Abstract
Mutualistic interactions can be just as important to community dynamics as antagonistic species interactions like competition and predation. Because of their large effects on both abiotic and biotic environmental variables, resource mutualisms, in particular, have the potential to influence plant communities. Moreover, the effects of resource mutualists such as nitrogen-fixing rhizobia on diversity and community composition may be more pronounced in nutrient-limited environments. I experimentally manipulated the presence of rhizobia across a nitrogen gradient in early assembling mesocosm communities with identical starting species composition to test how the classic mutualism between nitrogen-fixing rhizobia and their legume host influence diversity and community composition. After harvest, I assessed changes in α-diversity, community composition, β-diversity, and ecosystem properties such as inorganic nitrogen availability and productivity as a result of rhizobia and nitrogen availability. The presence of rhizobia decreased plant community diversity, increased community convergence (reduced β-diversity), altered plant community composition, and increased total community productivity. These community-level effects resulted from rhizobia increasing the competitive dominance of their legume host Chamaecrista fasciculata. Moreover, different non-leguminous species responded both negatively and positively to the presence of rhizobia, indicating that rhizobia are driving both inhibitory and potentially facilitative effects in communities. These findings expand our understanding of plant communities by incorporating the effects of positive symbiotic interactions on plant diversity and composition. In particular, rhizobia that specialize on dominant plants may serve as keystone mutualists in terrestrial plant communities, reducing diversity by more than 40 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While mutualisms have long been recognized as important drivers of population dynamics and evolutionary processes (e.g., Wolin 1985; Bronstein et al. 2003; Stanton 2003; Thompson 2005; Frederickson and Gordon 2009; Kay and Sargent 2009), there is now growing support for the importance of mutualisms to community patterns. Mutualisms can increase (e.g., Bshary 2003; Bastolla et al. 2009; Stein et al. 2009; Wurst et al. 2011; Rodriguez-Cabal et al. 2013) or decrease diversity and evenness (e.g., Clay and Holah 1999; Hartnett and Wilson 2002; Izzo and Vasconcelos 2005; Grover et al. 2008; Rudgers et al. 2010). For example, a cleaning symbiosis between cleaner wrasse and their client fish increases fish diversity in coral reefs (Bshary 2003), while invasive tall fescue grass and its endophytic fungus mutualist reduce plant and arthropod diversity (Clay and Holah 1999; Rudgers and Clay 2008). Identifying the factors underlying these contrasting diversity effects may yield a more predictive framework for the role of mutualisms in community ecology.
Resource mutualisms may be especially likely to influence communities because they alter the availability of limiting resources such as nitrogen and phosphorus (Afkhami et al. 2014). Mycorrhizal fungi and rhizobium bacteria are two common resource mutualists with which plants exchange carbohydrates for phosphorous and nitrogen, respectively. Mycorrhizal fungi have been shown to increase phosphorous uptake into a community by 44 % (van der Heijden et al. 2006a), thus alleviating phosphorous limitation to plants. Similarly, rhizobia can significantly increase soil nitrogen to the system through the fixation of atmospheric nitrogen (Vitousek and Walker 1989). Since resources are among the most important drivers of species coexistence and competitive outcomes, these large effects on resource dynamics may lead to large effects on communities (Maron and Connors 1996).
In particular, rhizobia not only directly influence the legume but they also have the potential to either inhibit or facilitate other species in the community by altering the abiotic and biotic environment (as outlined by van der Heijden et al. 2006b). The legume–rhizobium mutualism may provide the legume with a competitive advantage over non-leguminous species through access to an additional nitrogen pool (Morris and Wood 1989; van der Heijden et al. 2006b), or could decrease nitrogen limitation to other plants and promote coexistence both within a season or over longer time scales (Vandermeer 1989; Maron and Connors 1996; van der Heijden et al. 2006b; Fustec et al. 2010). Overall shifts in community composition, patterns of diversity, and changes in ecosystem function may occur due to these changes in competitive and nutrient dynamics, especially in low-nitrogen environments. For example, increased competition from the legume may reduce diversity while reduced nitrogen limitation to multiple species in the community may increase diversity. Yet, since these changes in competition and nutrient availability are not mutually exclusive, the overall effects of rhizobia on communities may be a result of the relative strengths of these potentially opposing forces.
Given the potential for strong effects of the legume–rhizobium mutualism on plant communities, it is important to study how rhizobia may influence species interactions and community patterns, especially when legumes are highly abundant in the community. In an elegant study that manipulated generalist rhizobia associating with multiple species of legume in experimental communities, van der Heijden et al. (2006b) found that rhizobia increased the evenness of communities by promoting coexistence of legumes. Surprisingly, the presence of rhizobia did not affect non-leguminous species, possibly because in this system legumes were subdominant species in communities dominated by grasses. Many other studies that focused on highly abundant species or mutualisms that interact with many species observed effects of mutualisms on entire communities (e.g., Clay and Holah 1999; Bshary 2003; Stein et al. 2009; Wurst et al. 2011; Bauer et al. 2012), suggesting that mutualisms may be especially likely to affect other community members when they involve dominant species or generalist mutualists.
Importantly, however, suitable mutualist partners may not always be available to hosts; for example, the distribution of compatible rhizobia to a particular legume species can vary across habitats (Odee et al. 1995; Larson and Siemann 1998; Tlusty et al. 2004; Thrall et al. 2007; Stanton-Geddes and Anderson 2011). In agricultural systems and for invasive species, this spatial heterogeneity in the soil biotic community can limit legume establishment (Lowther et al. 1987; Parker et al. 2006). Yet, rhizobia availability can also limit native species establishment or growth (Odee et al. 1995; Thrall et al. 2007; Stanton-Geddes and Anderson 2011). A study of 18 legume species across 12 sites in Kenya identified substantial spatial variation in the availability of suitable rhizobia across sites (Odee et al. 1995). Similarly, another study detected significant geographic variation in rhizobia presence, abundance, and effectiveness for two Australian Acacia species, with some sites completely devoid of compatible rhizobia (Thrall et al. 2007). This spatial variation in rhizobium availability may be especially important for species with a patchy distribution or those colonizing newly disturbed habitats. Given the variable distribution of compatible rhizobia and the large effects resource mutualisms can have on communities, the characteristic soil microbial community at a site could lead to differences in the assembly, diversity, and composition of a community in natural field environments.
The effects of rhizobia availability may be context-dependent on abiotic environmental variation (Bronstein 2009). For example, resource mutualisms can be especially beneficial to plants when nutrients are limiting, but in higher nutrient conditions this mutualism can shift to parasitism (Neuhauser and Fargione 2004). Rhizobia provide leguminous plants with a greater benefit from the association when nitrogen is limiting in the environment (Heath and Tiffin 2007; Lau et al. 2012). These varying legume responses to rhizobia availability across abiotic conditions may result in differing competitive dynamics between legumes and non-leguminous species. In nitrogen-rich environments, legumes produce fewer nodules and the competitive advantage is lost (Lauenroth and Dodd 1979; Vargas et al. 2000), while other species that compete more strongly for soil nitrogen may be at an advantage over legumes (Lawrence 1979). Since the effects of mutualists may not be consistent across environments, identifying the abiotic constraints of mutualist-driven community effects could lead to a more predictive understanding of the role of these positive interactions in ecosystems. While some studies have shown rhizobia to influence various community properties (e.g., van der Heijden et al. 2006b; Bauer et al. 2012), it remains necessary to identify environmental contexts when rhizobia have the greatest influence on plant communities.
I used a mesocosm experiment in which I manipulated the presence of rhizobia that associates with a focal dominant legume in simulated early assembling plant communities across a nitrogen gradient to ask: (1) how do rhizobia influence the dominance of a legume host, (2) do rhizobia affect α- and β-diversity, community composition, and productivity, and (3) are rhizobia effects on communities more pronounced when nitrogen is limiting?
Materials and methods
Study system
Chamaecrista fasciculata is an annual legume native to the Midwestern and Eastern United States. It is a pioneer species that establishes and can dominate grasslands and old-fields following disturbance (Holah and Alexander 1999; Galloway and Fenster 2000; Keller, personal observations). This legume is found in both highly disturbed sites and high-quality prairies at densities ranging from nearly 0 to 55 plants per m2 (Fenster 1991), with some populations containing more than 100 plants per m2 (Keller, personal observation). C. fasciculata forms a facultative mutualistic interaction with rhizobia, such as Bradyrhizobium elkanii, which provide the plant with fixed nitrogen in exchange for carbohydrates. C. fasciculata has a patchy distribution and compatible rhizobia are not found consistently across potential colonization sites. Limited rhizobia availability can affect C. fasciculata establishment and growth at some locations (Stanton-Geddes and Anderson 2011; Keller, unpublished).
Experimental design
I created mesocosms simulating early successional prairie communities and manipulated the presence of rhizobia at three different nitrogen levels in a 2 × 3 full-factorial design replicated three times (n = 18 mesocosms). Each mesocosm consisted of a 14.4-L pot filled with potting mix: 68.5 % soil (Metro Mix 360; SunGro Horticulture, Agawam, MA, USA), 24.5 % sand (Quikrete All Purpose Sand; Quikrete, Atlanta, GA, USA), and 7 % clay (Turface MVP; Profile Products, Buffalo Grove, IL, USA) simulating sandy soils characteristic of C. fasciculata habitat in the upper Midwestern United States. I planted each mesocosm with simulated native early successional prairie communities consisting of Chamaecrista fasciculata (12 individuals to create a density consistent with higher-density field observations of this early establishing legume) and four individuals each of Bromus kalmii (short-lived perennial C3 bunch grass), Danthonia spicata (short-lived perennial C3 bunch grass), Monarda punctata (short-lived perennial forb), Oenothera biennis (biennial forb), Potentilla arguta (perennial forb), and Vulpia octaflora (annual C3 grass). I sterilized all seeds with 95 % ethanol and germinated them in seedling flats filled with Metro Mix 360. Two weeks later, I transplanted seedlings into mesocosms placed in the Kellogg Biological Station (Hickory Corners, MI, USA) greenhouse. Individuals were placed at approximately 3 cm apart in the same arrangement across mesocosms. Seeds of C. fasciculata were greenhouse-reared progeny of field-collected seeds from 6 populations across the Midwestern United States in Illinois, Indiana, Michigan, and Ohio. Seeds from all other species were obtained from Native Connections (Three Rivers, MI, USA).
I manipulated the presence of rhizobia, B. elkanii (strain 6437, isolated at the University of Minnesota; Tlusty et al. 2004) by culturing rhizobia in TY liquid media for 5 days at 28 °C and then applying 1 mL of rhizobia inoculant diluted to ~2.5 × 106 cells/mL based on OD600 to the base of each C. fasciculata seedling. B. elkanii strain 6437 was isolated from a Minnesota population of C. fasciculata not included in the seed collection for this experiment; however, this strain successfully nodulated all C. fasciculata populations used in this experiment. Non-inoculated mesocosms received 1 mL of TY media without rhizobia to each C. fasciculata seedling.
I manipulated nitrogen availability at three levels: 0 g, 10 g, and 20 g N per m2 with the highest value representing high fertility sites in southwest Michigan (Foster and Gross 1998). Nitrogen was applied as ammonium nitrate granules on the soil surface, with half the total amount applied 1 week after mesocosm installation and the other half applied 3 months later. No-nitrogen treatments did not receive any nitrogen fertilization. To prevent phosphorous and potassium limitation across all three nitrogen treatments, all mesocosms received 10 g per m2 of P and K applied as superphosphate and potash respectively, with half applied at the same time as each nitrogen fertilization.
Data collection and analysis
After 6 months (to mimic a single growing season and provide sufficient time for interactions between individuals), I harvested the aboveground biomass in each mesocosm. Individuals of each species were sorted and counted, and biomass was dried at 65 °C for >2 days and weighed. I calculated α-diversity as Shannon diversity (H′), Simpson’s reciprocal index of diversity (1/D), and Pielou's evenness (J) for each mesocosm, each of which incorporate richness and abundance (biomass) data into a single measure. I took three 10-cm soil cores from each mesocosm, performed a KCl extraction, and estimated inorganic soil nitrogen availability with an Alpkem/OI Analytic Flow Solution IV analyzer (Model 3550) (see Eilts et al. 2011). I also examined some C. fasciculata roots from each mesocosm to confirm inoculation treatments, but was unable to completely measure belowground root productivity and the number of nodules produced due to the tight intermixing of plant roots between species. At the time of harvest, inoculated mesocosms exhibited successful nodulation and non-inoculated mesocosms were not contaminated by rhizobia.
To test how rhizobia and nitrogen influence diversity, aboveground productivity, individual species aboveground biomasses, relative abundances, and inorganic nitrogen availability, I performed ANOVA with rhizobia presence, nitrogen treatment, and the rhizobia × nitrogen interaction included as fixed factors. I used Pearson’s correlation coefficients to examine the pairwise relationships between C. fasciculata biomass and the biomass of competing species and between C. fasciculata biomass and diversity.
To test how rhizobia and nitrogen affect plant community composition, I performed PERMANOVA (‘adonis’ function of vegan using the Bray–Curtis distance measure with 9999 permutations) on species biomass, including rhizobia and nitrogen treatments and the interaction as fixed factors. Since mesocosms started with identical species composition and there were minimal extinctions resulting in similar richness values across mesocosms, I used abundance estimated from biomass with joint absences excluded to examine β-diversity as variation in community composition (sensu Anderson et al. 2011). Specifically, I analyzed among-mesocosm dissimilarity in composition by treatment by first creating a matrix of pairwise dissimilarities using the Bray–Curtis distance measure then using a multivariate test of Levene’s homogeneity of variances to calculate within-treatment dispersion (‘betadisper’ function of vegan). I then tested for differences between treatments using a permutation test (‘permutest’ function of vegan with 9999 permutations) (Anderson et al. 2006). Community composition was visualized using non-metric multidimensional scaling (NMDS) using the Bray–Curtis distance measure (‘metaMDS’ function of vegan) to explore changes in both location and dispersion effects between treatments. All analyses were performed in R with the car and vegan packages (3.0.2, R core development team; Fox and Weisberg 2011; Oksanen et al. 2013).
Results
Rhizobia reduced Shannon diversity and evenness by 43.3 and 46.2 %, respectively (Shannon diversity: F 1,12 = 25.98, P = 0.0003; Table 1; Fig. 1b; evenness: F 1,12 = 30.38, P = 0.0001; Table 1). Rhizobia reduced diversity primarily because of reductions in evenness since all mesocosms were started with the same number of species and few extinctions were observed during the experiment (there were no significant effects of rhizobia on richness, P > 0.05). Simpson’s diversity also declined by 37.7 % in mesocosms inoculated with rhizobia, further indicating an increase in dominance by few species (F 1,12 = 17.80, P = 0.001; Table 1).
Rhizobia altered plant community composition (F 1,12 = 14.75, P = 0.0003; Fig. 2; Table 1) because rhizobia increased the abundance of some species and caused reductions in other species. Rhizobia also significantly reduced β-diversity; variability among mesocosms in community composition was lower for rhizobia treatments than no rhizobia treatments (F 1,16 = 7.46, P = 0.014; Fig. 2), suggesting that rhizobia caused communities to converge. The observed effects of rhizobia on diversity and composition likely result because rhizobia increased C. fasciculata competitive dominance, increasing its relative abundance from 60 to 84 % of the community (F 1,12 = 15.43, P < 0.01; Fig. 3). Rhizobia inoculation also tended to decrease the relative abundance of all species in the mesocosms (Fig. 3), with O. biennis (F 1,12 = 10.75, P < 0.01) and M. punctata (F 1,12 = 4.68, P = 0.05) experiencing significant reductions in relative biomass.
Effects of rhizobia on community composition in experimental mesocosms indicating differences in both community composition and dispersion (β-diversity) between treatments, visualized with non-metric multidimensional scaling (NMDS) based on biomass of each species per mesocosm. Each point represents either a non-inoculated mesocosm (rhizobia absent, white circles) or inoculated mesocosm (rhizobia present, gray triangles)
Average relative community composition of a rhizobia non-inoculated and b inoculated mesocosms for all seven species (in order): C. fasciculata (dark gray), M. punctata (light gray), B. kalmii (vertical barring), O. biennis (horizontal barring), P. arguta (dotted), V. octaflora (black), and D. spicata (white)
Overall, rhizobia significantly increased total community productivity (mean ± SE: no rhizobia: 26.4 ± 3.9 g/mesocosm; rhizobia: 62.9 ± 3.4 g/mesocosm) (F 1,12 = 41.71, P < 0.001), but this was due to increased C. fasciculata biomass in inoculated mesocosms since rhizobia did not affect subdominant community productivity (P > 0.05). Rhizobia increased C. fasciculata biomass (F 1,12 = 43.43, P < 0.001; Fig. 1a), and increased C. fasciculata biomass was associated with decreased Shannon diversity (r = −0.92, P < 0.001). Rhizobia inoculation reduced O. biennis biomass (F 1,12 = 10.5, P < 0.01) but tended to marginally increase B. kalmii biomass (F 1,12 = 3.79, P = 0.075). Moreover, O. biennis biomass was negatively correlated with C. fasciculata biomass (r = −0.53, P = 0.02), suggesting that rhizobia reduced O. biennis biomass by increasing competition from C. fasciculata. All other species (D. spicata, M. punctata, P. arguta and V. octaflora) were not directly influenced by the treatments applied (P > 0.1), but the change in biomass of some species were correlated with changes in other species. As B. kalmii increased, M. punctata biomass marginally decreased (r = −0.44, P = 0.06). Increased M. punctata biomass was correlated with decreased P. arguta biomass (r = −0.48, P = 0.04). Biomass of the short-lived grasses D. spictata and V. octaflora were positively correlated (r = −0.81, P < 0.001).
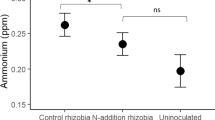
Rhizobia effects on plant communities were consistent across nitrogen treatments (non-significant rhizobia × nitrogen interaction, P > 0.1; Table 1). Nitrogen main effects did not significantly affect productivity, diversity, or composition (all P > 0.1; Table 1). Total available nitrogen did not vary across rhizobia or nitrogen treatments or their interactions (all P > 0.1).
Discussion
Resource mutualism affects diversity
I show that, similar to predators and ecosystem engineers, mutualists have the potential to be keystone species. In this system, rhizobia act as a keystone mutualist by decreasing plant diversity and evenness, altering community composition, and driving convergence in community structure. Like the classic keystone species Pisaster starfish, which alters diversity by influencing the abundance of a dominant intertidal competitor (Paine 1966, 1969), rhizobia influence diversity by changing the abundance of the dominant plant competitor C. fasciculata. However, while Pisaster decreased the abundance of the dominant competitor, relaxing competition and promoting diversity, rhizobia increased the competitive dominance of C. fasciculata, thus inhibiting diversity.
This short-term (6 months) study shows effects of mutualism on early community structure; however, transient dynamics can be important to the long-term successional trajectory of a community through priority effects (Fukami and Nakajima 2013). Here, the reduction in diversity observed in my study may be a transient response to the immediate success of the early successional dominant legume. Over longer time scales, increasing nitrogen levels during succession may help promote the establishment of other species and decrease the dominance of the legume (e.g., Tilman 1987; Chapin et al. 1994; del Moral and Rozzell 2005). How the effects of rhizobia on diversity and convergence in community structure observed here influence longer-term community assembly processes requires further study.
Rhizobia reduced both α-diversity and β-diversity. Shannon diversity decreased, and there was also more convergence in community composition between inoculated mesocosms. Rhizobia likely drove greater community similarity by dramatically increasing C. fasciculata dominance from 60 to 84 % of the community. Conversely, in the absence of rhizobia, there was greater divergence in community composition between mesocosms as subdominants experienced less competition from C. fasciculata and there was greater variation in subdominant species growth. This is consistent with research showing reduced β-diversity with increased competitive dominance (Hillebrand et al. 2008). For example, invasion by the dominant ant Anoplolepis gracilipes reduces β-diversity of ant–plant mutualists and local arthropods (Savage and Whitney 2011).
While a mutualism reduces diversity in this and several other systems, numerous other studies have found the opposite pattern—that mutualists increase diversity. These contrasting effects of mutualists on diversity may be explained by the degree of specificity of the mutualistic interaction (Rudgers and Clay 2008). While my study does not permit the exploration of the effects of generalist mutualists, mutualists that associate with many species in a community frequently increase overall species diversity by increasing fitness of many species and promoting coexistence by minimizing average fitness differences across species (sensu Chesson 2000). For example, arbuscular mycorrhizal fungi increase diversity by benefiting numerous subdominant species in phosphorous-limited tallgrass prairies, especially when the competitively dominant species does not greatly benefit from AMF (Collins and Foster 2009). Similarly, generalist ant seed dispersal mutualists promote diversity (Gove et al. 2007), and declines in the abundance of similar ant generalist mutualists reduced diversity and altered community composition in the South African fynbos (Christian 2001). In contrast, specialist mutualists that associate with a single host species may make their partner species more competitive and decrease diversity, especially when their partner is a dominant species such as in the system studied here. For example, a specialized aphid–ant mutualism where ants tend honeydew-producing aphids on Populus trees increased ant abundance causing reduced arthropod diversity (Wimp and Whitham 2001). Also, endophytic fungi reduce diversity by making their host, the dominant plant tall fescue, even more competitive (Clay and Holah 1999) by altering small mammal herbivory (Rudgers et al. 2007).
C. fasciculata can be a dominant species in disturbed habitats, and rhizobia appear to increase that dominance. Moreover, the rhizobia mutualism is not generalist across many species in my experimental communities (C. fasciculata was the only legume in these mesocosms); thus, this specialized interaction confers a unique competitive advantage to C. fasciculata over the other species. In a similar study, Bauer et al. (2012) tested how mycorrhizae and rhizobia influence simulated prairie communities, finding that mycorrhizae increased diversity while rhizobia altered community composition. Bauer’s results are consistent with some results presented here: rhizobia induce shifts in community composition mediated through the legume; however, Bauer did not detect any effects of rhizobia on diversity. In Bauer’s experiment, however, legumes were not dominant, and rhizobia were generalists interacting with multiple leguminous species. Similarly, another study that manipulated generalist rhizobia associating with multiple species of subdominant legumes in experimental communities dominated by grasses found that rhizobia increased community evenness by promoting coexistence of legumes (van der Heijden et al. 2006b). The degree of rhizobium specificity and partner dominance may explain the contrasting patterns between these studies and the findings presented here.
Mechanisms of resource mutualism effects on community patterns
When associating with a dominant legume, rhizobia can positively affect other community members by relaxing nitrogen limitation on the entire community or can negatively affect competing plants by conferring competitive advantages solely to legume species. Both of these mechanisms may alter community composition and diversity. In this study, rhizobia tended to have both positive and negative effects on competitors, suggesting that both mechanisms may act simultaneously. The decline in some species, such as O. biennis, with increasing C. fasciculata biomass indicates that increased competition due to rhizobia may negatively impact other species, possibly through reduced nutrient, water, or light availability. In contrast, facilitation from rhizobia increasing nitrogen availability to competitors (directly via increased inputs or indirectly by reducing legume competition for soil nitrogen) may cause biomass increases in other species like B. kalmii. These differences in subdominant species responses could be due to varying degrees of niche overlap with C. fasciculata.
Numerous studies have shown that rhizobia are less beneficial in fertilized soils (e.g., Naisbitt and Sprent 1993; Heath and Tiffin 2007). Therefore, I expected rhizobia effects to be more pronounced in nitrogen-limited mesocosms compared to nitrogen-fertilized mesocosms. However, rhizobia effects on diversity, composition, and biomass were consistent across nitrogen treatments. Also, surprisingly, rhizobia and nitrogen fertilization did not change soil nitrogen availability despite successful nodulation, and experimental nitrogen treatments did not affect diversity or community composition. One possibility is that plants were using nitrogen quickly and allocating resources belowground, which was not measurable in this experiment due to the dense root matrix that was formed by the end of the experiment. Alternatively, small sample sizes may have limited statistical power for detecting nitrogen effects. Consistent with this latter hypothesis, while not significant, aboveground biomass tended to be highest in the high nitrogen treatment (mean ± SE: high-nitrogen: 47.8 ± 9.8 g/mesocosm; mid-nitrogen: 42.7 ± 10.1 g/mesocosm; no-nitrogen: 43.4 ± 8.0 g/mesocosm), and similar nitrogen treatments did significantly influence aboveground productivity in a separate experiment (Keller, in preparation).
Conclusions
In sum, rhizobia can be a keystone mutualist in communities, reducing both α- and β-diversity and altering community composition. As communities assemble, an early colonizing legume may become dominant and substantially drive subsequent species interactions depending on the biotic soil conditions of the site. Further incorporating the effects of positive symbiotic interactions on plant communities will help increase our understanding of community dynamics by looking beyond only negative interactions such as predation and competition. In particular, more research is needed to explore how plant diversity and community composition may change over time and whether facilitative effects follow these initial reductions in diversity as soil nitrogen concentrations increase following legume senescence. Additionally, it is important to consider how generalist versus specialist mutualists may differentially influence community diversity, composition, and even stability. If this system is any indication, specialist mutualists that affect a dominant competitor may be especially likely to be keystone mutualists.
References
Afkhami ME, Rudgers JA, Stachowicz JJ (2014) Multiple mutualist effects: conflict and synergy in multispecies mutualisms. Ecology 95:833–844
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28
Bastolla U, Fortuna MA, Pascual-Garcia A, Ferrera A, Luque B, Bascompte J (2009) The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 458:1018–1020
Bauer JT, Kleczewski NM, Bever JD, Clay K, Reynolds HL (2012) Nitrogen-fixing bacteria, arbuscular mycorrhizal fungi, and the productivity and structure of prairie grassland communities. Oecologia 170:1089–1098
Bronstein JL (2009) The evolution of facilitation and mutualism. J Ecol 97:1160–1170
Bronstein JL, Wilson WG, Morris WF (2003) Ecological dynamics of mutualist/antagonist communities. Am Nat 162:S24–S39
Bshary R (2003) The cleaner wrasse, Labroides dimidiatus, is a key organism for reef fish diversity at Ras Mohammed National Park. Egypt. J Anim Ecol 72:169–176
Chapin FS, Walker LR, Fastie CL, Sharman LC (1994) Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol Monogr 64:149–175
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Christian CE (2001) Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 413:635–639
Clay K, Holah J (1999) Fungal endophyte symbiosis and plant diversity in successional fields. Science 285:1742–1744
Collins CD, Foster BL (2009) Community-level consequences of mycorrhizae depend on phosphorous availability. Ecology 90:2567–2576
del Moral R, Rozzell LR (2005) Long-term effects of Lupinus lepidus on vegetation dynamics at Mount St. Helens. Plant Ecol 181:203–215
Eilts JA, Mittelbach GG, Reynolds HL, Gross KL (2011) Resource heterogeneity, soil fertility, and species diversity: effects of clonal species on plant communities. Am Nat 177:574–588
Fenster CB (1991) Gene flow in Chamaecrista fasciculata (Leguminosae) II. Gene establishment. Evolution 45:410–422
Foster BL, Gross KL (1998) Species richness in a successional grassland: effects of nitrogen enrichment and plant litter. Ecology 79:2593–2602
Fox J, Weisberg S (2011) An R Companion to applied regression, 2nd edn. Sage, Thousand Oaks
Frederickson ME, Gordon DM (2009) The intertwined population biology of two Amazonian myrmecophytes and their symbiotic ants. Ecology 90:1595–1607
Fukami T, Nakajima M (2013) Complex plant-soil interactions enhance species diversity by delaying community convergence. J Ecol 101:316–324
Fustec J, Lesuffleur F, Mahieu S, Cliquet J (2010) Nitrogen rhizodeposition of legumes. A review. Agron Sustain Dev 30:57–66
Galloway LF, Fenster CB (2000) Population differentiation in an annual legume: local adaptation. Evolution 54:1173–1181
Gove AD, Dunn RR, Majer JD (2007) A keystone ant species promotes seed dispersal in a ‘diffuse’ mutualism. Oecologia 153:687–697
Grover CD, Dayton KC, Menke SB, Holway DA (2008) Effects of aphids on foliar foraging by argentine ants and the resulting effects on other arthropods. Ecol Entomol 33:101–106
Hartnett DC, Wilson GWT (2002) The role of mycorrhizas in plant community structure and dynamics: lessons from grasslands. Plant Soil 244:319–331
Heath KD, Tiffin P (2007) Context dependence in the coevolution of plant and rhizobial mutualists. Proc R Soc Lond B 274:1905–1912
Hillebrand H, Bennett DM, Cadotte MW (2008) Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology 89:1510–1520
Holah JC, Alexander HM (1999) Soil pathogenic fungi have the potential to affect the co-existence of two tallgrass prairie species. J Ecol 87:598–608
Izzo TJ, Vasconcelos HL (2005) Ants and plant size shape the structure of the arthropod community of Hirtella myrmecophila, an Amazonian ant-plant. Ecol Entomol 30:650–656
Kay KM, Sargent RD (2009) The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annu Rev Ecol Evol Syst 40:637–656
Larson JL, Siemann E (1998) Legumes may be symbiont-limited during old-field succession. Am Midl Nat 140:90–95
Lau JA, Bowling EJ, LE Gentry, Glasser PA, Monarch EA, Olesen WM, Waxmonsky J, Young RT (2012) Direct and interactive effects of light and nutrients on the legume–rhizobia mutualism. Acta Oecol 39:80–86
Lauenroth WK, Dodd JL (1979) Response of native grassland legumes to water and nitrogen treatments. J Range Manag 32:292–294
Lawrence DB (1979) In: Linn R.M(ed). Primary versus secondary succession at Glacier Bay National Monument, southeastern Alaska. Proceedings of the first conference on scientific research in the national parks. National Park Service Transactions and Proceedings Series 5, US Department of the Interior, Washington, pp. 213–224
Lowther WL, Johnson DA, Rumbaugh MD (1987) Distribution and symbiotic effectiveness of Rhizobium meliloti in rangeland soils of the intermountain west. J Range Manag 40:264–267
Maron JL, Connors P (1996) A native nitrogen-fixing shrub facilitates weed invasion. Oecologia 105:302–312
Morris WF, Wood DM (1989) The role of lupine in succession on Mount St. Helens: facilitation or inhibition? Ecology 70:697–703
Naisbitt T, Sprent JI (1993) The long-term effects of nitrate on the growth and nodule structure of the Caesalpinioid herbaceous legume Chamaecrista fasciculata. J Exp Bot 44:829–836
Neuhauser C, Fargione JE (2004) A mutualism-parasitism continuum model and its application to plant-mycorrhizae interactions. Ecol Model 177:337–352
Odee DW, Sutherland JM, Kimiti JM, Sprent JI (1995) Natural rhizobial populations and nodulation status of woody legumes growing in diverse Kenyan conditions. Plant Soil 173:211–224
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) vegan: Community Ecology Package. R Package version 2.0-10 http://cran.r-project.org/package=vegan
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75
Paine RT (1969) A note on trophic complexity and community stability. Am Nat 103:91–93
Parker MA, Malek W, Parker IM (2006) Growth of an invasive legume is symbiont limited in newly occupied habitats. Divers Distrib 12:563–571
Rodriguez-Cabal MA, Barrios-Garcia MN, Amico GC, Aizen MA, Sanders NJ (2013) Node-by-node disassembly of a mutualistic interaction web driven by species introductions. Proc Natl Acad Sci USA 110:16503–16507
Rudgers JA, Clay K (2008) An invasive plant-fungal mutualism reduces arthropod diversity. Ecol Lett 11:831–840
Rudgers JA, Holah J, Orr SP, Clay K (2007) Forest succession suppressed by an introduced plant-fungal symbiosis. Ecology 88:18–25
Rudgers JA, Savage AM, Rúa MA (2010) Geographic variation in a facultative mutualism: consequences for local arthropod composition and diversity. Oecologia 163:985–996
Savage AM, Whitney KD (2011) Trait-mediated indirect interactions in invasions: unique behavioral responses of an invasive ant to plant nectar. Ecosphere 2(9):106
Stanton ML (2003) Interacting guilds: moving beyond the pairwise perspective on mutualisms. Am Nat 162:S10–S23
Stanton-Geddes J, Anderson CG (2011) Does a facultative mutualism limit species range expansion? Oecologia 167:149–155
Stein C, Rißmann C, Hempel S, Renker C, Buscot F, Prati D, Auge H (2009) Interactive effects of mycorrhizae and a root hemiparasite on plant community productivity and diversity. Oecologia 159:191–205
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Thrall PH, Slattery JF, Broadhurst LM, Bickford S (2007) Geographic patterns of symbiont abundance and adaptation in native Australian Acacia–rhizobia interactions. J Ecol 95:1110–1122
Tilman D (1987) Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr 57:189–214
Tlusty B, Grossman JM, Graham PH (2004) Selection of rhizobia for prairie legumes used in restoration and reconstruction programs in Minnesota. Can J Microbiol 50:977–983
van der Heijden MGA, Streitwolf-Engel R, Riedl R, Siegrist S, Neudecker A, Ineichen K, Boller T, Wiemken A, Sanders IR (2006a) The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol 172:739–752
van der Heijden MGA, Bakker R, Verwaal J, Scheublin TR, Rutten M, van Logtestijn R, Staehelin C (2006b) Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol Ecol 56:178–187
Vandermeer J (1989) The ecology of intercropping systems. Cambridge University Press, Cambridge
Vargas MAT, Mendes IC, Hungria M (2000) Response of field-grown bean (Phaseolus vulgaris L.) to Rhizobium inoculation and nitrogen fertilization in two Cerrados soils. Biol Fertil Soils 32:228–233
Vitousek PM, Walker LR (1989) Biological invasion by Myrica Faya in Hawai’i : plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr 59:247–265
Wimp GM, Whitham TG (2001) Biodiversity consequences of predation and host plant hybridization on an aphid-ant mutualism. Ecology 82:440–452
Wolin CL (1985) The population dynamics of mutualistic systems. In: Boucher DH (ed) The biology of mutualism. Oxford University Press, New York, pp 248–269
Wurst S, Gebhardt K, Rillig MC (2011) Independent effects of arbuscular mycorrhiza and earthworms on plant diversity and newcomer plant establishment. J Veg Sci 22:1021–1030
Acknowledgments
I greatly thank J. Lau for help with all aspects of this study; J. Rudgers, J. Mellard, S. Magnoli and two anonymous reviewers for providing many suggestions for improving this manuscript; T. Bassett, M. Coder, M. Hammond, R. Prunier, E. Schultheis, T. Suwa, C. terHorst, and D. Weese for many helpful comments on the manuscript and greenhouse assistance; and J. Stanton-Geddes for providing the rhizobia strain. This work was funded by the National Science Foundation Graduate Research Fellowship Program, Michigan State University Plant Sciences Fellowship, and the Kellogg Biological Station G.H. Lauff and T. Wayne and K. Porter Research Awards. This is KBS contribution #1733.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Russell Monson.
Rights and permissions
About this article
Cite this article
Keller, K.R. Mutualistic rhizobia reduce plant diversity and alter community composition. Oecologia 176, 1101–1109 (2014). https://doi.org/10.1007/s00442-014-3089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3089-1