Abstract
Phenology often determines the outcome of interspecific interactions, where early-arriving species often dominate interactions over those arriving later. The effects of phenology on species interactions are especially pronounced in aquatic systems, but the evidence is largely derived from experimental studies. We examined whether differences in breeding phenology between two pond-breeding salamanders (Ambystoma annulatum and A. maculatum) affected metamorph recruitment and demographic traits within natural populations, with the expectation that the fall-breeding A. annulatum would negatively affect the spring-breeding A. maculatum. We monitored populations of each species at five ponds over 4 years using drift fences. Metamorph abundance and survival of A. annulatum were affected by intra- and interspecific processes, whereas metamorph size and date of emigration were primarily influenced by intraspecific effects. Metamorph abundance, snout–vent length, date of emigration and survival for A. maculatum were all predicted by combinations of intra- and interspecific effects, but often showed negative relationships with A. annulatum metamorph traits and abundance. Size and date of metamorphosis were strongly correlated within each species, but in opposite patterns (negative for A. annulatum and positive for A. maculatum), suggesting that the two species use alternative strategies to enhance terrestrial survival and that these factors may influence their interactions. Our results match predictions from experimental studies that suggest recruitment is influenced by intra- and interspecific processes which are determined by phenological differences between species. Incorporating spatiotemporal variability when modeling population dynamics is necessary to understand the importance of phenology in species interactions, especially as shifts in phenology occur under climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order of arrival by different species into a particular habitat has long been recognized as an important factor in ecological interactions (Connell and Slatyer 1977). More recently, increased attention has focused on arrival patterns due to documented shifts in phenology (i.e. the timing of life history events, such as breeding migrations) that have been linked to climate change (Miller-Rushing et al. 2010; Yang and Rudolf 2010). Phenology is important to species interactions because the level of synchrony between species (or temporal overlap) can influence the intensity or outcome of these processes; for example, prey may become more susceptible to predators or competitors may suffer from increased resource overlap (Encinas-Viso et al. 2012; Griffin et al. 2011; Revilla et al. 2013; Yang and Rudolf 2010). Phenology can also influence species interactions when it acts synergistically with other factors, such the density of competitors, predators or resources, resulting in complex ecological networks (Alford 1989; Hunter and Elkinton 2001; Kardol et al. 2013). Thus, an improved understanding of how shifts in phenology affect species interactions is imperative to predict future population and community level processes under climate change (Blois et al. 2013).
With regards to interactions between antagonistic species, such as competition or predation, species that arrive early into a habitat often dominate the interactions with those that arrive later, ultimately affecting population and community structure of the latter (Almany 2003; Geange and Stier 2009; Shorrocks and Bingley 1994; Stier et al. 2013; Wilbur and Alford 1985). Dominance by early-arriving species in interspecific interactions can occur because of size asymmetries that exist a priori or which develop during ontogeny, which often confer competitive or predatory advantages to the early-arriving species. These size differences can ultimately determine whether phenological patterns result in competition or predator–prey interactions (Geange and Stier 2009; Urban 2007a; Wissinger 1992). Early-arriving species may also modify habitat conditions or preemptively use resources, thereby limiting their availability to species arriving later and thus promoting dominance of the former (Connell and Slatyer 1977; Kardol et al. 2013). While it is often difficult to determine these advantages separately, sufficient evidence exists to support the general pattern of early-arrival dominance in many communities.
The temporal pattern of arrival to ponds for breeding, or breeding phenology, has a strong influence on the outcomes of both competitive and predatory interactions in lentic freshwater habitats (e.g. Alford and Wilbur 1985; Blaustein and Margalit 1996; Orizaola et al. 2013; Padeffke and Suhling 2003; Urban 2007a). For pond-breeding salamanders, breeding phenology affects larval size-structure, which can lead to larvae of early-breeding species preying upon larvae of those breeding later due to size asymmetries that develop through ontogeny. Nonconsumptive predatory effects and enhanced competitive abilities for prey resources can also exist for larvae of early breeders over subsequent cohorts of those breeding later (e.g. Boone et al. 2002; Eitam et al. 2005; Segev and Blaustein 2007; Urban 2007a). Positive effects of early-arriving species can occur as well if early-arriving species reduce the densities of those arriving later, which reduces competition among the latter group due to thinning effects (Alford 1989; Anderson and Semlitsch 2014; Morin 1983). In predator–prey systems, the presence of species which breed later could also be expected to benefit early-breeding species as a food resource, resulting in enhanced growth rates or decreased times to metamorphosis when there are high densities of late larvae to consume, although to our knowledge this relationship has yet to be shown empirically in natural systems.
The bulk of our understanding of how phenology influences interspecific interactions among amphibians has been established from small-scale experimental investigations. The ability of these experiments to describe natural processes occurring in un-manipulated populations is less well known. The majority of long-term studies that have estimated the population vital rates (e.g. reproduction and survival) necessary to fully understand the impact of species interactions on population dynamics have mostly examined single-species systems (Berven 2009; Schmidt et al. 2012; Wissinger et al. 2010), with few focusing on interspecific effects (Semlitsch et al. 1996). Investigations of interactions between specific ontogenetic stages have found differences in phenology to be strong selective forces (Segev and Blaustein 2007; Stenhouse et al. 1983; Urban 2007a), but the ultimate impacts on recruitment are unknown. Several studies have followed multiple species through metamorphosis (Semlitsch et al. 1996; Stenhouse 1987; Todd et al. 2011; Todd and Winne 2006), but explicit tests of interspecific interactions on population dynamics have been infrequent or at only one study location, providing little information about spatiotemporal variation in the outcome of interactions. This information is important because the persistence or exclusion of those species which arrive later may differ across spatial and temporal scales due to the length of temporal overlap, prior residency and spatial aggregation at each site (Lawler and Morin 1993; Porensky et al. 2012; Shorrocks and Bingley 1994; Urban 2007a). The effects may ultimately influence population spatial structure and contribute to long-term coexistence.
We have examined recruitment and demographic traits (size at and date of metamorphosis) of metamorphs of two pond-breeding salamanders, the ringed (Ambystoma annulatum) and spotted salamander (A. maculatum), which differ in breeding phenology. Adults of A. annulatum breed in the fall, whereas those of A. maculatum breed in the spring, making the larvae of the former an intraguild predator and hatchlings and larvae of the latter an intraguild prey (Anderson and Semlitsch 2014; Hocking et al. 2008; Semlitsch et al. 2014). We monitored populations of these species for 4 consecutive years at five ponds to enumerate both adult and metamorph abundances, as well as the timing of each species’ movements into and out of ponds. We compared models representing different biotic processes to gain an understanding of how both abundance and phenology influenced metamorph recruitment and traits. Based on previous research on interactions of fall- and spring-breeding species (Anderson and Semlitsch 2014; Stenhouse 1985, 1987; Stenhouse et al. 1983; Urban 2007a), we expected asymmetric interspecific effects to be present between these two species (A. annulatum superior over A. maculatum). We also expected positive effects from A. maculatum on A. annulatum metamorph traits and abundance that would indicate the latter species benefits from the presence of the former as a food resource. Finally, by following several natural populations through multiple years of recruitment, we were able to analyze how stochasticity (i.e. spatiotemporal variation) contributes to metamorph responses.
Methods
Study area
Populations of both A. annulatum and A. maculatum were monitored at five constructed wildlife ponds (surface area 160–330 m2) at the Daniel Boone Conservation Area (DBCA; 1,424.5 ha) in Warren County, Missouri, USA from 2004 to 2007 (Semlitsch et al. 2009). DBCA is a secondary growth forest primarily composed of an oak (Quercus spp.) and hickory (Carya spp.) overstory, with a sugar maple (Acer saccharum) and red cedar (Juniperus virginiana) understory. Fifty-one constructed ponds exist at DBCA, with a mean interpond distance of 235 m (±142 m) (see map in Semlitsch et al. 2014). The five focal ponds are 27–47 years old, fish-free and permanent in most years, have an interpond distance of 879 m (±541 m) and are used by up to 14 amphibian species (Hocking et al. 2008; Semlitsch et al. 2008, 2009). Both focal salamanders are forest-associated species, occupying ponds with greater canopy closure and with a greater percentage of forest in the surrounding habitat (Peterman et al. 2014). Most breeding adults of A. annulatum arrive at ponds from August to October at the DBCA, while A. maculatum breeding adults arrive in late February–March (Semlitsch et al. 2014). Eggs of A. annulatum hatch in late October and November, and the aquatic larvae overwinter in ponds; emigration of metamorphs occurs primarily in May and June (Semlitsch et al. 2014). The eggs of A. maculatum hatch in March and April, and most larvae complete metamorphosis by the end of the following summer (Hocking et al. 2008). Marbled salamanders (A. opacum) also occurred at DBCA, but were present at low frequency (<1 % of total metamorphs and adults) and were excluded from this analysis.
Sampling
Drift fences that completely encircled each of the five focal ponds were constructed and positioned 1–3 m from the highwater mark, with paired pitfall traps spaced 8 m apart on each side of the fence. A sponge was placed in each trap to prevent desiccation of the captured animals, and a wooden lid was placed 2.5 cm above the lip of the pitfall trap to minimize predation by mammals. All pitfall traps were checked at minimum every 48 h, and every 24 h following rain events, from mid-February to mid-November each year of the study. The sex of each incoming adult was determined by cloacal examination before it was released inside the fence, and all animals received a toe-clip cohort mark as part of an ongoing mark–recapture population study. Snout–vent length (SVL, in mm) was measured on a subset of adults (N = 4,536). Body shape, coloration and number of costal grooves were used to identify metamorphosing juvenile salamanders to species (Trauth et al. 2004), which were also measured for SVL (n = 4,455). Small individuals caught entering the ponds that could be confused with metamorphs were kept on the outside of the drift fence. Immature individuals and those whose sex was not distinguishable were excluded from analysis in this study (approximately 3 % of captures for each species).
Each female entering the pond during the breeding season was recorded and received a toe-clip cohort mark representative of the first year in which it bred during the study. Some adult A. annulatum were caught immigrating/emigrating several times during the breeding season (i.e. daily movement to and from the pond). To account for this movement when estimating reproductive effort, we counted the number of females that were caught incoming at the fence that had already received that year’s cohort mark at each pond, which was approximately 10 % for adult females; abundance for all ponds and years was therefore multiplied by 0.9 to make a more conservative estimate of breeding females of A. annulatum. We calculated reproductive effort by multiplying the number of breeding females by an average clutch size [390 ± 16 eggs for A. annulatum (Hutcherson et al. 1989) and 224 ± 15 for A. maculatum (Shoop 1974)]. This total number was then used as the number of embryos deposited by breeding females at each pond–year combination. Aquatic survival for each species within each year was then calculated by dividing the total number of metamorphs captured at the pond drift fence by the estimated number of eggs laid at each pond.
Analysis
We compared a priori mixed models for each species, which represented hypothesized biological processes that would affect each of four response variables: metamorph abundance (the number captured emigrating), metamorph SVL, metamorph date of emigration and survival (number of metamorphs/number of embryos). All models included pond and year as random effects. We modeled survival using a binomial error distribution (Warton and Hui 2011) and abundance with a Poisson distribution. We used the individual data points for metamorph SVL and date of emigration, as the random effects would account for temporal pseudoreplication, and modeled the responses with Gaussian error structures. All models were implemented using the lme4 package in R, and ranked using the corrected Akaike information criterion (AICc) (Bates et al. 2013; R Development Core Team 2013).
The candidate set of models included combinations of adult abundance and timing of breeding and, in some cases, also metamorph abundance and traits (see below; Tables 1, 2). All covariates were centered and scaled to allow for parameter estimates to be directly comparable. We used metamorph abundance and traits as predictor variables within models because this could be a more biologically-relevant estimate of the larval density experienced by A. maculatum and A. annulatum than initial density (e.g. SVL of metamorphs may have been governed by final density rather than initial density). Similarly, the size of A. annulatum metamorphs and their emigration date should influence their predatory potential on A. annulatum, as these factors would influence gape limitations and temporal overlap (Anderson and Semlitsch 2014; Urban 2007a), thus justifying their inclusion as predictors in models. Furthermore, in a survey of approximately 100 ponds in similar landscapes in Missouri, larval and metamorph densities were correlated within each species; therefore, our use of metamorph abundance would likely be indicative of the strength of larval interactions (RD Semlitsch, unpublished data). We acknowledge that these relationships only imply correlation, however, and direct causation cannot be determined from our analysis.
Model formulations
Intraspecific model
For each species, covariates included the abundance of adult female conspecifics (a proxy for intraspecific larval density-dependence; Scott 1994) and the mean date of adult female breeding migration, with the expectation that later breeding would reduce survival in each species. For SVL and date of metamorph emigration, metamorph abundance was also included as an estimate of final density in ponds, which often affects these two traits in experimental studies (Anderson and Semlitsch 2014; Scott 1994).
Interspecific model
For A. annulatum, covariates included the abundance of breeding females of A. maculatum and the date of their breeding immigration. Negative correlations with the response variables would suggest that A. maculatum is having a competitive effect. For A. maculatum, the three metamorph traits of A. annulatum (metamorph abundance, SVL and date of emigration) were included as covariates, all of which would be proxies for predator abundance, size and phenology (Anderson and Semlitsch 2014; Urban 2007a).
Joint effects model
For A. annulatum, this model examined the hypothesis that facilitation is occurring and included adult abundance and timing of breeding for both species, as well as metamorph abundance of A. annulatum in the SVL and emigration models. If A. maculatum was acting as a food source, abundance of female A. maculatum would be a proxy for hatchling density and therefore should be positively associated with metamorph abundance of A. annulatum. The timing of A. maculatum breeding should be negatively associated with A. annulatum metamorph abundance; as A. annulatum breeds later, there would be less opportunity for A. annulatum to consume hatchlings and thus survive at higher rates.
For A. maculatum, the joint effects model included the abundance, SVL and date of emigration of metamorphs of A. annulatum, and the female abundance and date of breeding of A. maculatum. These covariates describe how many predators (heterospecific metamorph abundance) and prey existed (conspecific female abundance), predator size (heterospecific metamorph SVL) and how long they overlapped (date of heterospecific emigration, plus date of female breeding in A. maculatum).
Density model
For A. annulatum, this model included abundance of con- and heterospecific adult females, as well as metamorph abundance for the response variables SVL and date of emigration. This model tests a general relationship of density-dependence commonly observed in experimental studies of amphibian interactions, where increasing larval density (approximated through the combination of female and metamorph abundance) tends to increase the date of metamorphosis and to decrease both size at metamorphosis and the number of metamorphs produced (Scott 1994; Semlitsch et al. 1996). For A. maculatum, the abundances of adults of both species and of metamorphs of A. annulatum were included in all models, and metamorph abundance of A. maculatum was included as a proxy for final intraspecific density for models in which the responses were SVL and date of emigration.
Phenology model
This model incorporates the date of breeding for conspecific and heterospecific adult females, as well as metamorph emigration date of A. annulatum for the response variables of abundance, SVL and survival of A. maculatum. This model examines the hypothesis that the timing of ontogenetic events is the only factor that affects metamorph recruitment and traits by influencing the degree of temporal overlap (Alford 1989; Encinas-Viso et al. 2012; Yang and Rudolf 2010).
The overall fit of the top model was assessed by calculating marginal and conditional r 2 values (Nakagawa et al. 2013). Marginal r 2 incorporates only the fixed effects in a model, and the conditional r 2 includes the contributions of the random effects with the fixed effects, which in our models indicate the contributions of spatiotemporal processes. While the interpretation of these values is not identical to traditional r 2 values, they do provide information on how much variation the fixed effects explain relative to a model that incorporates random effects. We also acknowledge that the high data to covariate ratio for the abundance and survival models (only 20 data points with up to seven predictors) could lead to overparameterization. However, the marginal r 2 values were relatively low, which indicates that adding extra covariates did not result in substantially over-fitted models.
Results
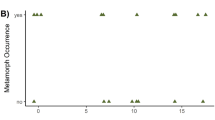
Across all ponds and years, mean (± standard deviation) abundance of metamorphs of A. annulatum and A. maculatum was 139.2 ± 155.7 and 88.6 ± 87.1, respectively. The SVL of metamorphs of A. annulatum was greater than that of A. maculatum (39.16 ± 3.4 vs. 31.39 ± 4.6 mm, respectively; Fig. 1a). The mean Julian date of metamorphosis for A. annulatum and A. maculatum was days 148 ± 6.8 and 224 ± 19.0, respectively (Fig. 1b). Mean SVL and Julian date of emigration showed significant and opposite patterns for each species, with SVL and emigration date showing a negative relationship for A. annulatum and a positive relationship for A. maculatum (Fig. 1c). Abundance of adult females averaged 229.73 ± 140.7 and 184.9 ± 120.2 for A. annulatum and A. maculatum, respectively. The mean Julian date of adult female immigration was 254 ± 8.2 for A. annulatum and 77 ± 8.9 for A. maculatum. Survival (abundance of metamorphs/no. of eggs laid) for both species was extremely low and was always <1 %. The mean temporal overlap of the two species was 66.1 ± 9.6 (range 50–81) days, which was calculated by subtracting the mean emigration date of A. annulatum metamorphs from the mean date of A. maculatum breeding immigration. This slightly overestimates the overlap of free-swimming larvae of both species due to the several weeks it would take for eggs of A. maculatum to hatch (Petranka 1998); an overlap of 30–60 days represents a more realistic range of overlap between larvae.
Ringed salamander (Ambystoma annulatum; N = 2,698) and spotted salamander (A. maculatum, N = 1,758) metamorph snout-vent length (SVL, in mm; a), Julian date (day) of metamorph emigration (b) and metamorph SVL–emigration date relationship (c). Bars in b represent a 3-days totals. For reference, Day 150 is equivalent to 30 May
For metamorph abundance of A. annulatum, the best supported model was the joint effects model, which contained both intra- and interspecific effects (adult abundance and timing of breeding of both species (Table 1). Conspecific female abundance and date of breeding both showed positive relationships with metamorph abundance, whereas the timing of heterospecific female breeding showed a weaker negative relationship, indicating that as A. maculatum bred later, recruitment in A. annulatum was higher (Table 3). For metamorph SVL, the phenology model was the most supported, which included the date of metamorph emigration and the date of breeding for both con- and heterospecific females (Table 1). The date of metamorph emigration and conspecific female breeding date were both negatively correlated with metamorph SVL, but the former was twice as strong as the latter (Table 3). Date of metamorph emigration was best predicted by the density model (Table 1); metamorphs were predicted to emigrate later when more conspecific and heterospecific females bred in each season (Table 3). The joint effects model predicted the survival of A. annulatum, which increased when greater numbers of females of both species bred, increased when A. annulatum bred later and decreased when A. maculatum bred later (Tables 1, 3).
The joint effects model was the top model for all responses (metamorph abundance, SVL, date of emigration and survival) of A. maculatum (Table 2). This model included the metamorph traits and abundance of A. annulatum and the abundance of adult females and timing of breeding for A. maculatum. The relationships between interspecific effects of A. annulatum and metamorph abundance and survival of A. maculatum were all negative, whereas a positive relationship existed for the date of breeding of A. maculatum and their metamorph abundance (Table 4). Metamorph survival in A. maculatum also declined with increasing numbers of conspecific females that bred, but did not vary with A. annulatum metamorph abundance (Table 4). Metamorph SVL of A. maculatum showed a positive relationship with the abundance and traits of A. annulatum metamorphs (Table 4), with the strongest effect on SVL occurring with emigration date of A. annulatum; metamorph SVL of A. maculatum increased the most when A. annulatum metamorphs emigrated later. Date of emigration for A. maculatum showed a negative relationship with the abundance of A. annulatum metamorphs, whereas all other variables in the joint effects model showed a positive relationship (Table 4). The two strongest positive relationships with the date of emigration in this species were conspecific female breeding date and A. annulatum metamorph emigration date (Table 4).
Discussion
Overwhelming experimental evidence in aquatic communities suggests that the order of arrival of organisms into an environment substantially affects the outcome of species interactions and community assembly (Geange and Stier 2009; Hernandez and Chalcraft 2012; Stier et al. 2013; Wissinger 1992). For pond-breeding salamanders, results from several experimental and short-term field studies suggest breeding phenology differences should result in significant negative effects from fall-breeding species on spring-breeding species due to size asymmetries that exist between larvae (Segev and Blaustein 2007; Urban 2007a). We found that combinations of intra- and interspecific effects best explained metamorph abundance and survival in natural populations of each species. The relationships between each effect and the response variables also supports the hypothesis that fall-breeding A. annulatum negatively affects recruitment of the spring-breeding A. maculatum, matching the results of experimental studies (Anderson and Semlitsch 2014). Our results also indicate metamorph size and date of emigration within each species were affected by different processes (largely intraspecific effects for A. annulatum and combinations of intra- and interspecific interactions for A. maculatum), which contribute to different size–date relationships that have implications on terrestrial survival and adult fitness.
Greater numbers of conspecific females of A. annulatum resulted in increased survival and abundance of metamorphs, similar to data reported in other studies (Semlitsch et al. 1996). Later breeding also increased metamorph abundance and survival of A. annulatum, potentially due to reduced predation by ectothermic predators in colder water temperatures in the fall. A positive relationship between the reproductive effort of A. maculatum and A. annulatum recruitment was also observed, supporting the hypothesis that A. maculatum is a food source for A. annulatum that increases their survival (Anderson and Semlitsch 2014). The timing of A. maculatum breeding may also be important for A. annulatum, as later A. maculatum breeding migrations resulted in increased A. annulatum metamorph abundance, suggesting that a separation of the larval period may potentially reduce negative interspecific interactions on early breeding species. Other studies have also suggested that a decreasing overlap may release species from negative interactions (Encinas-Viso et al. 2012; Revilla et al. 2013; Yang and Rudolf 2010). Overall, the combination of these two interspecific effects suggest that there may be a complex relationship between phenology and abundance, where metamorph production of A. annulatum increases when there are more prey (A. maculatum hatchlings), as long as they do not overlap for long periods of time that would increase competition with the A. maculatum that escape via a size refugia (Urban 2007b). The date of emigration for A. annulatum also increased when more females bred the previous fall, which matches data reported from experimental studies showing density-dependent metamorphic rates (Anderson and Semlitsch 2014). The density model had relatively low explanatory power, even when accounting for the random effects, suggesting factors other than biotic interactions are also important for emigration. In particular, metamorph emigration date is likely influenced more by rainfall patterns than by biotic interactions, as individuals can undergo metamorphosis but not emigrate until favorable terrestrial conditions exist (Semlitsch et al. 1996; Todd and Winne 2006).
Metamorph abundance and survival of A. maculatum were negatively correlated with the metamorph traits and abundance of A. annulatum at our study site. Based on this model, greater abundance, later emigration dates (i.e. greater temporal overlap) and larger sizes of A. annulatum metamorphs all were negatively associated with A. maculatum metamorph abundance/survival. Abundance and survival of metamorphs increased when A. maculatum bred later, however. The direction of these effects supports results from previous studies which suggest that the density and size of predators, as well as the synchrony of temporal overlap of predators and prey, are important to the outcome of interspecific interactions (Anderson and Semlitsch 2014; Griffin et al. 2011; Stier et al. 2013; Urban 2007a). Metamorph SVL for A. maculatum showed positive relationships with the size of A. annulatum metamorphs and the date of their emigration—but not with their abundance. This indicates that when A. annulatum were bigger and remained aquatic for longer, A. maculatum had reduced survival and metamorphosed at a larger size, which could be evidence for thinning effects (Van Buskirk and Yurewicz 1998). Furthermore, A. maculatum metamorphosed earlier at higher abundances of A. annulatum metamorphs, but did so at smaller sizes, indicating a potential cost on future fitness (Semlitsch et al. 1988). One potential factor that may confound our results is that the abundance of larval A. annulatum when A. maculatum bred may be different than metamorph abundance of A. annulatum. Larval abundance is the more biologically relevant predator density at the time of A. maculatum egg hatching, but was not measured in our study. Our use of metamorph abundance as an indication of predation level within the pond is likely an underestimation of the predatory effects of A. annulatum, which would only strengthen the argument of their negative effects.
External factors, such as predation by other species (i.e. aquatic invertebrates), hydroperiod and habitat quality, may determine the strength of interspecific interactions in more heterogeneous landscapes/ponds than those studied at DBCA (Amarasekare 2003). The hydroperiod of a pond is often considered to be the most important influence on larval abundance/survival and the amount of forested habitat as the most important influence on juvenile/adult survival (Rittenhouse et al. 2009; Rothermel and Semlitsch 2006; Wellborn et al. 1996). The five ponds within our study site all had nearly permanent hydroperiod regimes, which can lead to a build-up of invertebrate predators (Wellborn et al. 1996). Larval interactions may be more important in ephemeral ponds, as density-dependent processes would potentially be greater due to reduced densities of invertebrate predators and higher larval densities in these habitats. Intraguild predation would then be the dominant interspecific effect in these ponds where larval A. annulatum are the top predators, which ultimately could have significant top-down impacts on population dynamics and traits of spring-breeding species (Urban 2007a, 2010). The terrestrial habitat was also relatively homogenous in terms of forest cover, although management activities did occur at DBCA (see Semlitsch et al. 2009), indicating that accounting for spatial patterns alone may be uninformative if spatial variation in habitat factors is low. In other locations, predicted larval abundance of A. maculatum was twofold that of A. annulatum in ponds surrounded by increasing amounts of forested habitat (Peterman et al. 2014). Thus, we predict that in a landscape of heterogeneous habitats (i.e. a gradient of hydroperiod cycles and varying terrestrial habitat types), concomitant variation in the strength of species interactions would occur due to varying densities (Amarasekare 2003).
The relationship of size and date of metamorphosis for metamorphs of the fall-breeding A. annulatum was negative, whereas metamorphs of A. maculatum, the spring-breeder, showed a positive relationship between the two responses. Size at and timing of metamorphosis are critical components to amphibian fitness (Scott 1994; Semlitsch et al. 1988), and our data show that differences in breeding phenology may result in differential patterns of selection on characters necessary for survival in a terrestrial environment. For A. annulatum, strong intraspecific competitors may dominate larval resource competition and/or prey upon conspecifics (Nyman et al. 1993), resulting in individuals that metamorphose early and at a large size. Individuals that cannot acquire sufficient resources as rapidly (i.e. poorer competitors) emigrate smaller and later with a reduced long-term fitness. Emerging early and large as a metamorph may reduce the risk of desiccation in the terrestrial environment as they would have a smaller body surface area:volume ratio, and spring temperatures would be lower than those of early summer (Peterman et al. 2013b; Semlitsch et al. 1988). Selection appears to operate in the opposite direction for A. maculatum. Early in ontogeny, individuals experience competition from conspecifics simultaneous to intraguild predation from resident species such as A. annulatum; the latter species can also deplete resource levels that would negatively affect A. maculatum (Anderson and Semlitsch 2014; Hernandez and Chalcraft 2012; Urban 2007a). A. maculatum individuals thus experience a trade-off, where those which undergo early metamorphosis escape negative aquatic conditions but do so at a cost of small juvenile size, which in turn is linked to reduced terrestrial survival (Rothermel and Semlitsch 2006). Individuals of A. maculatum that remain aquatic experience reduced larval competition as other conspecifics undergo metamorphosis (Semlitsch and Caldwell 1982), and they have access to greater food resources as invertebrate and vertebrate prey levels increase throughout the summer. In ephemeral habitats, this risky strategy increases the potential for mortality due to pond drying, however. Interestingly, individuals that metamorphosed at the same time for each species (late A. annulatum and early A. maculatum) were approximately the same size, indicating that life history differences of the two species may intensify their interactions as late-stage larvae and as metamorphs at the margins of their respective emigration distributional patterns. While little is known about juvenile terrestrial competition, equal-sized individuals may experience greater interspecific competition as they would potentially be selecting similar-size burrows, unless there are other site-selection and/or behavioral differences between the two species (e.g. increased aggressiveness), which are currently unknown.
Understanding spatiotemporal variation in population dynamics is critical to accurately predict fluctuations in abundance that occur across space and time. In our study, accounting for spatiotemporal patterns (i.e. differences in marginal and conditional r 2 values) varied from no improvement to explaining up to an additional 70 % of the variance, further highlighting the need to account for many factors when examining natural populations. Examinations of spatial and temporal patterns in amphibian assemblages often rely on presence/absence data or abundance of aquatic stages, with primary foci on population turnover and little emphasis on actual recruitment out of the aquatic habitat (Hecnar and M’Closkey 1996; Van Buskirk 2005; Werner et al. 2007). Juvenile salamanders are considered the important life history stage for population growth rates (Biek et al. 2002; Harper et al. 2008; Vonesh and De la Cruz 2002); therefore, understanding the parameters that affect spatiotemporal variation in recruitment and size is critical. As many species occur within spatially structured populations (i.e. metapopulations), understanding the factors (biotic and abiotic) that lead to spatial variability in recruitment is important for identifying mechanisms that differentiate high- and low-quality populations (i.e. sources and sinks). The designation of these categories may change between years based on the temporal variation we observed, necessitating multi-year monitoring of populations (Peterman et al. 2013a). Additionally, long-term monitoring of multiple populations would yield greater insight into natural fluctuations of abundance and may be necessary to understand variability in recruitment and long-term persistence (Semlitsch et al. 1996; Whiteman and Wissinger 2005).
Shifts in phenology due to climate change have been documented in many species (Miller-Rushing et al. 2010; Yang and Rudolf 2010), especially in amphibian breeding cycles (Todd et al. 2011). Therefore, an increased understanding of how phenology affects species interactions is critical to predict changes in community assembly patterns. Changes in temporal overlap or synchrony can result in novel interactions or decouple necessary interactions, such as plant-pollinators or pulses in food resources essential to consumers (Yang and Rudolf 2010). The translation of pairwise or guild interactions into community-level effects in which they are embedded are often difficult to determine, but could have important ecological and evolutionary consequences (Gilman et al. 2010). This study also provides baseline information on the phenology and abundance patterns of two interacting species that can provide a yardstick for understanding future variation in phenology, as well as act as a framework for future investigations (Rafferty et al. 2013; Visser and Both 2005). Incorporating spatiotemporal variability would also facilitate more developed and broad conclusions regarding which processes influence variation in population dynamics, especially as climate shifts occur. Although it is often difficult to monitor and/or tease apart the multitude of factors affecting natural populations, concurrent experimental studies with observations of natural populations are necessary to understand when and where species interactions become relevant in their effects on population and community dynamics.
References
Alford RA (1989) Variation in predator phenology affects predator performance and prey community composition. Ecology 70:206–219
Alford RA, Wilbur HM (1985) Priority effects in experimental pond communities: competition between Bufo and Rana. Ecology 66:1097–1105
Almany GR (2003) Priority effects in coral reef fish communities. Ecology 84:1920–1935
Amarasekare P (2003) Competitive coexistence in spatially structured environments: a synthesis. Ecol Lett 6:1109–1122. doi:10.1046/j.1461-0248.2003.00530.x
Anderson TL, Semlitsch RD (2014) High intraguild predator density induces thinning effects on and increases temporal overlap with prey populations. Popul Ecol 56:265–273. doi:10.1007/s10144-013-0419-9
Bates D, Maechler M, Bolker BM (2013) lme4: Linear mixed-effects models using S4 classes, R package version 1.0-5. Available at: http://CRAN.R-project.org/package=lme4
Berven KA (2009) Density dependence in the terrestrial stage of wood frogs: evidence from a 21-year population study. Copeia 2009:328–338
Biek R, Funk WC, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv Biol 16:728–734
Blaustein L, Margalit J (1996) Priority effects in temporary pools: nature and outcome of mosquito larva-toad tadpole interactions depend on order of entrance. J Anim Ecol 65:77–84
Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S (2013) Climate change and the past, present, and future of biotic interactions. Science 341:499–504. doi:10.1126/science.1237184
Boone MD, Scott DE, Niewiarowski PH (2002) Effects of hatching time for larval ambystomatid salamanders. Copeia 2002:511–517
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
Eitam A, Blaustein L, Mangel M (2005) Density and intercohort priority effects on larval Salamandra salamandra in temporary pools. Oecologia 146:36–42. doi:10.1007/s00442-005-0185-2
Encinas-Viso F, Revilla TA, Etienne RS (2012) Phenology drives mutualistic network structure and diversity. Ecol Lett 15:198–208. doi:10.1111/j.1461-0248.2011.01726.x
Geange SW, Stier AC (2009) Order of arrival affects competition in two reef fishes. Ecology 90:2868–2878
Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25:325–331. doi:10.1016/j.tree.2010.03.002
Griffin KA, Hebblewhite M, Robinson HS, Zager P, Barber-Meyer SM, Christianson D et al (2011) Neonatal mortality of elk driven by climate, predator phenology and predator community composition. J Anim Ecol 80:1246–1257
Harper EB, Rittenhouse TA, Semlitsch RD (2008) Demographic consequences of terrestrial habitat loss for pool-breeding amphibians: predicting extinction risks associated with inadequate size of buffer zones. Conserv Biol 22:1205–1215. doi:10.1111/j.1523-1739.2008.01015.x
Hecnar SJ, M’Closkey RT (1996) Regional dynamics and the status of amphibians. Ecology 77:2091–2097
Hernandez JP, Chalcraft DR (2012) Synergistic effects of multiple mechanisms drive priority effects within a tadpole assemblage. Oikos 121:259–267. doi:10.1111/j.1600-0706.2011.19221.x
Hocking DJ, Rittenhouse TAG, Rothermel BB, Johnson JR, Conner CA, Harper EB et al (2008) Breeding and recruitment phenology of amphibians in Missouri oak-hickory forests. Am Midl Nat 160:41–60
Hunter AF, Elkinton JS (2001) Interaction between phenology and density effects on mortality from natural enemies. J Anim Ecol 68:1093–1100
Hutcherson JE, Peterson CL, Wilkinson RF (1989) Reproductive and larval biology of Ambystoma annulatum. J Herpetol 23:181–183
Kardol P, Souza L, Classen AT (2013) Resource availability mediates the importance of priority effects in plant community assembly and ecosystem function. Oikos 122:84–94. doi:10.1111/j.1600-0706.2012.20546.x
Lawler SP, Morin PJ (1993) Temporal overlap, competition, and priority effects in larval anurans. Ecology 74:174–182
Miller-Rushing AJ, Hoye TT, Inouye DW, Post E (2010) The effects of phenological mismatches on demography. Philos Trans R Soc Lond Ser B Biol Sci 365:3177–3186. doi:10.1098/rstb.2010.0148
Morin PJ (1983) Predation, competition, and the composition of larval anuran guilds. Ecol Monogr 53:120–138
Nakagawa S, Schielzeth H, O’Hara RB (2013) A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. doi:10.1111/j.2041-210x.2012.00261.x
Nyman S, Wilkinson RF, Hutcherson JE (1993) Cannibalism and size relations in a cohort of larval ringed salamanders (Amybstoma annulatum). J Herpetol 27:78–84
Orizaola G, Dahl E, Nicieza AG, Laurila A (2013) Larval life history and anti-predator strategies are affected by breeding phenology in an amphibian. Oecologia 171:873–881. doi:10.1007/s00442-012-2456-z
Padeffke T, Suhling F (2003) Temporal priority and intra-guild predation in temporary waters: an experimental study using Namibian desert dragonflies. Ecol Entomol 28:340–347
Peterman WE, Earl JE, Rittenhouse TAG, Semlitsch RD (2013a) Demographic network and multi-season occupancy modeling of Rana sylvatica reveal spatial and temporal patterns of population connectivity and persistence. Landsc Ecol 28:1601–1613. doi:10.1007/s10980-013-9906-9
Peterman WE, Locke JL, Semlitsch RD (2013b) Spatial and temporal patterns of water loss in heterogeneous landscapes: using plaster models as amphibian analogues. Can J Zool 91:135–140
Peterman WE, Anderson TL, Drake DL, Ousterhout BH, Semlitsch RD (2014) Maximizing pond biodiversity across the landscape: a case study of larval ambystomatid salamanders. Anim Conserv 17:275–285. doi:10.1111/acv.12090
Petranka JW (1998) Salamanders of the US and Canada. Smithsonian Institution Press, Washington, DC
Porensky LM, Vaughn KJ, Young TP (2012) Can initial intraspecific spatial aggregation increase multi-year coexistence by creating temporal priority? Ecol Appl 22:927–936
R Development Core Team (2013) R: a language and environment for statistical computing, 3.0.2 edn. R Foundation for Statistical Computing, Vienna
Rafferty NE, Caradonna PJ, Burkle LA, Iler AM, Bronstein JL (2013) Phenological overlap of interacting species in a changing climate: an assessment of available approaches. Ecol Evol 3:3183–3193. doi:10.1002/ece3.668
Revilla T, Encinas-Viso F, Loreau M (2013) (A bit) Earlier or later is always better: phenological shifts in consumer–resource interactions. Theor Ecol 7:149–162. doi:10.1007/s12080-013-0207-3
Rittenhouse TA, Semlitsch RD, Thompson FR III (2009) Survival costs associated with wood frog breeding migrations: effects of timber harvest and drought. Ecology 90:1620–1630
Rothermel B, Semlitsch R (2006) Consequences of forest fragmentation for juvenile survival in spotted (Ambystoma maculatum) and marbled (Ambystoma opacum) salamanders. Can J Zool 84:797–807
Schmidt BR, Hodl W, Schaub M (2012) From metamorphosis to maturity in complex life cycles: equal performance of different juvenile life history pathways. Ecology 93:657–667
Scott DE (1994) The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 75:1383–1396
Segev O, Blaustein L (2007) Priority effects of the early breeding fire salamander on the late breeding banded newt. Hydrobiologia 583:275–283. doi:10.1007/s10750-006-0565-6
Semlitsch RD, Caldwell JP (1982) Effects of density of growth, metamorphosis, and survivorship in tadpoles of Scaphiopus holbrooki. Ecology 63:905–911
Semlitsch RD, Scott DE, Pechmann JH (1988) Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69:184–192
Semlitsch R, Scott D, Pechmann J, Gibbons J (1996) Structure and dynamics of an amphibian community: evidence from a 16-year study of a natural pond. In: Cody ML, Smallwood JA (eds) Long-term studies of vertebrate communities. Academic Press, San Diego, pp 217–248
Semlitsch RD, Conner CA, Hocking DJ, Rittenhouse TA, Harper EB (2008) Effects of timber harvesting on pond-breeding amphibian persistence: testing the evacuation hypothesis. Ecol Appl 18:283–289
Semlitsch RD, Todd BD, Blomquist SM, Calhoun AJK, Whitfield Gibbons J, Gibbs JP et al (2009) Effects of timber harvest on amphibian populations: understanding mechanisms from forest experiments. Bioscience 59:853–862. doi:10.1525/bio.2009.59.10.7
Semlitsch RD, Anderson TL, Ousterhout BH, Osbourn MS (2014) Structure and dynamics of ringed salamander (Ambystoma annulatum) populations in Missouri. Herpetologica 70:14–22
Shoop CR (1974) Yearly variation in larval survival of Ambystoma maculatum. Ecology 55:440–444
Shorrocks B, Bingley M (1994) Priority effects and species coexistence: experiments with fungal-breeding Drosophila. J Anim Ecol 63:799–806
Stenhouse SL (1985) Interdemic variation in predation on salamander larvae. Ecology 66:1706–1717
Stenhouse SL (1987) Embryo mortality and recruitment of juveniles of Ambystoma maculatum and Ambystoma opacum in North Carolina. Herpetologica 43:496–501
Stenhouse SL, Hairston NG, Cobey AE (1983) Predation and competition in Ambystoma larvae: field and laboratory experiments. J Herpetol 17:210–220
Stier A, Geange SW, Hanson KM, Bolker B (2013) Predator density and timing of arrival affect reef fish community assembly. Ecology 94:1057–1068
Todd BD, Winne CT (2006) Ontogenetic and interspecific variation in timing of movement and responses to climatic factors during migrations by pond-breeding amphibians. Can J Zool 84:715–722. doi:10.1139/z06-054
Todd BD, Scott DE, Pechmann JH, Gibbons JW (2011) Climate change correlates with rapid delays and advancements in reproductive timing in an amphibian community. Proc R Soc B Biol Sci 278:2191–2197. doi:10.1098/rspb.2010.1768
Trauth SE, Robison HW, Plummer MV (2004) The amphibians and reptiles of Arkansas. University of Arkansas Press, Fayetteville
Urban MC (2007a) Predator size and phenology shape prey survival in temporary ponds. Oecologia 154:571–580. doi:10.1007/s00442-007-0856-2
Urban MC (2007b) Risky prey behavior evolves in risky habitats. Proc Natl Acad Sci USA 104:14377–14382. doi:10.1073/pnas.0704645104
Urban MC (2010) Microgeographic adaptations of spotted salamander morphological defenses in response to a predaceous salamander and beetle. Oikos 119:646–658
Van Buskirk J (2005) Local and landscape influence on amphibian occurrence and abundance. Ecology 86:1936–1947
Van Buskirk J, Yurewicz KL (1998) Effects of predators on prey growth rate: relative contributions of thinning and reduced activity. Oikos 82:20–28
Visser ME, Both C (2005) Shifts in phenology due to climate change: the need for a yardstick. Proc R Soc Lond Ser B Biol Sci 272:2561–2569. doi:10.1098/rspb.2005.3356
Vonesh J, De la Cruz O (2002) Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia 133:325–333. doi:10.1007/s00442-002-1039-9
Warton DI, Hui FK (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Werner EE, Yurewicz KL, Skelly DK, Relyea RA (2007) Turnover in an amphibian metacommunity: the role of local and regional factors. Oikos 116:1713–1725. doi:10.1111/j.2007.0030-1299.16039.x
Whiteman HH, Wissinger SA (2005) Amphibian population cycles and long-term data sets. In: Lannoo MJ (ed) Amphibian declines: conservation status of US species. California University Press, Los Angeles, pp 177–184
Wilbur HM, Alford RA (1985) Priority effects in experimental pond communities: responses of Hyla to Bufo and Rana. Ecology 66:1106–1114
Wissinger SA (1992) Niche overlap and the potential for competition and intraguild predation between size-structured populations. Ecology 73:1431–1444
Wissinger S, Whiteman HH, Denoel M, Mumford ML, Aubee CB (2010) Consumptive and nonconsumptive effects of cannibalism in fluctuating age-structured populations. Ecology 91:549–559
Yang LH, Rudolf VH (2010) Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol Lett 13:1–10. doi:10.1111/j.1461-0248.2009.01402.x
Acknowledgments
We thank the many volunteers and technicians who helped monitor drift fences, especially K. Malone, J. Bardwell, B. Scheffers, E. Wengert, J. Sias, and L. Rehard, and J. Briggler and G. Raeker of the Missouri Department of Conservation. We also thank R. Alford and two anonymous reviewers whose comments greatly improved this manuscript. This project was supported by NSF DEB 0239943 and conducted under MU-ACUC 3368.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ross Andrew Alford.
Rights and permissions
About this article
Cite this article
Anderson, T.L., Hocking, D.J., Conner, C.A. et al. Abundance and phenology patterns of two pond-breeding salamanders determine species interactions in natural populations. Oecologia 177, 761–773 (2015). https://doi.org/10.1007/s00442-014-3151-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3151-z