Abstract
The stable N isotopic composition of individual amino acids (SIAA) has recently been used to estimate trophic positions (TPs) of animals in several simple food chain systems. However, it is unknown whether the SIAA is applicable to more complex food web analysis. In this study we measured the SIAA of stream macroinvertebrates, fishes, and their potential food sources (periphyton and leaf litter of terrestrial C3 plants) collected from upper and lower sites in two streams having contrasting riparian landscapes. The stable N isotope ratios of glutamic acid and phenylalanine confirmed that for primary producers (periphyton and C3 litter) the TP was 1, and for primary consumers (e.g., mayfly and caddisfly larvae) it was 2. We built a two-source mixing model to estimate the relative contributions of aquatic and terrestrial sources to secondary and higher consumers (e.g., stonefly larva and fishes) prior to the TP calculation. The estimated TPs (2.3–3.5) roughly corresponded to their omnivorous and carnivorous feeding habits, respectively. We found that the SIAA method offers substantial advantages over traditional bulk method for food web analysis because it defines the food web structure based on the metabolic pathway of amino groups, and can be used to estimate food web structure under conditions where the bulk method cannot be used. Our result provides evidence that the SIAA method is applicable to the analysis of complex food webs, where heterogeneous resources are mixed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The biological production fuels energy dynamics through an ecosystem (Lindeman 1942) via the trophic pathways composed of the prey-predator relationships involving spatial and temporal variations (Winemiller 1990). In most freshwater (e.g., stream) ecosystems associated with terrestrial and/or ocean ecosystems, biological production is supported by in situ primary production (e.g., periphytic algae attached to a substrate, hereafter “periphyton”) as well as organic materials derived from other sources (e.g., terrestrial leaf litter) and these determine food web structure (Hynes 1970; Fisher and Likens 1973; Vannote et al. 1980; Nakano and Murakami 2001). Aquatic invertebrates are diverse consumers in stream food webs, such as algal-grazing specialists (e.g., Heptageniidae larva—mayfly), leaf-shredding specialists (e.g., Lepidostomatidae larva—caddisfly), and predatory generalists (e.g., Perlidae larva—stonefly) (Cummins 1973; Takemon 2005). The reliance of animals on resources implies dynamic flow of material and energy among ecosystems (Baxter et al. 2005; Carpenter et al. 2005). Animals that have multiple dietary pathways (so-called omnivores) often dominate communities and occupy non-integer trophic positions (TPs), suggesting that in natural trophic networks the prey-predator relationships form a tangled food web rather than a simple food chain (Marczak et al. 2007; Thompson et al. 2007).
Analyses of stable isotope ratios of C (δ13C) and N (δ15N) have contributed to the development of food web research during the last 30 years (e.g., Minagawa and Wada 1984; Fry 1991; Post et al. 2000). Animals’ bulk-tissue δ13C (δ13CBulk) and δ15N (δ15NBulk) values have been used as indicators of food sources and TPs, respectively, because δ13C values can distinguish primary producers [e.g., aquatic algae vs. terrestrial plants (Deines 1980)], and δ15N values increase with higher TP (e.g., Vander Zanden and Rasmussen 2001; Post 2002). Therefore, biplots for δ13CBulk and δ15NBulk reveal food web structure in terms of resource importance and trophic pathways. However, in stream ecosystems the δ13CBulk of periphyton (primary producers) is sometimes too variable to enable assessment of the food sources for animals (Ishikawa et al. 2012), and for δ15NBulk the isotope enrichment factor per trophic level (TL) of stream invertebrates is likely smaller and more variable than that of other animals (Bunn et al. 2013). To better understand the food web structure in stream ecosystems, a novel technique enabling analysis of food sources and TPs will be indispensable.
Techniques for the measurement of the stable N isotopic composition of amino acids (SIAA) have recently been developed and applied to estimating the TPs of various animals (e.g., McClelland and Montoya 2002; Popp et al. 2007; Miller et al. 2013). In amino acid metabolism, glutamic acid is subject to deamination and transamination, which leads to increased isotope enrichment per TL [trophic enrichment factor (TEF) = 8.0 ‰ in δ15N]. In contrast, phenylalanine retains its amino group during metabolism as animals cannot synthesize phenylalanine themselves, resulting in little isotope enrichment per TL (TEF = 0.4 ‰ in δ15N) (Chikaraishi et al. 2009). The fairly constant TEFs in glutamic acid and phenylalanine have been observed in several food chain systems, including feeding experiments performed by Chikaraishi (2011) (quad-TLs—plant leaf > caterpillar and bee > wasp > hornet) and Steffan et al. (2013) (penta-TLs—apple leaves > apple aphid > hover fly > parasitoid > hyperparasitoid). Therefore, the TP of an animal in a single food chain can be determined using the following simple equation, with small deviations in estimates of TP (1σ ~ 0.12) (Chikaraishi et al. 2009):
where δ15NGlu and δ15NPhe are the stable N isotope ratios of glutamic acid and phenylalanine of an animal, respectively, and β is the difference between δ15NPhe and δ15NGlu for a primary producer (baseline) in the food chain [i.e., −3.4 for aquatic algae, +8.4 for terrestrial C3 plants (Chikaraishi et al. 2009, 2010a, 2011)]. Thus, in a single food chain the TP of an animal can be estimated only from its δ15NGlu and δ15NPhe values, without the data on the δ15NGlu and δ15NPhe values of the baseline (Chikaraishi et al. 2009).
The applicability of the SIAA method for the estimation of TPs has been tested for animals in simple ecosystems [e.g., a single food chain involving cabbage, caterpillar, and wasp (Chikaraishi et al. 2011)]. Few studies applying the SIAA method to complex food webs (e.g., where both aquatic- and terrestrial-derived resources potentially contribute to the diet of animals) have been reported [cf., reconstruction of marine and terrestrial paleoenvironments (Naito et al. 2010)]. In stream food webs where aquatic and terrestrial resources are mixed, the proportion of resources derived from aquatic and terrestrial food chains can be used in the estimation of the TP of animals (e.g., macroinvertebrates and fishes), because aquatic and terrestrial primary producers have distinctive β values in Eq. 1. In this study we test the applicability of the SIAA method for analyzing stream food webs, with the assumption of constant TEFs in δ15NGlu (8.0 ‰) and δ15NPhe (0.4 ‰) (Chikaraishi et al. 2009) for stream invertebrates and fishes. We build a two-source mixing model using the δ15NGlu and δ15NPhe values of periphyton, leaf litter of terrestrial C3 plants, and animals to estimate both resource importance and trophic pathways in stream food webs.
Materials and methods
Study sites and sample collection
In November (winter) 2011 and May (summer) 2012, stream macroinvertebrates, fishes, and their potential food sources (periphyton and leaf litter of terrestrial C3 plants) were collected from upper and lower sites of the Yasu River and the Ado River, central Japan (Table A1, Figs. A1, A2). The Yasu River is the largest watershed in the Lake Biwa basin: the upper site is pristine while the lower site is affected by urban development. The concentration and N isotope ratios of dissolved NO3 − increase in the downstream direction in the Yasu River (Ohte et al. 2010). The Ado River is the third largest watershed in the Lake Biwa basin. The natural landscape has been retained throughout its length, and the concentration and N isotope ratios of dissolved NO3 − do not greatly change along its course in the Ado River (Ohte et al. 2010). Several plants with C3 photosynthesis (Cupressaceae and Fagaceae) dominate the riparian vegetation at each of the study sites.
Aquatic invertebrates and fishes were collected at each site using a hand net. We also randomly collected several submerged river cobbles, which were rinsed gently with distilled water prior to collecting the periphyton from the cobble surface, using a brush and distilled water. The resulting slurry was placed into a 100-mL polypropylene bottle (three to five replicates per site). The leaf litter of terrestrial C3 plants (hereafter “C3 litter”, mainly Fagaceae and Ericaceae), was collected from several leaf packs within the stream at each site: the exception was the lower site of the Yasu River in November, where no leaf packs were present; on this occasion, rather than C3 litter we collected particulate organic material (POM) using a surber net (mesh size 1,000 μm) placed vertically in the current in the center of the channel. Neither C3 litter nor POM included C4 plants. All samples were held on ice in the dark until further processing in the laboratory. Gut contents of the invertebrates were not eliminated because some of them had already died during transportation. We identified and categorized invertebrates into functional feeding groups (FFGs; grazer, shredder, filter feeder, predator, and other invertebrates). Isotope measurements were based on single invertebrates where the body size was large enough for analysis (i.e., >3.0 mg dry weight per individual), or were based on several individuals belonging to the same family, which were combined to form the sample for analysis. All samples were freeze dried, and each was ground into a fine powder prior to analysis.
Bulk stable C and N isotope measurements
We measured the δ13CBulk and δ15NBulk of periphyton, C3 litter, invertebrates, and fishes. Each sample was packed into a tin capsule, and the δ13CBulk and δ15NBulk (‰) were measured using a Flash EA1112 elemental analyzer connected to a Delta XP isotope ratio mass spectrometer (Thermo Fisher Scientific, Waltham, MA) with a Conflo III interface (Thermo Fisher Scientific). The δ13C and δ15N values were reported relative to that of Vienna Pee Dee belemnite (VPDB) and atmospheric N2 (air), respectively. Data were corrected using internal standards (CERKU-01 DL-alanine—δ13CVPDB = −25.36 ‰, δ15NAir = −2.89 ‰; CERKU-02 L-alanine—δ13CVPDB = −19.04 ‰, δ15NAir = +22.71 ‰; CERKU-03 glycine—δ13CVPDB = −34.92 ‰, δ15NAir = +2.18 ‰) that were corrected to multiple international standards (Tayasu et al. 2011). The SDs of the δ13CBulk and δ15NBulk measurements were within 0.10 and 0.14 ‰, respectively.
Amino acid purification and stable N isotope measurement
For compound-specific isotope analysis, amino acids in all samples were purified by HCl hydrolysis followed by N-pivaloyl/isopropyl (Pv/iPr) addition, according to the improved procedures of Chikaraishi et al. (2007). In brief, samples of animals (~3 mg) and periphyton, POM, and C3 litter (~20 mg) were hydrolyzed in 12 mol L−1 HCl at 110 °C for 12 h. The hydrolysates were filtrated through a pipette stuffed with quartz wool, washed with n-hexane/dichloromethane (3:2, v/v) to remove large particles and hydrophobic constituents (e.g., lipids), respectively, and evaporated to dryness under a N2 stream. After derivatization with thionyl chloride/2-propanol (1:4, v/v) at 110 °C for 2 h and pivaloyl chloride/dichloromethane (1:4, v/v) at 110 °C for 2 h, and liquid–liquid extraction with 0.5 mL of n-hexane/dichloromethane (3:2, v/v) and 0.2 mL of distilled water, the Pv/iPr derivatives of amino acids were dissolved in dichloromethane.
We measured the stable N isotopic composition of amino acids following the modified method of Chikaraishi et al. (2010b). In brief, the δ15N values of the individual amino acids were determined by gas chromatography/combustion/isotope ratio mass spectrometry (GC/C/IRMS) using a Delta V plus isotope ratio mass spectrometer (Thermo Fisher Scientific) coupled to a gas chromatograph (GC7890A; Agilent Technologies, Santa Clara, CA) via a modified GC-Isolink interface consisting of combustion and reduction furnaces. The amino acid derivatives were injected into the GC column using a Gerstel PTV injector in solvent vent mode. The PTV temperature program was as follows: 50 °C (initial temperature) for 0.25 min, heating from 50 to 270 °C at the rate of 600 °C min−1, isothermal hold at 270 °C for 10 min. The combustion was performed in a microvolume ceramic tube with CuO, NiO, and Pt wires at 1,030 °C, and the reduction was performed in a microvolume ceramic tube with reduced Cu wire at 650 °C. The GC was equipped with an Ultra-2 capillary column (50 m, 0.32 mm internal diameter, 0.52-μm film thickness; Agilent Technologies). The GC oven temperature was programmed as follows: 40 °C (initial temperature) for 2.5 min, increase at 15 °C min−1 to 110 °C, increase at 3 °C min−1 to 150 °C, increase at 6 °C min−1 to 220 °C, held at the final temperature for 14 min. The carrier gas (He) flow rate through the GC column was 1.4 mL min−1. The CO2 generated in the combustion furnace was removed using a liquid N trap. Standard mixtures of at least five amino acids (δ15N ranging from −6.27 to +22.71 ‰) were analyzed every one to six samples to confirm the reproducibility of the isotope measurements. Analytical errors (1σ) of the standards were better than 0.7 ‰ with a sample quantity of 60 ng N.
Estimation of periphyton contribution and TP
Two-isotope and two-source mixing models are widely used in various ecological studies including food web research (e.g., Fry 2006). Using δ15NBulk and δ13CBulk values of periphyton (average of three to five replicates), C3 litter, and animals at each site, the local periphyton contributions to animals relative to C3 litter (f) were calculated using Eq. 2 (see Appendices for more details on algebraic procedures):
where 0 ≤ f ≤ 1 and δ15NBulk[A], δ13CBulk[A], δ15NBulk[L], δ13CBulk[L], δ15NBulk[P], and δ13CBulk[P] are δ15NBulk and δ13CBulk of animal [A], those of C3 litter [L], and those of periphyton [P] in each site, respectively. ΔN and ΔC are TEFs for δ15NBulk (3.4 ‰) and δ13CBulk (0.8 ‰), respectively (Vander Zanden and Rasmussen 2001). Using Eq. 2, the TPs of animals were estimated according to Eq. 3:
Using the δ15NGlu and δ15NPhe values of periphyton (average of three to five replicates), C3 litter, and animals at each site, the local periphyton contributions to animals relative to C3 litter (g) were calculated in the same manner:
where 0 ≤ g ≤ 1 and δ15NGlu[A], δ15NPhe[A], δ15NGlu[L], δ15NPhe[L], δ15NGlu[P], and δ15NPhe[P] are δ15NGlu and δ15NPhe of animal [A], those of C3 litter [L], and those of periphyton [P] in each site, respectively. ΔGlu and ΔPhe are TEFs for δ15NGlu (8.0 ‰) and δ15NPhe (0.4 ‰), respectively (Chikaraishi al. 2009). Using Eq. 4, the TPs of animals were estimated according to Eq. 5:
Animals for which the percentage contributions of periphyton were calculated to be >100 % or <0 % were removed from the analysis (seven of a total of 87 data points). Data on C3 litter were not available for the lower site of the Yasu River in November and consequently the periphyton contributions and TPs of animals at this site were not calculated (11 of a total of 87 data points). All statistical analyses and graphing were performed using R 2.14.2 software (R Development Core Team 2012), with the significance level set α = 0.01.
Results
δ15NBulk and δ13CBulk
ANOVA showed that the δ15NBulk values of periphyton were significantly different between the two sites (upper sites vs. lower sites; p < 0.001), but were not different between the two seasons (November vs. May; p = 0.14) or between the two rivers (Yasu vs. Ado; p = 0.20). In both November and May the δ15NBulk values of periphyton in the Yasu River were significantly lower at the upper site (−2.4 ± 0.76 ‰, mean ± 1 SD, n = 7) than the lower site (+5.9 ± 1.95 ‰, n = 8) [Tukey’s honest significant difference (HSD), p < 0.001 in both seasons]. In contrast, the δ15NBulk values of periphyton in the Ado River were not significantly different between the upper site (+0.5 ± 0.68 ‰, n = 9) and the lower site (+1.7 ± 0.45 ‰, n = 8) (Tukey’s HSD, p = 0.35 in November and p = 0.10 in May; Figs. 1, 2). The δ13CBulk values of periphyton showed large intra-site variations (5–10 ‰) in all sites, while those of the C3 litter remained relatively constant among sites (ca. −30 ‰) (Figs. 1, 2). For animals, the δ13CBulk values fell mostly between the δ13CBulk values of periphyton and C3 litter. An exception was the lower site of the Ado River in November, where the δ13CBulk values of some animals were higher than those of periphyton (Fig. 1). The δ15NBulk values of invertebrates fell mostly between the δ15NBulk values of primary producers (i.e., periphyton and C3 litter) and fishes. An exception was the lower site of the Yasu River in November, where the δ15NBulk values of periphyton were higher than those of invertebrates (Fig. 1). Overall, the amount of animals’ δ15NBulk and δ13CBulk data that could be used for the calculation of the two-source mixing model was larger in May (31 of a total of 37 data points) than in November (20 of a total of 36 data points).
Biplot for the bulk stable isotope ratios of C (δ13CBulk) and N (δ15NBulk) of animals and their potential food sources collected in November 2011. Filled diamonds Periphyton, filled squares terrestrial C3 litter, cross surrounded by a square particulate organic material (POM), open diamond grazer, open square shredder, open circle filter feeder, open triangle predator, open inverted triangle other invertebrates, filled star demersal fish (goby), open star other fishes
Biplot for δ13CBulk and δ15NBulk of animals and their potential food sources collected in May 2012. Symbols and abbreviations are as described in Fig. 1
Primary producers
ANOVA showed that the δ15NPhe values of periphyton were significantly different between the two sites (p < 0.001), but were not different between the two seasons (p = 0.10) or between the two rivers (p = 0.04). In both November and May, the δ15NPhe values of periphyton in the Yasu River were significantly lower at the upper site (−4.2 ± 1.80 ‰, n = 6) than the lower site (+4.8 ± 2.52 ‰, n = 8) (Tukey’s HSD, p < 0.001 in both seasons). In contrast, the δ15NPhe values of periphyton in the Ado River were not significantly different between the upper site (−1.4 ± 1.92 ‰, n = 8) and the lower site (−0.9 ± 1.05 ‰, n = 8) (Tukey’s HSD, p > 0.99 in both seasons; Figs. 3, 4). The differences between the δ15NGlu and δ15NPhe values of periphyton were relatively constant (+3.7 ± 1.69 ‰, n = 30), and not significantly different from those reported for aquatic primary producers [+3.4 ± 0.9 ‰, n = 25 (Chikaraishi et al. 2009)] (Wilcoxon test, W = 327, p = 0.42). However, the differences between the δ15NGlu and δ15NPhe values of the C3 litter (−10.7 ± 1.31 ‰, n = 7) were significantly different from those reported for terrestrial C3 plants [−8.4 ± 1.6 ‰, n = 17 (Chikaraishi et al. 2010a)] (Wilcoxon test, W = 104, p = 0.005). The difference between δ15NGlu and δ15NPhe values of POM collected from the lower site of the Yasu River on November (+7.4 ‰) was higher than those of aquatic primary producers (+3.4 ‰) and terrestrial C3 plants (−8.4 ‰) (Fig. 3c), indicating that POM included not only primary producers, but also living and/or dead heterotrophs.
Biplot for the stable N isotope ratios of glutamic acid (δ15NGlu) and phenylalanine (δ15NPhe) of animals and their potential food sources, collected in November 2011. Aquatic and terrestrial baselines [trophic level (TL) = 1] are indicated as solid lines [aquatic, δ15NGlu − δ15NPhe = +3.4; terrestrial C3, δ15NGlu − δ15NPhe = −8.4 (Chikaraishi et al. 2009, 2010a, b)]. Stepwise enrichments of δ15NGlu (+8.0 ‰) and δ15NPhe (+0.4 ‰) along with trophic levels are shown as dashed lines (TL = 2) and dotted lines (TL = 3) for both aquatic and terrestrial food chains. Symbols are as described in Fig. 1
Primary consumers
The δ15NPhe values of primary consumers (mayfly and caddisfly larvae; an exception was the larvae of the leaf-shredding caddisfly Lepidostoma japonicum) in the Yasu River were much lower at the upper site (−4.5 ± 2.57 ‰, n = 5) than the lower site (+6.2 ± 2.35 ‰, n = 7), while in the Ado River the δ15NPhe values of primary consumers were slightly lower at the upper site (−0.2 ± 1.64 ‰, n = 7) than the lower site (+1.1 ± 0.59 ‰, n = 7). For grazing mayflies (larvae of Heptageniidae spp. and Baetis spp.) the δ15NGlu values were approximately 8 ‰ higher than those of local periphyton, while the δ15NPhe values were similar to the periphyton values, and thus they were located near the line of aquatic TL = 2 (Figs. 3, 4). The two-source mixing model showed that the reliance of mayflies on periphyton was 90 ± 6.5 % (n = 9; Fig. 5a) with the TP of 2.1 ± 0.08 (n = 9; Fig. 5b). The δ15NGlu and δ15NPhe values of filter-feeding caddisflies (larvae of Hydropsychidae spp. and Stenopsyche marmorata) showed large variations among sites and seasons, but their reliance on periphyton (87 ± 3.3 %, n = 8) and their TP (2.2 ± 0.14, n = 8) were less variable than those of other animals (Fig. 5). The δ15NPhe values of larvae of the leaf-shredding caddisfly L. japonicum were 10–15 ‰ higher than those of local periphyton, and were similar to that of C3 litter. The periphyton contribution to shredders was thus estimated to be 24 ± 16.9 % (n = 5; Fig. 5a) with the TP of 2.0 ± 0.27 (n = 5; Fig. 5b).
a Periphyton contribution to animals relative to terrestrial C3 litter (%), estimated using a stable N isotopic composition of individual amino acids (SIAA)-based two-source mixing model (see Eq. 4). Periphyton contributions to periphyton (n = 13) and C3 litter (n = 7) were fixed at 100 and 0 %, respectively. Grazer (G; n = 9), predator (P; dragonfly n = 4, stonefly n = 18, dobsonfly n = 5), other invertebrates O; n = 4), filter feeder (F; n = 8), shredder (S; n = 5), goby (n = 10), other fishes (n = 6). b Trophic position (TP) of animals based on the mixing proportion of aquatic (periphyton) and terrestrial (C3 litter) resources estimated using a SIAA-based two-source mixing model (see Eq. 5). Box Inter-quartile (Q1 and Q3), bar median, whisker most extreme data point that is no more than 1.5-fold the inter-quartile range. Outliers are shown where applicable
Secondary consumers and fishes
The δ15NGlu values of secondary consumers were similar to those of grazers and filter feeders (Fig. 5). As with the primary consumers, the δ15NPhe values of secondary consumers (i.e., predatory larvae—the dragonfly Gomphidae spp.; the stoneflies Kamimuria tibialis, Chloroperlidae spp., Paragnetina tinctipennis, Oyamia lugubris, Niponiella limbatella; and the dobsonfly Protohermes grandis) in the Yasu River were much lower at the upper site (−0.9 ± 1.09 ‰, n = 5) than the lower site (+6.3 ± 1.61 ‰, n = 7), while in the Ado River there was only a small difference between the upper site (+1.3 ± 0.94 ‰, n = 15) and the lower site (+1.5 ± 1.39 ‰, n = 7). Dragonfly, stoneflies, and dobsonfly were 85 ± 8.5 % (n = 4), 81 ± 9.0 % (n = 18), and 82 ± 10.0 % (n = 5) reliant on periphyton, respectively (Fig. 5a). The TPs of predators (dragonfly 2.3 ± 0.10, stoneflies 2.5 ± 0.25, dobsonfly 2.3 ± 0.18) were higher than those of primary consumers, but were <3 (Fig. 5b). Larvae of the crane fly (Tipulidae spp., FFG not specified) were 70 ± 9.0 % (n = 4; Fig. 5a) reliant on periphyton with the TP of 2.5 ± 0.23 (n = 4; Fig. 5b). Fishes, including demersal goby (Rhinogobius spp.) and other fishes (trout Oncorhynchus masou ishikawae; chub Nipponocypris temminckii; and minnow Rhynchocypris spp.) were 77 ± 8.0 % (n = 10) and 78 ± 10.9 % (n = 6) reliant on periphyton, respectively (Fig. 5a). The TPs in our data set were highest for fishes (Fig. 5b), including for goby (3.1 ± 0.28, n = 10) and the other fishes (2.8 ± 0.25, n = 6).
The amount of animals’ δ15NGlu and δ15NPhe data that could be used for calculation of the two-source mixing model was similar between November (36 of a total of 39 data points) and May (33 of a total of 37 data points). ANOVA showed that the periphyton contributions (relative to the C3 litter) to animals were significantly different between the two seasons and the two rivers, and among animal groups, but were not significantly different between the two sites (Table A2). The TPs of animals were significantly different between seasons, sites (marginally), and among animal groups, but were not significantly different between rivers (Table A3). The TPs of animals in November were significantly lower than those in May (Tukey’s HSD, p < 0.01).
Comparisons between bulk and SIAA methods
Based on Eqs. 2, 3, 4, 5, TPs estimated from δ15NBulk and δ13CBulk values and from δ15NGlu and δ15NPhe values were compared and a different pattern was observed between November and May (Fig. 6). The amount of data for November was small because the δ15NBulk and δ13CBulk values of periphyton were too variable to construct a two-source mixing model for estimating the relative contributions of periphyton and C3 litter to animals (Fig. 1): approximately half of the animals' data were removed from the analysis because the estimated periphyton contributions exceeded 100 %. Furthermore, the bulk-estimated TPs for November were different from the SIAA-estimated TPs: the SIAA-estimated TPs ranged from 2 to 3, while the bulk-estimated TPs varied widely from 1 to 4 (Fig. 6a). On the other hand, as the δ13CBulk values of animals for May were between those of periphyton and C3 litter, and the δ15NBulk values of animals were higher than those of periphyton and C3 litter (Fig. 2), in most cases the periphyton contribution to animals, and their TPs, were estimated. The TPs for May, estimated using the bulk and SIAA methods, were more alike than those for November, although for several primary consumers (grazers and shredders) the bulk method provided TP estimates <2 (Fig. 6b).
Discussion
The SIAA is useful for understanding the structure of stream food webs. This conclusion was reached by comparing the resource reliance and TPs determined using bulk and SIAA methods for a range of variable stream conditions (upper vs. lower parts of the streams, pristine vs. urbanized landscapes, and summer vs. winter). One important assumption of the linear mixing model based on δ15NBulk and δ13CBulk values is that dietary N and C are assimilated by animals in the same proportions (Phillips and Koch 2002), although the C:N ratios of animals and those of their diets are not necessarily identical in natural food webs (Post 2002). The SIAA method does not rely on this assumption because the biplot for δ15NGlu and δ15NPhe defines the food web structure based on the metabolic pathway of amino groups.
Our seasonal data showed two contrasting results for the bulk methods. The δ15NBulk and δ13CBulk values for May could be used to estimate relative contributions of periphyton and C3 litter to animals, and the bulk-estimated TPs were well correlated with the SIAA-estimated TPs (Fig. 6b), suggesting that both methods are applicable to stream food web analysis. However, the bulk method was not applicable to analyzing stream food webs in November, because the δ15NBulk values of some animals were lower than those of periphyton (e.g., Lower Yasu; Fig. 1), and because the δ13CBulk values of some animals were not between those of periphyton and C3 litter (e.g., Lower Ado; Fig. 1). As noted in many reports, variations in TEF of δ15NBulk among taxa and variations in the δ13CBulk values of periphyton may have caused problems in the analysis of stream food webs (McCutchan et al. 2003; Dekar et al. 2009; Ishikawa et al. 2012; Bunn et al. 2013). In November, the bulk-estimated TPs were not consistent with the SIAA-estimated TPs, and the former provided contradictory results in some animals (e.g., the TPs of some invertebrates were <2; Fig. 6a). In contrast, our results using the SIAA method met the assumptions that the δ15NGlu values of animals are higher than those of primary producers, and that the δ15NPhe values of animals fall between those of periphyton and C3 litter (Figs. 3, 4). The results indicate that both periphyton and C3 litter support stream food webs, and that animals at higher TPs integrate aquatic and terrestrial food chains.
The δ15NPhe values of periphyton were variable among sites, probably reflecting in situ nutrient conditions (Pastor et al. 2013). In the Yasu River the δ15NBulk and δ15NPhe values of periphyton were higher at the lower site than the upper site, but this was not the case for the Ado River. The result is consistent with the pattern of elevation of δ15N–NO3 along the Yasu River reflecting anthropogenic N loading in the urbanized watershed (Ohte et al. 2010). As the δ15NPhe values of primary producers reflect the δ15N values of inorganic N (e.g., δ15N–NO3) (Chikaraishi et al. 2009), the intra-site variation in δ15NPhe values of periphyton suggests that either δ15N values of inorganic N or isotope fractionation between inorganic N and algae vary within a site. On the other hand, the δ15NPhe values of C3 litter were much higher than those of periphyton, and corresponded to or were below the terrestrial C3 baseline (TL = 1), which was expected on the basis of the results of Chikaraishi et al. (2010a, 2011). The C3 plants synthesize lignin from phenylalanine through the phenylpropanoid pathway, but aquatic algae do not (Bender 2012). Kinetic isotope fractionation from phenylalanine to lignin may result in elevated δ15NPhe values relative to δ15N values of other amino acids (e.g., glutamic acid) in C3 plants, and consequently relative to δ15NPhe values of aquatic algae. Our results suggest that both aquatic and terrestrial primary producers have large δ15NGlu and δ15NPhe variations as several previous studies have shown (e.g., Chikaraishi et al. 2009, 2011; Naito et al. 2013). Further studies will be necessary to elucidate what controls the large variations in the δ15NGlu and δ15NPhe values of primary producers in different environments.
The δ15NGlu values of grazers were approximately 8.0 ‰ higher than those of periphyton, while the δ15NPhe values of both were similar, suggesting that grazing animals occupy the position of TL = 2 in the aquatic food chain. On the other hand, the δ15NPhe values of shredders were slightly lower than those of C3 litter, suggesting that leaf-shredding animals are partly subsidized by 15NPhe-depleted aquatic resources. The two-source mixing model indicated that the periphyton contribution to predators was less than that to grazers, suggesting that predators rely on both aquatic and terrestrial resources. It also indicated that the TPs of predators were higher than those of grazers and shredders, but were <3, suggesting that the larvae of dragonfly, stonefly, and dobsonfly are not completely carnivores, but are partly omnivores. This result is consistent with previous gut content analysis showing that the larvae of two stoneflies (O. lugubris and K. tibialis) feed on both animals and algae (Miyasaka and Genkai-Kato 2009). In contrast, as the larvae of dragonfly and dobsonfly have highly specialized mouthparts for eating animal prey, and their guts include animals exclusively (Hayashi 1988; Takemon 2005), our TP estimates of dragonfly and dobsonfly larvae were lower than those predicted based on diet. In most cases the TPs of fishes were >2 but <3, suggesting that their diet includes autotrophs and heterotrophs derived from both aquatic and terrestrial food webs, and that they assimilate both animal- and plant-derived proteins.
In this study we assumed constant TEFs in δ15NGlu (ΔGlu = 8.0 ‰) and δ15NPhe (ΔPhe = 0.4 ‰) for stream invertebrates and fishes, based on the metabolic theory of amino acids and several empirical observations (Chikaraishi et al. 2009, 2011; Steffan et al. 2013). The results suggested that this assumption is reasonable for primary consumers (i.e., grazers and shredders), while it should be examined for secondary and higher consumers (e.g., the larvae of dragonfly and dobsonfly) in further studies. Indeed, the value of ΔGlu − ΔPhe is reported as lower than 7.6 ‰ between some animals and their potential food sources [e.g., penguin 3.4–3.8 ‰ (Lorrain et al. 2009); stingray and shark 5.0 ± 0.6 ‰ (Dale et al. 2011)]. In addition, a feeding experiment indicated that the value of δ15NGlu − δ15NPhe in harbor seal is only 4.3 ‰ higher than the value of their exclusive diet (wild herring) (Germain et al. 2013).
The seasonal differences in periphyton contributions to animals suggest that high in-stream production in summer and/or large inputs of terrestrial resources in winter are reflected in the biomass of animals (Nakano and Murakami 2001). The TPs of animals were also slightly different between seasons, probably because the predator species analyzed were different between November and May: for example, the dominant stoneflies were K. tibialis in November (TP = 2.3 ± 0.19, n = 8), but were N. limbatella in May (TP = 2.6 ± 0.30, n = 6). We did not expect that the periphyton contributions would be lower in the Yasu River than in the Ado River, because the watershed of the former is more urbanized and has a higher dissolved NO3 − concentration (Ohte et al. 2010), which would increase in-stream primary production. In addition, we did not find a significant difference in the periphyton contributions between upper and lower sites, suggesting that N transfer pathway in food webs does not greatly change along a river continuum.
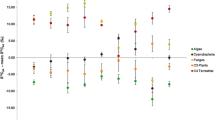
Most ecosystems are open, and the movement of materials and energy among them plays an important role in several ecological processes [e.g., the addition of extra resources make food webs more complex (Polis et al. 1997; Nakano and Murakami 2001)]. Although the number of studies using the SIAA method for estimating the TPs of animals has recently increased (e.g., McClelland and Montoya 2002; Popp et al. 2007; Miller et al. 2013), these studies have been limited to simple food chain systems [to our knowledge, exceptions are a few archaeological studies (Naito et al. 2010, 2013; Styring et al. 2010)] because aquatic and terrestrial primary producers have distinctive δ15N differences between source amino acids (e.g., phenylalanine) and trophic amino acids (e.g., glutamic acid) (Chikaraishi et al. 2009, 2010a). We overcome this limitation by applying a two-source mixing model to stream food webs involving mixed aquatic and terrestrial resources. Our data suggest novel applications of the SIAA method, in addition to estimating the TPs of animals, assessing the relative contributions of aquatic and terrestrial resources to animals (Fig. 7); this structure is central to understanding how aquatic and terrestrial food chains are incorporated into stream ecosystems. Furthermore, amino acids are fundamental to the transfer of N within and among ecosystems (Bender 2012). Based on these advantages, we conclude that a mixing model using the SIAA method can provide useful information for the analysis of complex food webs and N cycling in natural ecosystems.
Two-dimensional food web structure in stream ecosystems estimated from δ15NGlu and δ15NPhe. Periphyton, n = 13; terrestrial C3 litter, n = 7; grazer, n = 9; shredder, n = 5; filter feeder, n = 8; other invertebrates, n = 4; predator, n = 27; demersal fish (goby), n = 10; other fishes, n = 6. Bars indicate SDs. Symbols are as described in Fig. 1
References
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biol 50:201–220
Bender DA (2012) Amino acid metabolism, 3rd edn. Wiley-Blackwell, Oxford
Bunn SE, Leigh C, Jardine TD (2013) Diet–tissue fractionation of δ15N by consumers from streams and rivers. Limnol Oceanogr 58:765–773
Carpenter SR, Cole JJ, Pace ML, Van de Bogert M, Bade DL, Bastviken D, Gille CM, Hodgson JR, Kitchell JF, Kritzberg ES (2005) Ecosystem subsidies: terrestrial support of aquatic food webs from C-13 addition to contrasting lakes. Ecology 86:2737–2750
Chikaraishi Y, Kashiyama Y, Ogawa NO, Kitazato H, Ohkouchi N (2007) Biosynthetic and metabolic controls of nitrogen isotopic composition of amino acids in marine macroalgae and gastropods: implications for aquatic food web studies. Mar Ecol Prog Ser 342:85–90
Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, Miyashita H, Kitazato H, Ohkouchi N (2009) Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Meth 7:740–750
Chikaraishi Y, Ogawa NO, Ohkouchi N (2010a) Further evaluation of the trophic level estimation based on nitrogen isotopic composition of amino acids. In: Ohokouchi N, Tayasu I, Koba K (eds) Earth, life, and isotopes. Kyoto University Press, Kyoto, pp 37–51
Chikaraishi Y, Takano Y, Ogawa NO, Ohkouchi N (2010b) Instrumental optimization of compound-specific isotope analysis of amino acids by gas chromatography/combustion/isotope ratio mass spectrometry. In: Ohokouchi N, Tayasu I, Koba K (eds) Earth, life, and isotopes. Kyoto University Press, Kyoto, pp 367–386
Chikaraishi Y, Ogawa NO, Doi H, Ohkouchi N (2011) 15N/14N ratios of amino acids as a tool for studying terrestrial food webs: a case study of terrestrial insects (bees, wasps, and hornets). Ecol Res 26:835–844
Cummins KW (1973) Trophic relations of aquatic insects. Annu Rev Entomol 18:183–206
Dale JJ, Wallsgrove NJ, Popp BN, Holland KN (2011) Nursery habitat use and foraging ecology of the brown stingray Dasyatis lata determined from stomach contents, bulk and amino acid stable isotopes. Mar Ecol Prog Ser 433:221–236
Deines P (1980) The isotopic composition of reduced organic carbon. In: Fritz P, Fontes JC (eds) Handbook of environmental isotope geochemistry. The terrestrial environment, A, vol 1. Elsevier, Amsterdam, pp 329–406
Dekar MP, Magoulick DD, Huxel GR (2009) Shifts in the trophic base of intermittent stream food webs. Hydrobiologia 635:263–277
Fisher SG, Likens GE (1973) Energy flow in Bear Brook, New Hampshire: an integrative approach to stream ecosystem. Ecol Monogr 43:421–439
Fry B (1991) Stable isotope diagrams of freshwater food webs. Ecology 72:2293–2297
Fry B (2006) Stable isotope ecology. Springer, New York
Germain LR, Koch PL, Harvey J, McCarthy MD (2013) Nitrogen isotope fractionation in amino acids from harbor seals: implications for compound-specific trophic position calculations. Mar Ecol Prog Ser 482:265–277
Hayashi F (1988) Prey selection by the dobsonfly larva, Protohermes grandis (Megaloptera: Corydalidae). Freshwater Biol 20:19–29
Hynes HBN (1970) The ecology of stream insects. Annu Rev Entomol 15:25–42
Ishikawa NF, Doi H, Finlay JC (2012) Global meta-analysis for controlling factors on carbon stable isotope ratios of lotic periphyton. Oecologia 170:541–549
Lindeman RL (1942) The trophic-dynamic aspect of ecology. Ecology 23:399–418
Lorrain A, Graham B, Ménard F, Popp B, Bouillon S, van Breugel P, Cherel Y (2009) Nitrogen and carbon isotope values of individual amino acids: a tool to study foraging ecology of penguins in the Southern Ocean. Mar Ecol Prog Ser 391:293–306
Marczak LB, Thompson RM, Richardson JS (2007) Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88:140–148
McClelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83:2173–2180
McCutchan JM Jr, Lewis WM Jr, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
Miller MJ, Chikaraishi Y, Ogawa NO, Yamada Y, Tsukamoto K, Ohkouchi N (2013) A low trophic position of Japanese eel larvae indicates feeding on marine snow. Biol Lett 9:20120826
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidences and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Miyasaka H, Genkai-Kato M (2009) Shift between carnivory and omnivory in stream stonefly predators. Ecol Res 24:11–19
Naito YI, Honch NV, Chikaraishi Y, Ohkouchi N, Yoneda M (2010) Quantitative evaluation of marine protein contribution in ancient diets based on nitrogen isotope ratios of individual amino acids in bone collagen: an investigation at the Kitakogane Jomon site. Am J Phys Anthropol 143:31–40
Naito YI, Chikaraishi Y, Ohkouchi N, Drucker DG, Bocherens H (2013) Nitrogen isotopic composition of collagen amino acids as an indicator of aquatic resource consumption: insights from Mesolithic and Epipalaeolithic archaeological sites in France. World Archaeol 45:338–359
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98:166–170
Ohte N, Tayasu I, Kohzu A, Yoshimizu C, Osaka K, Makabe A, Koba K, Yoshida N, Nagata T (2010) Spatial distribution of nitrate sources of rivers in the Lake Biwa watershed, Japan: controlling factors revealed by nitrogen and oxygen isotope values. Water Resour Res 46:W07505
Pastor A, Peipoch M, Cañas L, Chappuis E, Ribot M, Gacia E, Riera JL, Marti E, Sabater F (2013) Nitrogen stable isotopes in primary uptake compartments across streams differing in nutrient availability. Environ Sci Technol 47:10155–10162
Phillips DL, Koch PL (2002) Incorporating concentration dependence in stable isotope mixing models. Oecologia 130:114–125
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Evol Syst 28:289–316
Popp BN, Graham BS, Olson RJ, Hannides CCS, Lott MJ, López-Ibarra G, Galván-Magaña F, Fry B (2007) Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson TE, Siegwolf RTW (eds) Stable isotopes as indicators of ecological change. Elsevier, Amsterdam, pp 173–190
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Post DM, Pace ML, Hairston NG (2000) Ecosystem size determines food-chain length in lakes. Nature 405:1047–1049
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org
Steffan SA, Chikaraishi Y, Horton DR, Ohkouchi N, Singleton ME, Miliczky E, Hogg DB, Jones VP (2013) Trophic hierarchies illuminated via amino acid isotopic analysis. PLoS ONE 8:e76152. doi:10.1371/journal.pone.0076152
Styring AK, Sealy JC, Evershed RP (2010) Resolving the bulk δ15N values of ancient human and animal bone collagen via compound-specific nitrogen isotope analysis of constituent amino acids. Geochim Cosmochim Acta 74:241–251
Takemon Y (2005) Life-type concept and functional feeding groups of benthos communities as indicators of lotic ecosystem conditions (in Japanese). Jpn J Ecol 55:189–197
Tayasu I, Hirasawa R, Ogawa NO, Ohkouchi N, Yamada K (2011) New organic reference materials for carbon and nitrogen stable isotope ratio measurements provided by Center for Ecological Research, Kyoto University and Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology. Limnology 12:261–266
Thompson RM, Hemberg M, Starzomski BM, Shurin JB (2007) Trophic levels and trophic tangles: the prevalence of omnivory in real food webs. Ecology 88:612–617
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Winemiller KO (1990) Spatial and temporal variation in tropical fish trophic networks. Ecol Monogr 60:331–367
Acknowledgments
We thank M. Itoh, K. Osaka, Y. Kohmatsu, H. Nakagawa, T. Egusa, S. Ishikawa, W. Hidaka, Y. Tamiya, and Y. Takaoka for their assistance in fieldwork. N. Tokuchi, N. Ohte, M. Kondoh, Y. Chikaraishi, N. Ohkouchi, R. O. Hall, and two anonymous reviewers provided valuable comments on an early draft of this manuscript. This research was supported by the Environment Research and Technology Development Fund (D-1102) of the Ministry of the Environment, Japan. Partial support was also provided by the River Fund (24-1215-022) in charge of the River Foundation and a Grant-in-Aid for Scientific Research (B) (no. 25291101). N. F. I. was supported by the Research Fellowship for Young Scientists (25-1021) of the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Robert O. Hall.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ishikawa, N.F., Kato, Y., Togashi, H. et al. Stable nitrogen isotopic composition of amino acids reveals food web structure in stream ecosystems. Oecologia 175, 911–922 (2014). https://doi.org/10.1007/s00442-014-2936-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2936-4