Abstract
Sympatric species that initially overlap in resource use are expected to partition the environment in ways that will minimize interspecific competition. This shift in resource use can in turn prompt evolutionary changes in morphology. A classic example of habitat partitioning and morphological differentiation are the Caribbean Anolis lizards. Less well studied, but nevertheless striking analogues to the Anolis are the Southeast Asian Draco lizards. Draco and Anolis have evolved independently of each other for at least 80 million years. Their comparison subsequently offers a special opportunity to examine mechanisms of phenotypic differentiation between two ecologically diverse, but phylogenetically distinct groups. We tested whether Draco shared ecological axes of differentiation with Anolis (e.g., habitat use), whether this differentiation reflected interspecific competition, and to what extent adaptive change in morphology has occurred along these ecological axes. Using existing data on Anolis, we compared the habitat use and morphology of Draco in a field study of allopatric and sympatric species on the Malay Peninsula, Borneo and in the Philippines. Sympatric Draco lizards partitioned the environment along common resource axes to the Anolis lizards, especially in perch use. Furthermore, the morphology of Draco was correlated with perch use in the same way as it was in Anolis: species that used wider perches exhibited longer limb lengths. These results provide an important illustration of how interspecific competition can occur along common ecological axes in different animal groups, and how natural selection along these axes can generate the same type of adaptive change in morphology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When taxa that exploit similar ecological resources come into contact, the ensuing competition between these taxa can exert considerable selection pressure for one or both to diverge in habitat use to minimize ecological competition. This shift can lead to phenotypic differentiation among taxa in ecologically relevant characters as each taxon becomes adapted to its change in habitat use, a phenomenon known as character displacement (Brown and Wilson 1956). Character displacement from interspecific competition has been viewed as an important engine of evolutionary diversification (Dayan and Simberloff 2005; Pfennig and Pfennig 2012; Stuart and Losos 2013). A classic example is the adaptive radiation of the Caribbean Anolis lizards. Sympatric Anolis species have diverged in perch use and other resources to minimize ecological overlap and subsequent competition (e.g., Losos 1990b, 1992; see also Stuart and Losos 2013). This is believed to have promoted the evolution of up to six different microhabitat specialists distinct in morphology and behavior, termed “ecomorphs” (Williams 1983). The Anolis are an especially powerful illustration of how ecological competition can drive morphological differentiation because of the level of replication exhibited within the group: the same set of Anolis microhabitat ecomorphs have independently evolved repeatedly on each of the four Greater Antillean islands (Losos et al. 1998).
Shared adaptive responses to common selection pressures in congeners are understandable given that closely related species share much of their genome. That is, the adaptive convergence of the Anolis ecomorphs on each island has presumably been facilitated by the fact that all of the lizards of this genus have inherited part of their genome from the same evolutionary ancestor, which has predisposed these lizards to adapt in similar ways (e.g., Wood et al. 2005). The extent to which more distantly related species (e.g., from different families) follow the same evolutionary trajectories when exposed to similar forms of selection is less clear. Increasing phylogenetic distance between taxa tends to accentuate the signature of stochasticity in evolutionary differentiation through processes such as genetic drift and random mutation (Lenormand et al. 2009). The history of past adaptations and different functional trade-offs or genetic correlations between aspects of the phenotype can also mean taxa from diverse phylogenetic backgrounds respond very differently to common selection pressures (e.g., Alfaro et al. 2005; Ord et al. 2011). In light of these variables, any similarity in the outcome of adaptive evolution between highly divergent, phylogenetic groups would reveal the extent that natural selection can override stochastic processes and historical contingencies to repeatedly produce similar phenotypes.
With this in mind, we examined whether similarities in habitat use—and subsequent similarities in selection—between two highly divergent lineages of lizard have produced the same adaptive changes in morphology, and whether interspecific competition along the same ecological axes has potentially played a role in diversification in both groups. These lineages were the Southeast Asian Draco lizards and the Greater Antillean Anolis lizards. As alluded to above, the adaptive radiation of the Anolis lizards and the relationship between interspecific competition, habitat use and morphological evolution have been extensively studied (reviewed by Losos 2009). We used these existing data on the Anolis lizards to benchmark a field study of the habitat use and morphology of the Draco lizards. This comparison of Draco and Anolis was pertinent for several key reasons. First, the two genera are the product of distinct evolutionary histories. Each genus is nested within separate, ecologically diverse families—the Agamidae and Iguanidae, respectively—that have not shared an evolutionary ancestor for 80-146 million years (Townsend et al. 2011; Daza et al. 2012; Mulcahy et al. 2012; Pyron and Burbrink 2014). Second, the two genera appear to be striking analogues in ecology and behavior (Lazell 1992). Both groups are diurnal, arboreal and insectivorous lizards that occupy a range of comparable and diverse tropical habitats (Schwartz and Henderson 1991; Grismer 2011), have social systems centered on males defending territories (Hairston 1957; Ord 2008), and communicate using elaborate visual displays that are remarkable in their similarity (Mori and Hikida 1994; Ord and Martins 2006). Third, both genera are species rich; Caribbean Anolis especially so with over 150 species (Losos 2009), while Draco is more moderately diverse with at least 45 species (McGuire and Dudley 2011). Finally, species in both genera are often found at high densities with several congeners at the same location (Inger 1983; Schwartz and Henderson 1991), implying interspecific competition is potentially important for Draco in the same way it has been for the Anolis lizards (Losos 1994).
We conducted our comparison of the evolutionary differentiation of the Anolis and Draco lizards in three parts. First, we compared the level of functionally relevant morphological differentiation among Draco species with comparison to the Anolis adaptive radiation. Second, we assessed whether morphological variation among Draco species exhibited patterns of adaptive evolution similar to the Anolis lizards. Finally, we examined the extent to which Draco species have shifted resource use in the presence of ecologically similar congeners, and whether this occurs along the same axes as the Anolis lizards.
In Anolis, interspecific competition has prompted microhabitat differentiation along several ecological axes, but predominately perch size and perch height (e.g., Schoener 1968; Pacala and Roughgarden 1982; Rummel and Roughgarden 1985; Losos et al. 1993). Shifts in perch use have in turn resulted in adaptive differentiation in morphology, particularly limb length (Losos et al. 1997, 2004) and, at its ultimate conclusion, this differentiation probably culminated in the evolution of the ecomorphs (Losos 2009). Specific adaptations in limb length to perch use result because lizards with shorter limbs are more agile on smaller, irregular-shaped perches, whereas lizards with longer limbs have higher running speeds and better jumping capabilities on wider, more even surfaces (e.g., Losos and Sinervo 1989; Irschick and Losos 1999; Toro et al. 2004). Limb length has often evolved in concert with tail length (Losos 1990a) and longer tails function to improve stability while jumping between perches (Higham et al. 2001; Kuo et al. 2012). Other axes of ecological divergence among sympatric Anolis include thermal microhabitat [e.g., perches in the sun vs. those in shade (see Williams 1983)] and diet [prey size (e.g., Schoener 1968; Pacala and Roughgarden 1985)].
To determine whether interspecific competition among Draco might have also occurred along these same ecological axes—perch choice, thermal environment, and diet—we examined whether Draco species showed evidence of greater partitioning in these variables in sympatry than populations of the same species in allopatry. We also supplemented this part of our investigation with a re-evaluation of data from Inger (1983) who recorded a similar range of ecological variables for sympatric Draco species at three other locations. If divergence in habitat use was evident between allopatric and sympatric populations in our study, then we expected the same ecological differentiation to be exhibited among species of the sympatric populations studied by Inger (1983).
A final point of relevance that adds an interesting dimension to the study of Draco and Anolis is that each of these two genera have evolved distinct key innovations that affect how the lizards move about their environments. Anolis lizards have evolved adhesive toepads, which probably helped the genus to radiate into an extensive array of ecological niches (Warheit et al. 1999). In contrast, Draco lizards rely on claws for their arboreal lifestyle. This use of claws in other arboreal lizards has been used to explain the evolution of lower levels of morphological differentiation when compared to Anolis (e.g., Warheit et al. 1999; see also Collar et al. 2010). However, modifications to the rib cage of Draco enable these lizards to extend a large membrane or wing between their front and back legs, and this wing allows lizards to glide over an impressive range [tens of meters (McGuire and Dudley 2005)]. Draco lizards are gracile in appearance, with elongated bodies and slender limbs, which presumably reflects the requirements of flight. Tail length may also play a role in enhancing stability or steering during gliding (Shine et al. 1998; T. J. O. and D. A. K., personal observations).
Nevertheless, both genera spend much of their time using the environment in a similar manner: e.g., walking and running along trunks and branches, and jumping between nearby perches (T. J. O. and D. A. K., personal observations). In this respect, we might expect similar directional changes in morphology with perch use in both genera. However, the evolution of toepads in Anolis may have facilitated the exploitation of more habitat niches in a given environment and greater evolutionary change in morphology, whereas in Draco the biomechanical requirements of gliding may have constrained the magnitude of evolutionary change possible in morphology. There have also been a number of previous studies on lizard groups more closely related to the Greater Antillean Anolis that have failed to document the same relationships between morphology and habitat use that are so apparent among the island anoles [e.g., mainland Anolis (Irschick et al. 1997); North American iguanids (Herrel et al. 2002); South American Liolaemus (Schulte et al. 2004; see also Vanhooydonck and Van Damme 1999; Zaaf and Van Damme 2001; Bickel and Losos 2002)]. That is, while some similarities in ecology and behavior predict convergent morphological adaptations to habitat use between Anolis and Draco, the divergent evolutionary histories of the two groups suggest morphological adaptation could have proceeded quite differently in the two taxa.
Materials and methods
Ecobehavior and morphological measurements of Draco
Field data
We studied ten species of Draco from the Malay Peninsula, Borneo and the Philippines. For four of the ten species, we surveyed two geographically separated populations. In one case—Draco sumatranus—the two populations were from the Malay Peninsula and Borneo and phylogenetically divergent (e.g., as genetically differentiated as Draco formosus and Draco obscurus; McGuire and Heang 2001; see Fig. 1). In the three remaining cases—Draco quinquefasciatus, Draco melanopogon and Draco cornutus—one population studied was not sympatric with any other Draco species, while the second population was sympatric with all three Draco species. For these three species, we explicitly surveyed populations that did and did not overlap with congeners to examine whether lizards shifted habitat use in the context of interspecific competition. The Malay population of D. sumatranus was also sympatric with another species (Draco fimbriatus), but this other species was not surveyed and no data were available on the extent to which it overlapped or differed in habitat use in allopatry or sympatry with D. sumatranus. For all species and populations, an average of 13 adult males and five adult females were sampled for ecobehavior measurements (range 2–41 males, 1–11 females; measurements included perch height, circumference and temperature—see next section), and an average of six adult males and four adult females sampled per species for morphological measurements (range 1–11 individuals for both sexes; measurements included body length, limb length and tail length—see next section). The allopatric population of D. quinquefasciatus was only sampled for ecobehavior data (two males, two females) and was not included in morphological comparative analyses because we had difficulty catching these lizards in dense forest. Sample sizes for each species and population as well as other information on habitat type and community structure relevant for the goals of our study are reported in Table S1 and Fig. S1.
a Inset Maximum clade credibility phylogeny from Collar et al. (2010) of the Draco species studied. The majority of nodes were supported by >0.95 Bayesian posterior probabilities. Branch lengths are proportional to time. b Combined phenogram of Draco and Greater Antillean Anolis based on ecobehavior and morphology. Anolis species are labeled by ecomorph class
We conducted surveys using the so-called Rand Census method developed for Anolis (Rand 1964, 1967). Perch site was based on the location of the first sighting of an individual. Data were only included in our analyses if that individual had not been initially disturbed by the presence of the researcher. The height of the perch was primarily measured using a TruPulse 200 laser range finder with inclinometer (accuracy 0.3 m). However, in a few instances (<5 % of measurements) a long fishing pole with a tape measure attached was used to make height measurements. Perch circumference was measured at shoulder height if the lizard was observed on a tree trunk. When lizards were spotted on a branch, we measured the branch if within reach or a nearby branch of equivalent size if out of reach. These protocols for measuring perch height and circumference were consistent with those used for compiling the Anolis data set (Losos 1990a). Surface temperatures of perches for the interspecific competition analysis were measured using an Extech Infra-red digital surface thermometer (accuracy 2.5–2.8 °C). Temperature readings were taken with the surface thermometer pointing at the perch site at a distance of roughly 10 cm. If the actual perch site was out of reach, temperature readings were taken for a nearby site equivalent in sunlight exposure and substrate.
To obtain external morphological measurements, lizards were caught using an extendible fishing pole (6 m) with a small nylon noose attached to the end. A single person (D. A. K.) then measured snout–vent length (SVL), tail length, forelimb length, hind limb length and hind toe length using digital calipers (see below for details on these measurements). SVL was measured from the tip of the nose to the vent opening; tail length was measured from vent opening to the tip of the tail; total forelimb length was measured from the armpit to the claw tip of the longest toe (the third toe); total hind limb length was measured from where the leg joins the body to the claw tip of the longest toe (the fourth); hind toe length was measured from where the fourth toe joins the foot to the tip of the claw. These characteristics complemented those in the Anolis data set, except hind toe length. We added the measure for toe length on the hind limb of Draco because it was potentially important in the ability of these lizards to move about vertical substrates (unlike Anolis who primarily rely on toepads, Draco exclusively use claws in arboreal locomotion). For analyses exclusively on Draco, we subtracted hind toe length from total hind limb length to assess the extent that limb length evolution as it related to perch use was specific to the limb, toe or both (all analyses comparing Anolis and Draco used total limb length).
Published data
We also included a re-examination of published data from Inger (1983). These data were collected at three locations on Borneo not surveyed by us and were separated by at least 180 km and had numerous Draco species in sympatry. Data collected by Inger (1983) included rough estimates of perch height and quantitative information on diet [average number of individuals per species used for perch height estimates, 86 (range 8, 227) males, 48 (range 5, 116) females; average number of stomach-flushed individuals per species used for diet analysis, 20, both sexes combined (range 10, 25)].
Ecobehavior and morphological measurements of Anolis
Data on the Greater Antillean Anolis were kindly provided by Jonathan Losos. These data covered 53 Anolis species from the four Greater Antillean islands (Cuba, Jamaica, Hispaniola and Puerto Rico). The data were only for adult males and included ecomorph assignments for each species named according to the type of microhabitat typically used by an ecomorph category. These ecomorphs were crown-giant, grass-bush, trunk, trunk-crown, trunk-ground, and twig anoles. There were also eight species of “unique” anoles, which were species that did not conform to any particular ecomorph category and had no convergent counterpart on any of the other Caribbean islands. Other data included SVL, tail length (excluding individuals with re-generated tails), total forelimb and total hind limb length (from the tip of the outermost toe to the point where the limb joined the body), perch height and perch circumference (see Losos 1990a).
Statistical analyses
Draco and Anolis ecobehavior and morphological differentiation
Our first objective was to compare the level of functionally relevant morphological differentiation among Draco species with comparison to the Anolis adaptive radiation. We followed classic work on Anolis and did this in two ways. First, we applied a hierarchical cluster analysis on ecobehavior and morphology to categorize differentiation in Draco with direct reference to Anolis. We labeled the Anolis species on the computed phenogram according to their ecomorph categories, not because we were specifically hoping to identify the same ecomorphs in Draco, but to provide a benchmark for the degree of phenotypic differentiation exhibited by Draco. If Draco species clustered separately from the Anolis on the phenogram, then greater similarity existed among Draco species than between Draco and Anolis. In addition, the length of the phenogram branches among taxa would indicate the overall level of differentiation among Draco compared to the Anolis ecomorphs. Conversely, if Draco were distributed throughout the phenogram, then Draco shared characteristics with the Anolis and the subsequent position of taxa on the phenogram would indicate the level of disparity among Draco relative to the disparity exhibited among the Anolis ecomorphs.
The cluster analysis was implemented in R version 2.15.1 (R Development Core Team, R Foundation for Statistical Computing, Vienna) using the hclust function and defined similarity among species according to the Euclidean distance of perch height, perch circumference, and size-free residuals of tail, forelimb and hind limb length. Size-free residuals were computed for Draco and Anolis separately using phylogenetic generalized least squares (PGLS) regressions of tail or limb length on SVL (PGLS was implemented in COMPARE 4.6b, see below). Size-free residuals were also used for graphical representations of data, but all regression analyses relied on a covariate of SVL.
Next, we examined the distribution of Draco and Anolis species in phenotypic space for the key axes of differentiation previously identified for the Greater Antillean Anolis lizards: perch height and circumference, and size-free residuals of total hind limb length and tail length.
Adaptive response of limb length to perch use
Our second objective was to assess whether morphological variation among Draco species exhibited patterns of adaptive evolution similar to the Anolis lizards. Specifically, whether there was a positive correlation between limb length—specifically hind limb length—and perch circumference. The magnitude of this relationship was also of interest because similarities in the amount of evolutionary change in limb length for a given unit of increase in perch size (i.e., similar slope) would imply that both genera have analogous functional demands on their morphology and have subsequently adapted in a convergent manner.
In this analysis, we performed a PGLS regression of total hind limb length on perch circumference for adult male Draco and adult male Anolis combined in the same analysis. This analysis included an interaction term to test for differences in slope between the genera. This interaction term was not found to be significantly different from zero (see “Results”), but this might have been due to low power resulting from the difference in sample sizes between the genera: 13 Draco taxa vs. 53 Anolis species. We therefore conducted a second set of regressions in which each genus was analyzed separately. We then compared the estimated variance associated with slope estimates to assess relative power.
The Anolis and Draco analyses described above only examined morphological variation in adult male lizards (which were the data available for Anolis), but our field study of Draco was inclusive of both sexes. We therefore conducted a final set of analyses on just the Draco data to test both male and female limb length evolution as a function of perch circumference. In these analyses, we also examined evidence for potential selection on both hind limb length and hind toe length.
For all phylogenetic analyses, we used the mitochondrial DNA Bayesian phylogenetic analysis of the agamid family created by Collar et al. (2010) that included an Anolis outgroup. This supertree was congruent in its resolution of the phylogenetic relationships among Draco species to a phylogeny developed previously for Draco by McGuire and Heang (2001). However, we relied on the supertree because the Anolis outgroup provided a means of combining the Draco and Anolis phylogenies together. The Anolis phylogeny was the mitochondrial DNA phylogeny developed by Nicholson et al. (2005) [reproduced in Losos (2009) with branch length information]. In order to merge the two phylogenies and retain information on branch lengths, we assumed the earliest common ancestor shared by the two genera was 80 million years ago (see “Introduction”) and subsequently scaled the branch lengths within the genera accordingly [for Draco, this was already incorporated into the supertree by having the Anolis outgroup; for Anolis, scaling was based on divergence times among species reported by Jackman et al. (2002)]. The final composite phylogeny was then pruned down to the species of interest using Mesquite version 2.74 (Maddison and Maddison 2010).
The Malay and Bornean populations of D. sumatranus were already included in the Collar supertree. For D. melanopogon and D. cornutus, we included both the sympatric and allopatric populations in our analyses because we expected variation in morphology and habitat use between these populations. These populations were positioned on the phylogeny as separate taxa with branch lengths based on the minimum divergence estimated for intra-island populations of Philippine Draco reported by McGuire and Heang (2001). We assessed the sensitivity of our analyses to this branch length estimate by re-running some analyses using a phylogeny that assumed the maximum estimated divergence between populations and results were qualitatively unchanged.
Phylogenetic generalized least squares regressions were performed using COMPARE 4.6b (Martins 2004), which assumes phenotypic evolution follows a Ornstein–Uhlenbeck model of evolution where the phenotype evolves towards some adaptive optimum, with the extent the phenotype can track this optimum estimated by an α parameter computed via maximum likelihood. When α approaches zero, phenotypic characteristics are tightly correlated to phylogeny and adaptive evolution towards the optima has been constrained. In this instance, much of the interspecific variation observed in phenotypes today can be attributed to evolutionary relationships among species. Very large α values (15.5+) indicate little phylogenetic signal in species data and phenotypic evolution has been free to vary adaptively and has tracked potential optima closely [e.g., an optimum class of ecomorph (Hansen and Martins 1996; Hansen 1997); see Ord and Martins (2006) for discussion on the advantages of PGLS with estimated α over other comparative methods].
Statistical significance of regression parameters was based on a one-tailed p-value because we had an a priori prediction that limb length should increase with increasing perch circumference.
Interspecific competition among sympatric Draco
Our third objective was to examine the extent that Draco species shift resource use in the presence of ecologically similar congeners, and whether this occurs along the same axes as for the Anolis lizards: perch type (height, circumference), perch temperature, and diet. This investigation was conducted in two parts. First, we compared differences in perch type and temperature between sympatric and allopatric populations of three species (D. quinquefasciatus, D. melanopogon and D. cornutus). Shifts in perch use as a result of interspecific competition should limit overlap among sympatric species in perch height, circumference, temperature or any combination of these characteristics, whereas overlap in these characteristics should be more likely among allopatric populations of the same two (or more) species. To quantify the magnitude of difference among species in perch use in allopatry and sympatry, we computed the standardized mean difference between two species, Cohen’s d, and an estimate of the 95 % confidence interval (CI) of this value using equations presented in Ord and Stamps (2009). These values were in turn converted into an r-value to provide a metric bounded between 0 and 1 [equations for converting Cohen’s d into an r-value are also given in Ord and Stamps (2009)]. Effect sizes for species comparisons with CIs that did not include zero were considered to represent biologically significant differences between species (equivalent to a two-tailed p-value <0.05).
However, it was possible that observed differences in habitat use between allopatric and sympatric populations could reflect site-specific differences in environment rather than divergences in habitat use induced by interspecific competition in sympatry (Grant 1972; Schluter and McPhail 1992). To evaluate this, we compared the distribution of perches used in allopatry to the perches used collectively by all species in sympatry. The range of perches used in sympatry (irrespective of the species) should represent the niche breadth for Draco available in that environment. If the type of perches used in allopatry for a given species falls within the range of perches available in the sympatric environment, then any shift in perch use in sympatry likely reflects the outcome of interspecific competition rather than a general difference in habitat between allopatric and sympatric sites. Conversely, if the distribution of perches used by a species in allopatry falls outside the range of perches available in the sympatric environment, then a shift in perch use could reflect differences in habitat and not interspecific competition.
Second, we supplemented the above analyses with a qualitative comparison of data presented in Inger (1983), which reported information on perch height and diet for several sympatric Draco species at three separate locations. If interspecific competition occurs in resource use, we expected species to differentiate in perch height and type of prey eaten in a manner very similar to our own field data (perch height) and previously documented for the Anolis lizards (perch height and diet).
Results
Ecobehavior and morphological differentiation in Draco and Anolis
Hierarchical cluster analysis revealed two main clusters of Draco species that corresponded to similarities in ecobehavior and morphology to the Anolis trunk-ground and trunk-crown ecomorphs (Fig. 1b). These two clusters of Draco species generally associated with the “Malaysian” and “Philippine” Draco radiations (the monophyletic lineages of D. quinquefasciatus to D. melanopogon inclusive, and Draco bimaculatus to Draco spilopterus inclusive; Fig. 1a). There were two exceptions: the Philippine D. bimaculatus clustered with the trunk-ground Malaysian Draco, and the Malaysian D. quinquefasciatus clustered with the trunk-crown Philippine Draco.
Plots of size-free residuals of tail length on total hind limb length again showed Draco distributed across morphospace shared by the Anolis trunk-crown and trunk-ground ecomorphs (Fig. 2; N.B. similarity to the Anolis crown-giants was not especially relevant because this ecomorph is quite similar to trunk-crowns—e.g., see Fig. 2a, b—and is defined largely by its dramatic size). A positive relationship between size-free residuals of tail and total hind limb length was also evident among Draco species that was consistent in direction (but not magnitude) to what has been previously documented for Anolis and that is also obvious across the Anolis ecomorphs in Fig. 2. Plots of perch height on perch circumference showed a general overlap in perch circumference of Draco with the trunk-ground and trunk-crown Anolis ecomorphs, but Draco tended to use the upper range of the perch heights exhibited by the Greater Antillean Anolis (Fig. 2b). Morphological differentiation between the sexes of Draco was generally minor. However, females did show a tendency for a wider range of perch heights among species than males (Fig. 2c), but this was not statistically significant (95 % CI for the coefficient of variation, male perches = 0.18–0.45, female perches = 0.30–0.81, n = 13 taxa).
Limb length differentiation with perch width
A phylogenetic regression that included Draco and Anolis in the same analysis (with a covariate for body size) showed a significant increase in total hind limb length with increased perch circumference among species (Table 1; Fig. 3a). The CIs of the interaction term overlapped zero indicating the relationship was virtually the same in both genera. Separate analyses on each genus confirmed the positive relationship between total hind limb length and perch circumference in both groups. The apparent difference in intercepts between the genera (Fig. 3a) reflected that Draco tend to have more elongated bodies than Anolis to accommodate wings. However, the slope estimated for Draco was remarkably close to the slope computed for the Anolis lizards (0.10 vs. 0.11). The variance associated with the slope estimate was very similar between the genera (a 95 % CI range of 0.24 for 13 Draco taxa and 0.18 for 53 Anolis species), which indicated that the lack of statistical significance for the interaction term in the first analysis (Draco and Anolis combined) was biological and not a reflection of low power.
Limb length differentiation among a adult male Draco species and Greater Antillean adult male Anolis species as a function of perch size, and b details of limb length differentiation among Draco species for adult male and female lizards. Trend lines were computed using a phylogenetic regression (Tables 1, 2)
The remaining phylogenetic analyses focused only on male and female Draco lizards. Adult male lizards continued to show a positive association between hind limb length (not including hind toe length) and perch circumference (Table 2; Fig. 3b). Adult males also exhibited a similar positive association between hind toe length and perch circumference (Table 2; Fig. 3b). There was no relationship between hind limb length or hind toe length and perch circumference in adult females (Table 2; Fig. 3b).
Habitat partitioning in sympatric Draco
Our comparison of allopatric and sympatric Draco provided marginal evidence for habitat partitioning among adult male lizards in perch use: sympatric D. cornutus and D. melanopogon seemed to diverge in perch height, while D. quinquefasciatus tended to shift to narrower perches (Fig. 4).
Habitat partitioning among three Draco species in allopatry and sympatry in relation to three ecological variables previously shown for other lizards to be important sources of interspecific competition. Allopatric includes populations that might have been parapatric with other Draco species (see Table S1)
In allopatry, D. cornutus and D. melanopogon tended to use different perch heights (r = 0.39) and perch widths (r = 0.32), but in sympatry, while both species were generally found on very similar-sized perches (r = 0.12), they seemed to exaggerate differences in the height of these perches, which was reflected in an effect nearly twice that of perch differences in allopatry (r = 0.71; Table 3a). For example, we frequently observed sympatric D. cornutus and D. melanopogon on the same trees, but perched at different heights.
The limited number of individuals sampled for the allopatric population of D. quinquefasciatus (two males, two females) made it inappropriate to compute CIs and effect size differences. Nevertheless, in contrast to D. cornutus and D. melanopogon, the primary axis of divergence in D. quinquefasciatus appeared to be perch circumference. For example, D. quinquefasciatus generally overlapped in perch height with D. melanopogon, but was found on much narrower perches (r = 0.49; Table 3a). This was consistent with our observations of D. quinquefasciatus and D. melanopogon using low perches on sometimes adjacent trees.
These shifts in perch height and perch circumference among sympatric species could not be explained by general differences in the availability of perches between allopatric and sympatric sites (Fig. S1): species had the same range of perch types available in both allopatric and sympatric environments. This suggests that interspecific competition was the mechanism that pushed species to shift in perch use in sympatry.
In contrast, perch temperature was not an axis of divergence in sympatry for any species. Instead, species were found on perches similar in temperature, which was consistent with our observations that these species were using perches in similar areas in the environment (forest edges; Table 3a; Fig. 4). Perch temperatures in allopatric environments, however, were quite different and reflected the broad differences in habitat type (see also Fig. S1). Allopatric D. cornutus were found in open mangroves, while allopatric D. melanopogon and D. quinquefasciatus were found in shade forests.
Adult females showed no evidence of habitat partitioning in any variable.
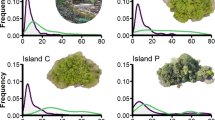
Inger (1983) provided no quantitative data on perch circumference, but his data on perch height reiterated that sympatric D. melanopogon and D. quinquefasciatus do not differentiate from one another in perch height (Fig. 5a). At all three locations surveyed, adult males of these two species overlapped extensively in their perch height distributions. Our data showed that D. melanopogon and D. quinquefasciatus seem to instead differentiate by perch circumference (Fig. 4a). However, Inger’s (1983) data also suggested a possible second axis of differentiation relating to diet between these species. He found the stomach contents of D. melanopogon consisted mostly of intermediate-sized prey (3–5 mm), while the stomach contents of D. quinquefasciatus were primarily smaller prey items (<3 mm in size; Fig. 5a, inset). Across all of the species surveyed by Inger, species tended to differentiate in either preferred perch height or the size of prey consumed (Fig. 5a). Female perch heights were broadly consistent with those of males among species, suggesting habitat partitioning was similar between the sexes. Comparison with our data on this point was difficult because there were only two species in common between our two studies for this analysis (D. melanopogon and D. quinquefasciatus), and both sexes in these species exhibit no differentiation in perch height (Fig. 4).
Re-examination of data presented in Inger (1983) on the perch heights of a adult male and b female lizards at Labang, Pesu and Nanga Tekalit on Borneo. Inger (1983) presented his data as the number of lizards for a given species observed at four broad height categories: 1–3, 3–6, 6–9 and 9+ m. Inger (1983) does not state how heights were measured, but we assumed heights were estimates made by eye. We created frequency distributions of these data by recoding Inger’s (1983) categories as 2, 5, 8 and 11 m, respectively (i.e., at 3-m increments) and fitting a smoothed line connecting the proportions of lizards for a given species at different heights using Excel. Inset Size of ants flushed from stomachs of both sexes at Nanga Tekalit. Arrows correspond to mode perch heights or prey size for each of the species
Discussion
We found both striking parallels in habitat use and morphology between the Southeast Asian Draco lizards and the Caribbean Anolis lizards, and important differences in how these patterns might have originated. Even within the relatively small subset of Draco species studied, there was an impressive level of morphological variation among species, especially in features related to perch use (specifically, the circumference of perches used). The 13 Draco taxa studied (corresponding to ten species) spanned the majority of hind limb lengths exhibited by the Anolis (all but the twig anoles; Fig. 2a) and used a broadly similar range of perch sizes (excluding the narrow perches used by the twig and grass-bush anoles; Fig. 2b). A virtually identical association existed between limb length and perch size in Draco and Anolis. The difference in intercepts apparent in Fig. 3 reflects a general difference in body length between the genera (Draco have longer bodies than Anolis to accommodate their wings), but both groups have comparable limb lengths when this difference in body length is considered (e.g., the factor for genus in Table 1 was not statistically significant). Furthermore, the slope of the relationship between limb length and perch size was almost identical in both groups. In Anolis, this association has been shown to be the product of adaptive evolution (Losos et al. 1997, 2000, 2004, 2006). It follows that this association in Draco between limb length and perch size likely reflects adaptation as well, although it is unknown at this stage the extent to which this differentiation has a plastic element to it. More specifically, it suggests the functional demands of habitat use on morphology are very similar in both groups, with lizards evolving longer legs to improve locomotion on wider (flatter) surfaces. The same unit of increase in perch size appears to have produced the same unit of increase in limb length in both genera (Table 2). This is quite extraordinary considering similar relationships between morphology and habitat use have not evolved in other, more closely related arboreal lizards to the Greater Antillean Anolis (e.g., Irschick et al. 1997; Herrel et al. 2002; Schulte et al. 2004). Furthermore, Draco and Anolis possess separate key innovations that impact how the lizards interact with their environment (adhesive toepads in Anolis and the ability to glide in Draco). Yet, even with these obvious differences in morphological innovation, natural selection has apparently still generated convergent limb lengths in Draco and Anolis.
Another point of comparison is the form of interspecific competition among species in each genus, although the similarities are more tentative. The Anolis lizards are a popular textbook example of how interspecific competition drives resource partitioning among sympatric species (e.g., Campbell and Reece 2002; Cain et al. 2008). The classic axes of this ecological differentiation are perch choice, thermal environment, and diet (reviewed by Losos 2009). There was some evidence that sympatric Draco diverge along one or possibly even two of these same axes. The primary axis of partitioning in Draco seems to be perch choice, as it is for Anolis [reviewed by Schoener (1974) and Losos (2009)]. There was a tendency for species of Draco to either differentiate in perch height (e.g., D. cornutus and D. melanopogon were often found on the same tree at different heights) or perch size (e.g., D. quinquefasciatus and D. melanopogon selected perches of similar height on neighboring trees that differed in trunk circumference). Inger’s (1983) data re-affirmed the partitioning of perch height among sympatric species (Fig. 5), but also highlighted diet as a possible second axis of differentiation. This differentiation in diet was unexpected because Draco is a well-known genus of ant specialists (Das 2010; Grismer 2011) and, at the locations we visited, ants were generally in high abundance implying food was unlikely to be a limiting resource for competition. Yet Inger’s (1983) data plainly show certain Draco species specialized on ants of certain size classes, and that these preferred size classes only differed between Draco species that overlapped in perch use. Further study of interspecific competition over food resources in Draco is clearly warranted, as well as determining the extent to which the observed differences in diet between species might occur by chance.
In general, however, the current data suggest Draco living in sympatric communities have potentially experienced interspecific competition over perch sites (and possibly food resources) and in ways that were analogous to the Caribbean Anolis lizards. While Draco tended to differ in perch choice in allopatry, these differences were accentuated in sympatry. This is expected from competition theory (May and MacArthur 1972; Pianka 1974). Species cannot overlap perfectly in resource use prior to contact otherwise interspecific competition in sympatry would restrict coexistence entirely (Grant 1972). It also suggests that species assortment, or the filtering of species through competitive exclusion, is less likely to be the primary force shaping community structure in Draco (at least for the sympatric community we studied). It is important to note, however, that there are a number of stringent criteria that need to be met before displacement can be confirmed over assortment (see Stuart and Losos 2013). While the degree of habitat partitioning exhibited by sympatric Draco might be consistent with the outcome of interspecific competition, these patterns need to be confirmed in more Draco communities. This would also provide the level of replication necessary for careful assessment of patterns against null models that assume partitioning arises by chance (e.g., Strong et al. 1979).
Assuming interspecific competition has occurred among sympatric Draco, whether it has subsequently resulted in character displacement remains to be tested. Our data did suggest that competition leading to shifts in perch size should result in character displacement in limb length. However, we were unable to confirm this directly because only one of the three species in our allopatric-sympatric comparison exhibited a shift in perch size—D. quinquefasciatus—and this was the one species for which we lacked morphological data in allopatry. The other two species displayed shifts in perch height, which would only impact morphology if it coincided with a change in perch size (e.g., the use of branches in the canopy) or perhaps gliding performance.
A final defining characteristic of the Greater Antillean Anolis is the ecomorph. We found Draco clustered into two distinct groups that shared key characteristics with the trunk-ground and trunk-crown Anolis ecomorphs. However, it would be premature to conclude that Anolis-like ecomorphs have evolved in Draco. The two trunk-ground and trunk-crown clusters in Draco generally corresponded to two monophyletic lineages (Fig. 1). This implies these two lineages differed consistently in habitat use and subsequent morphology. In general, Malaysian ‘trunk-grounds’ tended to use lower, wider perches and had longer hind limbs and tails, while Philippine ‘trunk-crowns’ tended to use higher, narrower perches and had shorter hind limbs and tails. There were two exceptions: D. quinquefasciatus was a Malaysian species that was more trunk-crown in behavior and appearance than its close relatives (especially in its use of narrow perches), and D. bimaculatus was a Philippine species that was more trunk-ground like than its close relatives. These two species might offer a tantalizing case of potential independent evolution, but an expanded investigation of the ecobehavior and morphology of the Draco genus as a whole is required if this conclusion is to carry any weight. Nevertheless, given that Draco generally used higher perches than Anolis trunk-ground species (Fig. 2b), it is notable that within the truncated range of perch heights used by Draco, there were still differences in perch height between the trunk-ground-like and the trunk-crown-like Draco that paralleled the direction (if not magnitude) of difference in perch height exhibited between the trunk-ground and trunk-crown Anolis.
Generally, though, the broad similarities in shared habitat use among Draco species within each lineage imply that niche conservatism has been important in shaping the evolution of Draco morphology (see Wiens et al. 2010), whereas this has not been the case for the Anolis (Losos et al. 2003). It should also be noted that the evolution of Anolis ecomorphs is largely an island-specific phenomenon, with mainland Anolis exhibiting few parallels in habitat use and morphology (Irschick et al. 1997; Pinto et al. 2008; Schaad and Poe 2010). Only three of the ten species of Draco we studied were island species (D. spilopterus, D. bimaculatus and D. reticulatus) with the remaining species found either on mainland Malaysia or Borneo. Perhaps the best opportunity for examining character displacement and ecomorph-like differentiation in Draco are the array of species distributed throughout the Philippine islands. Some islands were connected during the lower sea level of the late Pleistocene, but there were still many that remained isolated from one another during this period (Heaney 1986; McGuire and Alcala 2000). Furthermore, these island communities range from single species to as many as five sympatric species (McGuire and Alcala 2000) and should be priority targets for future research.
Caribbean Anolis have become a model group for the study of a range of fundamental questions in evolutionary ecology (reviewed by Losos 2009). We (this study) and others (Lazell 1992; Mori and Hikida 1994) have shown that the Southeast Asian Draco lizards, a group with gross differences in evolutionary history to the Anolis, exhibit important parallels in behavior, morphology, and community ecology. These parallels emphasize that convergent adaptive solutions in response to common selection pressures are possible among phylogenetically distant taxa, and reiterate that ecological axes dictating community structures in disparate groups are in fact predictable (Schoener 1974).
References
Alfaro ME, Bolnick DI, Wainwright PC (2005) Evolutionary consequences of many-to-one mapping of jaw morphology to mechanics in labrid fishes. Am Nat 165:E140–E154
Bickel R, Losos JB (2002) Patterns of morphological variation and correlates of habitat use in Chameleons. Biol J Linn Soc 76:91–103
Brown WL, Wilson EO (1956) Character displacement. Syst Zool 5:49–64
Cain ML, Bowman WD, Hacker SD (2008) Ecology. Sinauer, Sunderland
Campbell NA, Reece JB (2002) Biology, 6th edn. Cummings, Menlo Park
Collar DC, Schulte JA II, O’Meara BC, Losos JB (2010) Habitat use affects morphological diversification in dragon lizards. J Evol Biol 23:1033–1049
Das I (2010) A field guide to the reptiles of South-east Asia. New Holland, London
Dayan T, Simberloff D (2005) Ecological and community-wide character displacement: the next generation. Ecol Lett 8:875–894
Daza JD, Abdala V, Arias JS, Garcia-Lopez D, Ortiz P (2012) Cladistic analysis of Iguania and a fossil lizard from the late Pliocene of northwestern Argentina. J Herpetol 46:104–119
Grant PR (1972) Convergent and divergent character displacement. Biol J Linn Soc 4:39–68
Grismer LL (2011) Lizards of peninsular Malaysia, Singapore and their adjacent archipelagos. Chimaira, Frankfurt
Hairston NG (1957) Observations on the behaviour of Draco volans in the Philippines. Copeia 1957:262–265
Hansen TF (1997) Stabilizing selection and the comparative analysis of adaptation. Evolution 51:1341–1351
Hansen TF, Martins EP (1996) Translating between microevolutionary process and macroevolutionary patterns: the correlation structure of interspecific data. Evolution 50:1404–1417
Heaney LR (1986) Biogeography of mammals in SE Asia: estimates of rates of colonization, extinction and speciation. Biol J Linn Soc 28:127–165
Herrel A, Meyers JJ, Vanhooydonck B (2002) Relations between microhabitat use and limb shape in phrynosomatid lizards. Biol J Linn Soc 77:149–163
Higham TE, Davenport MS, Jayne BC (2001) Maneuvering in an arboreal habitat: the effects of turning angle on the locomotion of three sympatric ecomorphs of Anolis lizards. J Exp Biol 204:4141–4155
Inger RF (1983) Morphological and ecological variation in the flying lizards (genus Draco). Field Zool 18:1–33
Irschick DJ, Losos JB (1999) Do lizards avoid habitats in which performance is submaximal? The relationship between sprinting capabilities and structural habitat use in Caribbean anoles. Am Nat 154:293–305
Irschick DJ, Vitt LJ, Zani PA, Losos JB (1997) A comparison of evolutionary radiations in mainland and Caribbean Anolis lizards. Ecology 78:2191–2203
Jackman TR, Irschick DJ, de Queiroz K, Losos JB, Larson A (2002) Molecular phylogenetic perspective on evolution of lizards of the Anolis grahami series. J Exp Zool 294:1–16
Kuo C-Y, Gillis GB, Irschick DJ (2012) Take this broken tail and learn to jump: the ability to recover from reduced in-air stability in tailless green anole lizards [Anolis carolinensis (Squamata: Dactyloidae)]. Biol J Linn Soc 107:583–592
Lazell J (1992) New flying lizards and predictive biogeography of two Asian Archipelagos. Bull Mus Comp Zool 152:475–505
Lenormand T, Roze D, Rousset F (2009) Stochasticity in evolution. Trends Ecol Evol 24:157–165
Losos JB (1990a) Ecomorphology, performance capability, and scaling of West Indian Anolis lizards: an evolutionary analysis. Ecol Monogr 60:369–388
Losos JB (1990b) A phylogenetic analysis of character displacement in Caribbean Anolis lizards. Evolution 44:558–569
Losos JB (1992) A critical comparison of the taxon-cycle and character-displacement models of size evolution of Anolis lizards in the lesser Antilles. Copeia 1992:279–288
Losos JB (1994) Integrative approaches to evolutionary ecology: Anolis lizards as model systems. Annu Rev Ecol Syst 25:467–493
Losos JB (2009) Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. University of California Press, Berkeley
Losos JB, Sinervo B (1989) The effects of morphological and perch diameter on sprint performance of Anolis lizards. J Exp Biol 145:23–30
Losos JB, Marks JC, Schoener TW (1993) Habitat use and ecological interactions of an introduced and native species of Anolis lizard on Grand Cayman, with a review of the outcomes of anole introductions. Oecologia 95:525–532
Losos JB, Warheit KI, Schoener TW (1997) Adaptive differentiation following experimental island colonization in Anolis lizards. Nature 387:70–72
Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L (1998) Contingency and determinism in replicated adaptive radiations of island lizards. Science 279:2115–2118
Losos JB, Creer DA, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J (2000) Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54:301–305
Losos JB, Leal M, Glor RE, de Queiroz K, Hertz PE, Schettino LR, Lara AC, Jackman TR, Larson A (2003) Niche lability in the evolution of a Caribbean lizard community. Nature 424:542–545
Losos JB, Schoener TW, Spiller DA (2004) Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature 432:505–508
Losos JB, Schoener TW, Langerhans RB, Spiller DA (2006) Rapid temporal reversal in predator-driven natural selection. Science 314:1111
Maddison WP, Maddison DR (2010) Mesquite: a modular system for evolutionary analysis. http://mesquiteprogect.org
Martins EP (2004) COMPARE 4.6b: statistical analysis of comparative data. http://compare.bio.indiana.edu/
May RM, MacArthur RH (1972) Niche overlap as a function of environmental variability. Proc Natl Acad Sci USA 69:1109–1113
McGuire JA, Alcala AC (2000) A taxonomic revision of the flying lizards (Iguania: Agamidae: Draco) of the Philippine Islands, with a description of a new species. Herpetol Monogr 14:81–138
McGuire JA, Dudley R (2005) The cost of living large: comparative gliding performance in flying lizards (Agamidae: Draco). Am Nat 166:93–106
McGuire JA, Dudley R (2011) The biology of gliding in flying lizards (genus Draco) and their fossil and extant analogs. Integr Comp Biol 51:983–990
McGuire JA, Heang KB (2001) Phylogenetic systematics of Southeast Asian flying lizards (Iguania: Agamidae: Draco) as inferred from mitochondrial DNA sequence data. Biol J Linn Soc 72:203–229
Mori A, Hikida T (1994) Field observations on the social behavior of the flying lizard, Draco volans sumatranus, in Borneo. Copeia 1994:124–130
Mulcahy DG, Noonan BP, Moss T, Townsend TM, Reeder TW, Sites JW Jr, Wiens JJ (2012) Estimating divergence dates and evaluating dating methods using phylogenomic and mitochondrial data in squamate reptiles. Mol Phyl Evol 65:974–991
Nicholson KE, Glor RE, Kolbe JJ, Larson A, Hedges SB, Losos JB (2005) Mainland colonization by island lizards. J Biogeogr 32:929–938
Ord TJ (2008) Dawn and dusk ‘chorus’ in visually communicating Jamaican anole lizards. Am Nat 172:585–592
Ord TJ, Martins EP (2006) Tracing the origins of signal diversity in anole lizards: phylogenetic approaches to inferring the evolution of complex behaviour. Anim Behav 71:1411–1429
Ord TJ, Stamps JA (2009) Species identity cues in animal communication. Am Nat 174:585–593
Ord TJ, Charles GK, Hoffer RK (2011) The evolution of alternative adaptive strategies for effective communication in noisy environments. Am Nat 177:54–64
Pacala S, Roughgarden J (1982) Resource partitioning and interspecific competition in two two-species insular Anolis lizard communities. Science 217:444–446
Pacala S, Roughgarden J (1985) Population experiments with the Anolis lizards of St. Maarten and St. Eustatus. Ecology 66:129–141
Pfennig DW, Pfennig KS (2012) Evolution’s wedge: competition and the origins of diversity. University of California Press, Berkeley
Pianka ER (1974) Niche overlap and diffuse competition. Proc Natl Acad Sci USA 71:2141–2145
Pinto G, Mahler DL, Harmon LJ, Losos JB (2008) Testing the island effect in adaptive radiation: rates and patterns of morphological diversification in Caribbean and mainland Anolis lizards. Proc R Soc Lond B 275:2749–2757
Pyron RA, Burbrink FT (2014) Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol Letters 17:13–21
Rand AS (1964) Inverse relationship between temperature and shyness in the lizard Anolis lineatopus. Ecology 45:863–864
Rand AS (1967) Ecology and social organization in the iguanid lizard Anolis lineatopus. Proc US Natl Mus 122:1–77
Rummel J, Roughgarden J (1985) Effects of reduced perch-height separation on competition between two Anolis lizards. Ecology 66:430–444
Schaad EW, Poe S (2010) Patterns of ecomorphological convergence among mainland and island Anolis lizards. Biol J Linn Soc 101:852–859
Schluter D, McPhail JD (1992) Ecological character displacement and speciation in sticklebacks. Am Nat 140:85–108
Schoener TW (1968) The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology 49:704–726
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Schulte JA II, Losos JB, Cruz FB, Nunez H (2004) The relationship between morphology, escape behaviour and microhabitat occupation in the lizard clade Liolaemus (Iguanidae: Tropidurinae*: Liolaemini). J Evol Biol 17:408–420
Schwartz A, Henderson RW (1991) Amphibians and reptiles of the West Indies: descriptions, distributions and natural history. University of Florida Press, Gainesville
Shine R, Keogh S, Doughty P, Giragossyan H (1998) Costs of reproduction and the evolution of sexual dimorphism in a ‘flying lizard’ Draco melanopogon (Agamidae). J Zool 246:203–213
Strong DR Jr, Szyska LA, Simberloff DS (1979) Test of community-wide character displacement against null hypotheses. Evolution 33:897–913
Stuart YE, Losos JB (2013) Ecological character displacement: glass half full or half empty? Trends Ecol Evol 28:402–408
Toro E, Herrel A, Irschick DJ (2004) The evolution of jumping performance in Caribbean Anolis lizards: solutions to biomechanical trade-offs. Am Nat 163:844–856
Townsend TM, Mulcahy DG, Noonan BP, Sites JW Jr, Kuczynski CA, Wiens JJ, Reeder TW (2011) Phylogeny of iguanian lizards inferred from 29 nuclear loci, and a comparison of concatenated and species-tree approaches for an ancient, rapid radiation. Mol Phyl Evol 61:363–380
Vanhooydonck B, van Damme R (1999) Evolutionary relationships between body shape and habitat use in lacertid lizards. Evol Ecol Res 1:785–805
Warheit KI, Forman JD, Losos JB, Miles DB (1999) Morphological diversification and adaptive radiation: a comparison of two diverse lizard clades. Evolution 53:1226–1234
Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Davies TJ, Grytnes J-A, Harrison SP, Hawkins BA, Holt RD, McCain CM, Stephens PR (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13:1310–1324
Williams EE (1983) Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis. In: Huey RB, Pianka ER, Schoener TW (eds) Lizard ecology: studies of a model organism. Harvard University Press, Cambridge, pp 326–370
Wood TE, Burke JM, Rieseberg LH (2005) Parallel genotypic adaptation: when evolution repeats itself. Genetica 123:157–170
Zaaf A, van Damme R (2001) Limb proportions in climbing and ground-dwelling geckos (Lepidosauria, Gekkonidae): a phylogenetically informated analysis. Zoomorphology 121:45–53
Acknowledgments
We are especially grateful to Indraneil Das, Norhayati Ahmad, Arvin Diesmos and Pan Khang Aun for logistical support in the field and facilitating permits. Jim McGuire, Rafe Brown and Lee Grismer provided advice on potential field sites and focal species. We also thank Devi Stuart-Fox, Adnan Moussalli, Anna de Castro, Kenneth Calabia, Jia Cortes, Bea Javillonar and Saun Mabunay for assistance in the field, and Jonathan Losos for providing access to his data on Anolis and Dave Collar for providing his full supertree of the Agamidae. Jonathan Losos, Yoel Stuart, Jerry Husak, Jim McGuire, Luke Mahler, Lin Schwarzkopf and an anonymous reviewer also provided detailed comments on a previous version of this manuscript that greatly improved this article. This work was conducted under research permits from the Malaysian Economic Planning Unit, Sarawak State Planning Unit, Sarawak Forestry Department, Sarawak National Parks and Nature Reserves, and the Government of the Philippines through the Philippine Natural History Museum. This study was covered by the University of New South Wales (UNSW) Animal Care and Ethical Committee protocol no. 11/33b initially approved on 8 March 2011 and most recently reviewed on 28 February 2013. This work was financially supported by Evolution and Ecology Research Centre start-up funds and a UNSW SFRGP grant to T. J. O., a National Geographic Society grant to Devi Stuart-Fox, and an Australian Postgraduate Award and postgraduate research grant from the School of Biological, Earth and Environmental Sciences to D. A. K. All Draco data from this publication have been archived in the Dryad Digital Repository (http://www.dx.doi.org/10.5061/dryad.q1vf1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Lin Schwarzkopf.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ord, T.J., Klomp, D.A. Habitat partitioning and morphological differentiation: the Southeast Asian Draco lizards and Caribbean Anolis lizards compared. Oecologia 175, 651–666 (2014). https://doi.org/10.1007/s00442-014-2921-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2921-y