experience on adult host preferences in Polygonia c-album (Lepidoptera: nymphalidae): on the persistence of Hopkins’ host selection principle. Ecol Entomol 34:50–57
Joshi A, Thompson JN (1997) Adaptation and specialization in a two-resource environment in Drosophila species. Evolution 51:846–855
Kelber A (1999) Ovipositing butterflies use a red receptor to see green. J Exp Biol 202:2619–2630
Murphy SM (2004) Enemy-free space maintains swallowtail butterfly host shift. Proc Natl Acad Sci USA 101:18048–18052
Nylin S (1988) Host plant specialization and seasonality in a polyphagous butterfly, Polygonia c-album (Nymphalidae). Oikos 53:381–386
Nylin S, Janz N (1996) Host plant preferences in the comma butterfly (Polygonia c-album): do parents and offspring agree? Ecoscience 3:285–289
Pullin AS (1987) Changes in leaf quality following clipping and regrowth of Urtica dioica, and consequences for a specialist insect herbivore, Aglais urticae. Oikos 49:39–45
Reavey D (1992) Egg size, first instar behaviour and the ecology of Lepidoptera. J Zool 227:277–297
Salama HS, Dimetry NZ, Salem SA (1971) On the host preference and biology of the cotton leaf worm Spodoptera littoralis Bois. Z Angew Entomol 67:261–266
Seppänen EJ (1970) Suurperhostoukkien ravintokasvit. Werner Söderström Osakeyhtiö, Helsinki
Stamp NE (1980) Egg deposition patterns in butterflies: why do some species cluster their eggs rather than deposit them singly? Am Nat 115:367–380
Stefanescu C (1997) Migration patterns and feeding resources of the painted lady butterfly, Cynthia cardui (L.) (Lepidoptera, Nymphalidae) in the northeast of the Iberian peninsula. Miscell Zool 20:31–48
Stefanescu C (2001) The nature of migration in the red admiral butterfly Vanessa atalanta: evidence from the population ecology in its southern range. Ecol Entomol 26:525–536
Thomas J, Lewington R (2010) Butterflies of Britain and Ireland. British Wildlife, Gillingham
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Tolman T, Lewington R (1997) Collins butterfly guide. HarperCollins, London
Weingartner E, Wahlberg N, Nylin S (2006) Dynamics of host plant use and species diversity in Polygonia butterflies (Nymphalidae). J Evol Biol 19:483–491
Wiklund C (1984) Egg-laying patterns in butterflies in relation to their phenology and the visual apparancy and abundance of their host plants. Oecologia 63:23–29
Wiklund C, Friberg M (2008) Enemy-free space and habitat-specific host specialization in a butterfly. Oecologia 157:287–294
Wiklund C, Persson A (1983) Fecundity, and the relation of egg weight variation to offspring fitness in the speckled wood butterfly Pararge aegeria, or why don’t butterfly females lay more eggs? Oikos 40:53–63
Zalucki MP, Clarke AR, Malcolm SB (2002) Ecology and behavior of first instar larval Lepidoptera. Annu Rev Entomol 47:361–393
Acknowledgments
We would like to thank Emilie Linderoth, Åsa Sandström and Julia Carlsson for assistance in the laboratory and Martin Olofsson for constructing the device to measure leaf toughness. The study was supported by a grant from the Faculty of Science, Stockholm University, to NJ and a grant from the Swedish Research Council (VR) to SN (Grant 2011-5636). We also acknowledge support from the Strategic Research Programme Ekoklim at Stockholm University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jennifer Thaler.
Why this study and paper deserves to be honored as a Highlighted Student Paper: Through a unique comparison between several butterfly species we cast new light on the costs of polyphagy, but also show that the “generalist” and “specialist” categories hide substantial variation. The co-adaptation of female choice and larval abilities on the species level cannot only be explained by the specialist-generalist axis, but can be properly understood in a historical framework.
Rights and permissions
About this article
Cite this article
Schäpers, A., Nylin, S., Carlsson, M.A. et al. Specialist and generalist oviposition strategies in butterflies: maternal care or precocious young?. Oecologia 180, 335–343 (2016). https://doi.org/10.1007/s00442-015-3376-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3376-5