Abstract
Resource subsidies to opportunistic predators may alter natural predator–prey relationships and, in turn, have implications for how these predators affect co-occurring prey. To explore this idea, we compared the prey available to and eaten by a top canid predator, the Australian dingo (Canis lupus dingo), in areas with and without human-provided food. Overall, small mammals formed the majority of dingo prey, followed by reptiles and then invertebrates. Where human-provided food resources were available, dingoes ate them; 17 % of their diet comprised kitchen waste from a refuse facility. There was evidence of dietary preference for small mammals in areas where human-provided food was available. In more distant areas, by contrast, reptiles were the primary prey. The level of seasonal switching between small mammals and reptiles was also more pronounced in areas away from human-provided food. This reaffirmed concepts of prey switching but within a short, seasonal time frame. It also confirmed that the diet of dingoes is altered where human-provided food is available. We suggest that the availability of anthropogenic food to this species and other apex predators therefore has the potential to alter trophic cascades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Optimal foraging theories generally predict that animals should promote their fitness by foraging in ways that maximise their net rate of energy gain (Emlen 1966; MacArthur and Pianka 1966) or minimise the risk of starvation (Stephens and Krebs 1986). In its simplest form, maximising the rate of energy gain may be achieved by adopting efficient hunting behaviour or search strategies, exploiting food sources when they become available, and selecting ‘optimal’ prey (Stephens and Krebs 1986). By evaluating available prey, and selecting only the most profitable, predators’ diets should vary according to the quality and availability of alternative foods (Hughes 1993). However, selection can be influenced by prey preferences (Murdoch 1969), hunger, risk of injury (Hayward 2006) and natural fluctuations in prey availability (Dale et al. 1992). In human-modified environments, the dietary preferences of synanthropic animals may also be influenced by the availability of supplementary food (Fedriani et al. 2001). Understanding this dynamic is important, particularly when considering predators that are highly adaptable and whose foraging preferences and prey-switching behaviours might impact on the distributions, abundances and behaviour of co-occurring species.
One group of predators that frequently takes advantage of anthropogenic resources is free-roaming canids (Atwood et al. 2004; Gompper and Vanak 2008; Bino et al. 2010). From a management and conservation perspective, canids can have many detrimental effects where they occur at high densities, and can exert additional pressure on human industries and/or ecological communities (Sillero-Zubiri and Switzer 2004). For example, canids depredate livestock, damage infrastructure and spread disease (Baker et al. 2008). Ecologically, invasive or introduced canids can pose serious threats to native species if left uncontrolled, with many examples of extinctions occurring due to hyper-predation (Dickman 1996; Courchamp et al. 2000). Consequently, developing sound management and control programs for canids in human-dominated landscapes is a major priority (Fleming et al. 2001; Sillero-Zubiri and Switzer 2004).
The need to actively manage the impacts of canids has led to various levels of anthropogenic control throughout the ranges of some species. It has also led to the hypothesis that, where top-order or apex canids are removed, trophic cascades will be disrupted (Ripple et al. 2013). This is important to highlight because studies and reviews on trophic cascades have largely overlooked the role of human-resource subsidies (e.g. Estes et al. 2011), despite the obvious influence such resources could have on the abundance and distribution of top predators, including canids. Therefore, studies that provide insight into how human resources influence the ecology and behaviour of canids are critical to develop both practical management strategies and theoretical understanding about the roles of these predators in ecosystem functioning.
On the mainland of Australia, the dingo (Canis lupus dingo) has had a long association with humans since its introduction approximately 4,000 years ago (Smith and Litchfield 2009). The dingo is also often considered a top predator and potential trophic regulator (Johnson et al. 2007; Letnic and Koch 2010). Trophic regulation would occur if dingoes suppress the abundance or activity of the European red fox (Vulpes vulpes) and feral cat (Felis catus) sufficiently to enhance mammalian prey populations that are subject to suppression by them. As an opportunistic, generalist predator (Newsome et al. 1983; Corbett and Newsome 1987; Allen et al. 2012; Allen and Leung 2012), the dingo is an ideal candidate to investigate the effects of human-provided resources on prey populations because it is reasonable to assume that, where humans provide food, dingoes will exploit it. Therefore, given the dingo’s ecological flexibility, we investigated the dietary preferences of dingoes in the presence of anthropogenic resource subsidies. We considered that insights would be best achieved in a region of low productivity and where human-provided resources (food and water) were available in association with strongly localised human activity.
The Tanami Desert in central Australia provides such a site. There, commercial kitchen waste from two refuse facilities associated with mining operations potentially provides a substantial food supplement for local dingo populations. No other such refuse sites occur in the vast areas of the surrounding Tanami bioregion (~26,000 km2). We used this opportunity to quantify the use of human-provided resources by dingoes and the impacts that these resources might have on dingo diet selectivity.
First, we determined the overall dietary preferences of dingoes in the Tanami Desert. Due to above-average rainfall in the years preceding the study and the stimulatory effects of rain on numbers of native rodents (Dickman et al. 1999; Pavey et al. 2008), we hypothesised that small mammals would occur frequently in the diet of dingoes. Given also that reptiles were identified previously as major prey in the Tanami region (Paltridge 2002), and that seasonal changes in the availability of reptiles are dependent on temperature and thus highly predictable (Green and King 1978; Greer 1981; Paltridge 2002), we predicted further that they too would be consumed during the seasons in which they were most available.
Second, we determined the extent to which dingoes use anthropogenically derived foods by reviewing dietary preferences at and away from sites with human-provided food. We predicted that in areas with human-provided food, the dietary preferences of dingoes would be altered to include such food.
Third, we explored the relationship between the occurrence of prey in dingo diet and prey abundance in the wild. We predicted that dingoes would select prey according to availability and that preferences for natural prey would be independent of anthropogenic food availability.
Finally, we use the results to discuss the functional role of dingoes and opportunistic predators more generally where anthropogenic food subsidies are available.
Materials and methods
Study region and experimental design
The Tanami Desert (130°18′E, 20°30′S) is semi-arid and monsoonal with about 90 % of yearly rain received between November and April. Summers are very hot (mean maximum daily temperature up to 39 °C) and winters cold (mean minimum daily temperature down to 6.6 °C), with frosts occurring rarely. Rainfall is highly variable and was below average during this study at 219 and 263 mm in 2008 and 2009, respectively (Bureau of Meteorology 2010). In the preceding years, between August 2005 and July 2007, approximately 1,400 mm of rain fell. Our study was therefore conducted after a period of high rainfall during the low phase of a boom-bust cycle (Letnic and Dickman 2010).
At the time of the study, large quantities of food scraps from a commercial kitchen at the Granites mine were available daily to dingoes at a nearby waste facility (Newsome 2011). Food scraps were available also at a smaller waste facility at a second mine, DBS. In addition, calves and cattle (Bos spp.), carrion and small quantities of food from a domestic kitchen were available as food for dingoes at Tanami Downs cattle station. The Granites, DBS and Tanami Downs were therefore considered to be sites where human-resource subsidies were available, and seven other sites, with no human-resource subsidies, were stratified at increasing distances away (Fig. 1).
Overall dietary preferences
Dingo faeces were collected in April, August and November of 2008 and 2009 (i.e. from six trips). Due to the generally high rate of faecal deposition over small areas, it was possible to totally clear collection sites and then re-sample, allowing valid comparisons of diet to be made between trips. Because faeces were not cleared before the first trip, samples collected then that were decomposed and not intact were judged to be very old, and discarded. On following trips very old faeces were rarely found, which affirmed the categorisation used in the first trip.
Prior to identifying contents, faeces were oven dried at 100 °C, then washed in nylon bags to leave hard fragments of bones, hairs and human-derived rubbish. Dried remnants were placed on a sorting tray with quarter divisions, with one quarter split into 5 % divisions to allow for visual sorting. Collections of bones, hairs and other publicly available materials (Brunner and Coman 1974) were used for reference in the identification process. When material could not be identified to species level, it was grouped broadly or categorised as ‘unknown’. To separate feeding from grooming, faeces containing dingo bones were taken to indicate cannibalism and faeces with no dingo bones and fewer than ten dingo hairs to indicate grooming. Although arbitrary, this was likely a reasonable threshold: faeces usually contained fewer than ten hairs or far more than ten hairs plus dingo bone fragments. Because food from the refuse facility was mostly processed and lacked diagnostic bones or hairs, identification of items from this source in dingo faeces was rarely possible. However, the presence of plastic wrapping, foil, glass and/or paper in faeces confirmed the consumption of anthropogenic food.
To allow comparison with other studies, we calculated the ‘frequency of occurrence’ for individual prey species. For more detailed analysis, we modelled the odds ratio for the occurrence of species groups (Type) in each faecal sample. Types were based on taxonomic association; the primary categories we used were: birds, invertebrates, mammals, reptiles, rubbish and vegetation. To model the probability of occurrence of each type in faeces, we used a mixed effects logistic regression analysis. Denoting the probabilities of presence by p, the following model was fitted to the data:
Here logit(p) = log[p/(1 − p)] and the terms in bold were fitted as random effects. The model was fitted in R (R development Core Team 2009) using ASReml residual maximum likelihood for linear mixed models (Gilmour et al. 2002) in the package asreml (Butler 2009), with adjusted Wald statistics (Kenward and Roger 1997).
Use of anthropogenic food resources
To assess the significance of any trends, all Site × Trip combinations were subject to ordination using non-metric multi-dimensional scaling (nMDS), via function isoMDS in the library MASS (Venables and Ripley 2002) within R.
Faecal samples from the two mine sites were then grouped into one area category (Mine). The average probability of occurrence of each species group was then compared with those from Tanami Downs and with the remaining away sites.
To compare diet breadth between the three areas (above), the probabilities of occurrence of natural food items were estimated for each Area × Trip combination, and Brillouin’s index was calculated via repeated bootstrapping (Magurran 1988). To do this, we excluded rubbish and cattle from samples as these were the main forms of human-provided food. We plotted the slope of the average Brillouin index against sample size and assumed sample size sufficiency when plots plateaued. Differences between areas were assessed using two-way ANOVA.
Estimates of prey relative abundance
Prey abundance was sampled between October and November in 2008 and 2009 when both reptiles and small mammals were active. This timing allowed us to assess the abundance of these groups and compare their occurrence in faeces collected at the same times. For small prey species, we used data collected from 28 sites in 2008 and 27 sites in 2009, stratified within a sub-set of the study region (Fig. 1). The number of sites was proportional to the available land units (Domahidy 1990; Wilford and Butrovski 1999). At each site, 25 type-A Elliott traps (Elliott Scientific, Upwey, VIC) were placed 10 m apart in a straight line and baited with oats and peanut butter (Beer 1964). Four buried 20-L buckets (pitfall traps) with 10-m drift fences were also placed adjacent to the Elliott trap line, 50 m apart. All traps were opened for three consecutive nights, checked early each morning, and closed during the day (total 4,125 Elliott and 660 pitfall trap-nights over the 2 years). All trapped animals were identified to species, sexed and weighed.
The relative abundances of prey too large for our traps were estimated from footprints on tracking plots adjacent to roads, following Southgate et al. (2005). We set 300 × 200-m tracking plots at 22 of the trapping sites (above) and at an additional 35 locations (57 sites in total) (Fig. 1). To standardise effort, we searched each plot for 1 h and recorded the species and number of sets of footprints for each species detected.
Diet selectivity
To compare the availability of prey categories with their occurrence in dingo faeces, we examined the occurrence of all mammals (as well as the most common mammal species group: Muridae) and all reptiles (as well the most common reptile species groups: large skink, small skink and Varanidae) in traps and/or tracks across four sub-regions of the study region. The sub-regions were based on the minimum distance from the two main sites with human-provided food (DBS and the Granites), and were set with boundaries at 0–10, 10–25, 25–40 and >40 km from the nearest food subsidy. Because prey sampling did not extend to Tanami Downs or the Sangster’s site (Fig. 1), faecal samples from these two areas were excluded for all comparisons.
Logistic regression analysis, allowing for possible over-dispersion, was used to determine the incidence of each prey type in faeces within each sub-region. The model for the probability of finding the selected prey type in a faecal sample within a sub-region on a given trip, on the logit scale, is the sum of a baseline effect, an effect associated with the particular sub-region, and an effect associated with the particular trip of interest. That is,
Abundance data for the track plots and for the traps, corresponding to the number of observed tracks and observed trapped prey, respectively, were determined using log-linear Poisson modelling, allowing for possible over-dispersion and including an offset corresponding to the number (N) of track plots or the number of traps in the sub-region in a given year. For example, the expected number of tracks or traps within a sub-region on a particular trip was modelled as:
To enable graphical representation of dietary differences among sub-regions, using the logistic and log linear regression results (above), we calculated indices of prey selectivity. Ivlev’s electivity index (Ivlev 1961) has problems of non-linearity as it approaches the limiting values of +1 and −1, and is affected by small sample sizes (Strauss 1979; Lechowicz 1982). Therefore, we used Strauss’s linear index, L:
where r i is the relative occurrence of prey item i in a faeces sample and p i is the relative abundance of prey item i in the sub-region, both expressed as proportions (Strauss 1979). This index is linear across all values between +1 and −1. We used a randomisation procedure without replacement in the PopTools add-on for Microsoft Excel [i.e. the RESAMPLE procedure (Hood 2003)] followed by Monte-Carlo routines (10,000 iterations) to estimate 95 % confidence limits of the differences to make visual comparisons (Cumming 2009) of selectivity values (between −1 and +1) and also between sub-regions. Hence, negative values of L with confidence intervals not overlapping zero were taken to represent avoidance or inaccessibility of a particular prey item, zero indices to indicate no selectivity, and positive values with confidence intervals not overlapping zero to indicate prey preference.
Results
Overall diet and dietary preferences
Ingesta were identified from a total of 1,907 dingo faecal samples collected at ten sites in the Tanami region (Fig. 1). Mammals formed the majority of prey items by probability of occurrence, calculated as the unweighted average over sites and trips (63.1 %), followed by reptiles (51 %) and then invertebrates (32.8 %) (Table S1). The centralian blue-tongue lizard (Tiliqua multifasciata) occurred most frequently (22.5 % of faecal samples), followed by the desert mouse (Pseudomys desertor) (16.7 %). The probability of a faecal sample containing rubbish was relatively high (17.1 %) (Table S1).
When data were pooled and averaged over the 2 study years, mammal occurrence was consistently high (Fig. 2). However, there was evidence of seasonal change in predation on mammals and reptiles between trips. Reptile occurrence was markedly higher in faeces collected in April compared with those collected in cooler periods, particularly August, when reptiles are less active. Conversely, mammals were consumed at higher rates during the cooler months (Fig. 3).
Probability (0–1) of primary species groups occurring in the diet of dingoes in the Tanami Desert averaged over six trips. Means are shown ±95 % confidence intervals. n Number of faeces analysed per trip; for other abbreviations, see Fig. 2
Use of anthropogenic food resources
Analysis of prey group data via linear, mixed model, logistic regressions revealed strong Trip × Type interactions, averaged over sites. For the primary prey groups, for example, Wald tests showed that food types varied significantly between trips [F (30,205) = 2.97, P < 0.001]. To explore these trends, we subjected the predicted probabilities of occurrence for prey groups within each Site × Trip × Type to ordination via nMDS. The results suggest no clear pattern between dietary results for different trips within sites. However, there was clear separation between DBS and the Granites and all other sites, and secondly between Tanami Downs and all other sites, providing evidence for changes in the diet of dingoes where human activities occur (Fig. 4).
Pooling data into the three categories based on proximity to human-derived food subsidies revealed clear differences in the occurrence of food groups in faeces at mine sites (Fig. 5a) and Tanami Downs (Fig. 5b) as compared with all other areas (Fig. 5c). At the mine sites, mammals were consumed at higher rates than reptiles regardless of season (Fig. 5a). Away from the mine sites, reptiles were consumed at higher rates only at times when they were most active (Fig. 5c). At Tanami Downs, mammals (primarily cattle) dominated the diet of dingoes and there was little difference in consumption of other groups in different seasons (Fig. 5b).
Probability (0–1) of primary species groups occurring in the diet of dingoes at waste facilities around mine sites (a), at Tanami Downs (b), and away from mine sites (c) in the Tanami Desert averaged over six trips. Means are shown ±95 % confidence intervals. n Number of faeces analysed per trip; for abbreviations, see Fig. 2
The differences between areas with and without resource subsidies were reflected in dietary diversity, with significant differences occurring [F (2,10) = 11.44, P = 0.003] across the different areas. Mine site values (Brillouin index average = 2.51 ± 0.029) differed [F (1,5) = 17.34, P = 0.009] from those away from the mines (Brillouin index average = 2.83 ± 0.004) but not [F (1,5) = 4.23, P = 0.094] from those at Tanami Downs (Brillouin index average 2.06 ± 0.068).
Diet selectivity
In November 2008 and 2009 small mammals (358 and 1,033 captures, respectively) and reptiles (198 and 461 captures, respectively) formed the majority of trap captures. In 2008 and 2009, across the tracking plots, 94 and 69 tracks, respectively, were recorded for mammals, as were 96 and 89 tracks, respectively, for reptiles.
Based on Strauss’s index and corresponding 95 % confidence intervals, dingoes showed increasing selectivity for reptiles (and the large skink and Varanidae species groups) at distances up to 40 km from human-provided food (Fig. 6). In contrast, selectivity by dingoes for mammals (or the murid rodent and small skink species groups) remained unchanged with distance from human-provided food (Fig. 6).
Electivity index showing a greater preference by dingoes for reptiles (including large skinks and Varanidae) at increasing distances away from human-provided food (mine). There was no apparent difference in the preference for mammals (or murid rodents or small skinks) at increasing distances away from the mine
Discussion
Dingo faeces contained a wide variety of prey (45 species identified) and also significant amounts of human-derived rubbish that indicated scavenging at the waste facilities. This study is the first to report such a high occurrence of rubbish in the diet of dingoes across multiple years and seasons, although the Tanami Desert is not the only place where it is likely to occur. Despite this, wild prey still occurred in faeces from all collection areas, with mammals and then reptiles consumed most often (Fig. 2). The high proportion of mammals that we found is consistent with the results of other studies in arid Australia (Allen and Leung 2012). However, it was small mammals (<100 g) rather than large mammals that contributed to this trend. Further, the high occurrence of reptiles in the diet of dingoes in our study contrasts with general trends in dingo diet based on a review by Allen and Leung (2012) in which reptiles accounted for only 6 % of dingo diet.
In central Australia, Corbett and Newsome (1987) showed that dingo diet is related functionally to the relative abundances of major prey species. The main driver of small mammal abundance in this region is rainfall (Dickman et al. 1999). Hence, in good seasons with above-average rain, small mammals (e.g. rodents) can be important prey for dingoes (Pavey et al. 2008). This was demonstrated in our study, but the highest occurrence of small mammals was in winter (Fig. 3). Whilst interpretation of this trend would be assisted by estimates of the abundance of prey during winter, it can still be readily explained: reptile activity is temperature-dependent, with skinks and varanids being most active in summer between October and April (Green and King 1978; Greer 1981; Paltridge 2002).
Small mammals, by contrast, are available year round (Geiser 2004). Their high occurrence in dingo faeces during cooler periods, therefore, is not unexpected, especially as larger mammalian prey is relatively scarce and most reptiles are less active in winter (Green and King 1978; Christian et al. 1995). Nonetheless, the overall results suggest a dietary preference for reptiles over small mammals when both are active in summer, and thus reaffirm broad concepts of prey switching (Murdoch 1969, 1977; Oaten and Murdoch 1975). Additionally, our results provide a possible extension of the theory of alternation of predation proposed by Corbett and Newsome (1987) in that prey switching by dingoes may occur within a short, seasonal time frame. It also conforms with the general idea that animals will forage in ways that maximise their net rate of energy gain (Emlen 1966; MacArthur and Pianka 1966). High use of the waste facilities by dingoes was likely energetically optimal because there was a superabundant food supply. There was also no specific territorial defence of the focal resource by resident dingoes, allowing free access for transient individuals (Newsome et al. 2013a).
The occurrence of human-provided food resources at the mine sites was a major factor in our study. To interpret how this affected dingo prey selection, we explored the selection of natural prey in different areas and seasons. This was difficult because the presence of cattle at Tanami Downs could in itself affect prey availability, the prey eaten and the occurrence of prey in faeces. For example, localised water availability and piospheric grazing pressure by cattle may affect rodents and reptiles by reducing seed production, the availability of insects (Letnic et al. 2004) and herbage and cover (James et al. 1999). However, the impacts of mining operations are restricted to a much smaller area than those of free-ranging cattle, and because there are water sources throughout most of the study region (at bores, pipelines and old water tanks) and no major changes in habitat availability, the main difference at mine sites is the presence of waste food. This made comparisons between these and all other areas appropriate and compelling. From those comparisons, different dietary preferences were clearly evident at the mine sites, with mammals consumed at consistently high rates regardless of trip or season (Fig. 5a). This contrasts with sites away from the mine where dingoes displayed far greater levels of switching from small mammals to reptiles at times when reptiles were plentiful (Fig. 5c).
We explored the preference by dingoes for reptiles over mammals in relation to their availability during a subset of the study period and found that reptiles were selected more often away from human-provided food than close to it (Fig. 6). More sampling at different times would provide clearer insight; however, as sampling was conducted when both small mammals and reptiles are active (November), it was an ideal time to explore selectivity patterns. As the only difference between areas in the study region is the availability of human-provided food, our results suggest that the dietary preferences of dingoes differ when human-provided food is available. There are a number of ways to interpret this trend, but the most likely explanation is that the daily energetic requirements of dingoes around mine sites were met primarily by waste scavenging, allowing them to hunt small mammals (which may be more easily caught) rather than reptiles that are larger in size and more ‘optimal’ in that they return more energy per gram (Paltridge 2002). The overall choice within the small-mammal category also reflects preference for species that provide a trade-off between size and relative ease of capture. For example, the most readily eaten species, Pseudomys desertor, is relatively large (25 g) compared with the sandy inland mouse, Pseudomys hermannsburgensis (12 g) but likely much slower than the similar-sized spinifex hopping mouse, Notomys alexis (30 g) which is bipedal when moving fast (Dickman et al. 1999).
The hypothesis that human-provided foods alter the diet of dingoes is strengthened by the finding that dietary breadth was low around the mine sites. There were also clear differences in the occurrence of prey species in faeces collected from areas with human-provided food (mine sites and the pastoral station) compared with those well away from intensive human activity (Figs. 4, 5). For example, at mine sites, rubbish was consistently present in the diet with occurrence >60 % across all trips (Fig. 5a). Even higher occurrences of cattle (>90 %) were observed at Tanami Downs (Fig. 5b). Although the actual amount of human-provided food consumed around mine sites could not be fully quantified, large quantities of food were constantly available (Newsome 2011). Intensive foraging by dingoes living around the mine was corroborated also by frequent visual observations of them at the refuse tip at all times of the day, by mark-recapture data and by extensive radio-tracking of dingoes living around the mine (Newsome et al. 2013a, b, c). On Tanami Downs, cattle were the main prey of dingoes, but it is not known whether this was from direct predation or scavenging on carcasses.
There is global recognition that predators can have important roles in structuring ecosystems to benefit biodiversity (Ritchie and Johnson 2009; Terborgh and Estes 2010; Estes et al. 2011). For example, trophic regulation of ecosystems by dingoes may provide positive benefits to some native species (Johnson et al. 2007; Glen et al. 2007; Johnson and VanDerWal 2009; Letnic et al. 2009a, b, 2011; Letnic and Koch 2010), with benefits accruing from dingoes suppressing smaller introduced predators such as the European red fox and possibly also the feral cat. However, if access to human-provided food can influence dingo dietary preferences, then this, in association with other behavioural changes (Newsome et al. 2013a, b, c), could alter the roles that dingoes play in structuring ecosystems. Given that dingoes were considered formerly to be naturally sparse in the Tanami Desert (Breckwoldt 1988; Fleming et al. 2001), the level at which dingo populations are now supported by human activity could have important effects on populations of co-occurring predators and prey.
It is clear that readily available human-provided food assists dingoes in conserving energy that would otherwise be expended searching for and killing prey. However, predation pressure may not always be reduced by supplementing the diet of dingoes because added food also assists in maintaining population growth (Newsome et al. 2013c). Additionally, not all dingoes in a population are likely to live in close proximity to, and only consume, human-provided food even if it is readily available (Newsome et al. 2013a). Thus, in areas where natural resources are scarce and dingoes uncommon, the provision of human-provided food may maintain and increase an otherwise unsustainable dingo population. By mere presence alone, this could provide a net benefit for small prey, if suppression of mesopredators was mediated (Glen and Dickman 2005; Ritchie and Johnson 2009; Letnic et al. 2011) and dingoes did not prey substantially upon other populations (Allen and Fleming 2012).
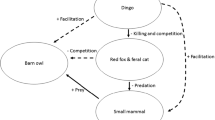
Alternatively, if dingoes are overabundant and hyper-predation occurs (Smith and Quin 1996; Courchamp et al. 2000), preferred prey items could be susceptible to decline. There is also a risk that if human-provided food resources are removed, and artificial water sources remain accessible, dingoes may switch in the short term to alternative prey until a new carrying capacity is attained (Brawata and Neeman 2011). To highlight these potential consequences we provide a conceptual model of trophic regulation when humans provide supplementary food and water to dingoes (Fig. 7).
Conceptual trophic cascade illustrating how the provision of food and water could affect predators and prey. Solid arrows are direct effects and broken arrows are indirect effects. The overall effect is either positive (+), negative (−) or variable (+/−) depending on the local circumstances. For example, if humans increase dingo abundance by providing supplementary food and water, then this, in association with other behavioural changes, could positively or negatively affect lower-order predators (e.g. foxes and cats) depending on the level of suppression by dingoes through competition, exclusion or direct predation. Hyper-predation by dingoes could negatively affect preferred prey (e.g. small mammals) and potentially also supplementary prey (e.g. reptiles). In areas where large or introduced herbivores are present they could be expected to be another link in the trophic web, particularly if predation of herbivores by dingoes reduces herbivory and plant biomass/understory complexity increases. a This study, b Newsome et al. (2014), c Newsome et al. (2013b), d Newsome et al. (2013a), e Newsome et al. (2013c)
In other parts of the world, human-induced perturbations influence the spatial distribution, density and diel activity patterns of coyotes (Fedriani et al. 2001; Atwood et al. 2004; Randa and Yunger 2006; Gehrt et al. 2009) and free-living stray or feral cats (Izawa et al. 1982; Izawa 1983; Denny et al. 2002). Rapid behavioural and ecological changes have also been documented in black bear (Ursus americanus) (Beckmann and Berger 2003) and raccoon (Procyon lotor) (Bozek et al. 2007) populations in areas where readily accessible human-provided garbage exists. Perhaps most relevant to the present study is the work of Bino et al. (2010) who demonstrated that home-range sizes and density of the European red fox are related directly to the availability of anthropogenic food resources around small villages. Such marked changes in behaviour and abundance of generalist predators could have numerous localised but unknown effects on ecological communities. To understand the functional role of dingoes and other top predators, it is therefore imperative to consider resource abundance and dispersion, especially in areas where human-provided food is abundant.
References
Allen BL, Fleming PJS (2012) Reintroducing the dingo: the risk of dingo predation to threatened vertebrates of western New South Wales. Wildl Res 39:35–50
Allen BL, Leung LK-P (2012) Assessing predation risk to threatened fauna from their prevalence in predator scats: dingoes and rodents in arid Australia. PLoS ONE 7:e36426
Allen L, Goullet M, Palmer R (2012) The diet of the dingo (Canis lupus dingo and hybrids) in north-eastern Australia: a supplement to the paper of Brook and Kutt (2011). Rangel J 34:211–217
Atwood TC, Weeks HP, Gehring TM (2004) Spatial ecology of coyotes along a suburban-to-rural gradient. J Wildl Manage 68:1000–1009
Baker PJ, Boitani L, Harris S et al (2008) Terrestrial carnivores and human food production: impact and management. Mammal Rev 38:123–166
Beckmann JP, Berger J (2003) Rapid ecological and behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. J Zool 261:207–212
Beer JR (1964) Bait preferences of some small mammals. J Mammal 45:632–634
Bino G, Dolev A, Yosha D et al (2010) Abrupt spatial and numerical responses of overabundant foxes to a reduction in anthropogenic resources. J Appl Ecol 47:1262–1271
Bozek CK, Prange S, Gehrt SD (2007) The influence of anthropogenic resources on multi-scale habitat selection by raccoons. Urban Ecosyst 10:413–425
Brawata RL, Neeman T (2011) Is water the key? Dingo management, intraguild interactions and predator distribution around water points in arid Australia. Wildl Res 38:426–436
Breckwoldt R (1988) The dingo: a very elegant animal. Angus & Robertson, North Ryde
Brunner H, Coman BJ (1974) The identification of mammalian hair. Inkata, Melbourne
Bureau of Meteorology (2010) Rabbit Flat, Northern Territory September 2010 daily weather observations
Butler D (2009) asreml: asreml() fits the linear mixed model. R package version 3.00
Christian KA, Corbett LK, Green B, Weavers BW (1995) Seasonal activity and energetics of two species of varanid lizards in tropical Australia. Oecologia 103:349–357
Corbett LK, Newsome AE (1987) The feeding ecology of the dingo. III. Dietary relationships with widely fluctuating prey populations in arid Australia: an hypothesis of alternation of predation. Oecologia 74:215–227
Courchamp F, Langlais M, Sugihara G (2000) Rabbits killing birds: modelling the hyperpredation process. J Anim Ecol 69:154–164
Cumming G (2009) Inference by eye: reading the overlap of independent confidence intervals. Stat Med 28:205–220
Dale BW, Adams LG, Bowyer RT (1992) Functional response of wolves preying on barren-ground caribou in a multiple-prey ecosystem. J Anim Ecol 63:644–652
Denny E, Yaklovlevich P, Eldridge MDB, Dickman CR (2002) Social and genetic analysis of a population of free-living cats (Felis catus L.) exploiting a resource-rich habitat. Wildl Res 29:405–413
Dickman CR (1996) Impact of exotic generalist predators on the native fauna of Australia. Wildl Biol 2:185–195
Dickman CR, Mahon PS, Masters P, Gibson DF (1999) Long-term dynamics of rodent populations in arid Australia: the influence of rainfall. Wildl Res 26:389–403
Domahidy G (1990) Hydrogeology of the granites—Tanami Mining Region. Power and Water Authority, Northern Territory
Emlen JM (1966) The role of time and energy in food preference. Am Nat 100:611–617
Estes JA, Terborgh J, Brashares JS et al (2011) Trophic downgrading of planet earth. Science 333:301–306
Fedriani JM, Fuller TK, Sauvajot RM (2001) Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography 24:325–331
Fleming PJS, Corbett LK, Harden R, Thomson P (2001) Managing the impacts of dingoes and other wild dogs. Bureau of Rural Sciences, Canberra
Gehrt SD, Anchor C, White LA (2009) Home range and landscape use of coyotes in a metropolitan landscape: conflict or coexistence? J Mammal 90:1045–1057
Geiser F (2004) The role of torpor in the life of Australian arid zone mammals. Aust Mammal 26:125–134
Gilmour AR, Gogel BJ, Cullis BR et al (2002) ASReml user guide release 1.0. VSN International, Hemel Hempstead, UK
Glen AS, Dickman CR (2005) Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol Rev 80:387–401
Glen AS, Dickman CR, Soulé ME, Mackey BG (2007) Evaluating the role of the dingo as a trophic regulator in Australian ecosystems. Austral Ecol 32:492–501
Gompper ME, Vanak AT (2008) Subsidized predators, landscapes of fear and disarticulated carnivore communities. Anim Conserv 11:13–14
Green B, King D (1978) Home range and activity patterns of the sand goanna, Varanus gouldii (Reptilia: Varanidae). Aust Wildl Res 5:417–424
Greer AE (1981) The biology and evolution of Australian lizards. Surrey Beatty, Sydney
Hayward MW (2006) Prey preferences of the spotted hyaena (Crocuta crocuta) and degree of dietary overlap with the lion (Panthera leo). J Zool 270:606–614
Hood G (2003) PopTools: software for analysis of ecological models. CSIRO, Canberra
Hughes R (1993) Diet selection: an interdisciplinary approach to foraging behaviour. Blackwell, Oxford
Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University, New Haven
Izawa M (1983) Daily activities of the feral cat Felis catus Linn. J Mammal Soc Jpn 9:219–227
Izawa M, Doi T, Ono Y (1982) Grouping patterns of feral cats (Felis catus) living on a small island in Japan. Jpn J Ecol 32:373–382
James CD, Landsberg J, Morton SR (1999) Provision of watering points in the Australian arid zone: a review of effects on biota. J Arid Environ 41:87–121
Johnson CN, VanDerWal J (2009) Evidence that dingoes limit abundance of a mesopredator in eastern Australian forests. J Appl Ecol 46:641–646
Johnson CN, Isaac JL, Fisher DO (2007) Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc R Soc B Biol Sci 274:341–346
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997
Lechowicz MJ (1982) The sampling characteristics of electivity indices. Oecologia 52:22–30
Letnic M, Dickman CR (2010) Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biol Rev 85:501–521
Letnic M, Koch F (2010) Are dingoes a trophic regulator in arid Australia? A comparison of mammal communities on either side of the dingo fence. Austral Ecol 35:167–175
Letnic M, Dickman CR, Tischler M et al (2004) The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. J Arid Environ 59:85–114
Letnic M, Crowther MS, Koch F (2009a) Does a top-predator provide an endangered rodent with refuge from an invasive mesopredator? Anim Conserv 12:302–312
Letnic M, Koch F, Gordon C et al (2009b) Keystone effects of an alien top-predator stem extinctions of native mammals. Proc R Soc B Biol Sci 276:3249–3256
Letnic M, Greenville A, Denny E et al (2011) Does a top predator suppress the abundance of an invasive mesopredator at a continental scale? Glob Ecol Biogeogr 20:343–353
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–609
Magurran A (1988) Ecological diversity and its measurement. University Press, Cambridge
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol Monogr 39:335–354
Murdoch WW (1977) Stabilizing effects of spatial heterogeneity in predator prey systems. Theor Popul Biol 11:252–273
Newsome TM (2011) The ecology of the dingo (Canis lupus dingo) in the Tanami Desert in relation to human resource subsidies. PhD thesis, the University of Sydney, Sydney
Newsome AE, Catling PC, Corbett LK (1983) The feeding ecology of the dingo. II. Dietary and numerical relationships with fluctuating prey populations in south-eastern Australia. Aust J Ecol 8:345–366
Newsome TM, Ballard G-A, Dickman CR et al (2013a) Home range, activity and sociality of a top predator, the dingo: a test of the resource dispersion hypothesis. Ecography 36:914–925
Newsome TM, Ballard G-A, Dickman CR et al (2013b) Anthropogenic resource subsidies determine space use by Australian arid zone dingoes: an improved resource selection modelling approach. PLoS ONE 8:e63931
Newsome TM, Stephens D, Ballard G-A et al (2013c) Genetic profile of dingoes (Canis lupus dingo) and free-roaming domestic dogs (C. l. familiaris) in the Tanami Desert, Australia. Wildl Res 40:196–206
Newsome TM, Ballard G-A, Crowther MS et al (2014) Dietary niche overlap of free-roaming dingoes and domestic dogs: the role of human-provided food. J Mammal 95 (in press). doi:10.1644/13-MAMM-A-145.1
Oaten A, Murdoch WW (1975) Switching, functional response, and stability in predator–prey systems. Am Nat 109:299–318
Paltridge R (2002) The diets of cats, foxes and dingoes in relation to prey availability in the Tanami Desert, Northern Territory. Wildl Res 29:389–403
Pavey CR, Eldridge SR, Heywood M (2008) Population dynamics and prey selection of native and introduced predators during a rodent outbreak in arid Australia. J Mammal 89:674–683
R development Core Team (2009) R: a language and environment for statistical computing. Version 2.92. R Foundation for Statistical Computing, Vienna
Randa LA, Yunger JA (2006) Carnivore occurrence along an urban-rural gradient: a landscape-level analysis. J Mammal 87:1154–1164
Ripple WJ, Wirsing AJ, Wilmers CC, Letnic M (2013) Widespread mesopredator effects after wolf extirpation. Biol Conserv 160:70–79
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Sillero-Zubiri C, Switzer D (2004) Management of wild canids in human dominated landscapes. In: Sillero-Zubiri C, Hoffmann M, Macdonald DW (eds) Canids foxes wolves jackals dogs status Survey Conservation Action Plan. IUCNSSC Canid Species Group, Information Press, Oxford, pp 257–266
Smith BP, Litchfield CA (2009) A review of the relationship between indigenous Australians, dingoes (Canis dingo) and domestic dogs (Canis familiaris). Anthrozoos 22:111–128
Smith AP, Quin DG (1996) Patterns and causes of extinction and decline in Australian conilurine rodents. Biol Conserv 77:243–267
Southgate RI, Paltridge RM, Masters P, Nano C (2005) An evaluation of transect, plot and aerial survey techniques to monitor the spatial pattern and status of the bilby (Macrotis lagotis) in the Tanami Desert. Wildl Res 32:43–52
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Strauss RE (1979) Reliability estimates for Ivlev’s Electivity Index, the forage ratio, and a proposed linear index of food selection. Trans Am Fish Soc 108:344–352
Terborgh J, Estes JA (2010) Trophic cascades: predator, prey, and the changing dynamics of nature. Island Press, Washington, DC
Venables W, Ripley B (2002) Modern applied statistics with S, 4th edn. Springer, New York
Wilford J, Butrovski D (1999) Tanami granites Regolith-landforms map series; (1:100,000 map scale). CRC, LEME, CSIRO, Wembley
Acknowledgments
This research was undertaken under the Animal Care and Ethics Authority O06/009 from Orange Animal Ethics Committee, clearance number A05020 from Charles Darwin University Animal Ethics Committee and permit number 33607 from Northern Territory Parks and Wildlife. The Central Land Council provided permit number CD004 for conducting research on Aboriginal Land. We adhered to all conditions. Work was funded and supported by Newmont Tanami Operations, the Invasive Animals Co-operative Research Centre and the Central Land Council. Many members of the Warlpiri community assisted in the field. Particular thanks to Shaun Wilson who assisted on most field trips. Prey availability data were collected as part of the Regional Biodiversity Monitoring Project funded by Newmont Tanami Operations. Many people assisted with that work, in particular those from the Central Land Council and Low Ecological Services. Rachel Paltridge and Steve Eldridge most thoroughly completed the identification of species in faecal samples collected on the first trip. Bill Low, Al Glen and Alex Diment provided valuable comments on early drafts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christopher N Johnson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Newsome, T.M., Ballard, GA., Fleming, P.J.S. et al. Human-resource subsidies alter the dietary preferences of a mammalian top predator. Oecologia 175, 139–150 (2014). https://doi.org/10.1007/s00442-014-2889-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2889-7