Abstract
The relationship between predators and prey is thought to change due to habitat loss and fragmentation, but patterns regarding the direction of the effect are lacking. The common prediction is that specialized predators, often more dependent on a certain habitat type, should be more vulnerable to habitat loss compared to generalist predators, but actual fragmentation effects are unknown. If a predator is small and vulnerable to predation by other larger predators through intra-guild predation, habitat fragmentation will similarly affect both the prey and the small predator. In this case, the predator is predicted to behave similarly to the prey and avoid open and risky areas. We studied a specialist predator’s, the least weasel, Mustela nivalis nivalis, spacing behavior and hunting efficiency on bank voles, Myodes glareolus, in an experimentally fragmented habitat. The habitat consisted of either one large habitat patch (non-fragmented) or four small habitat patches (fragmented) with the same total area. The study was replicated in summer and autumn during a year with high avian predation risk for both voles and weasels. As predicted, weasels under radio-surveillance killed more voles in the non-fragmented habitat which also provided cover from avian predators during their prey search. However, this was only during autumn, when the killing rate was also generally high due to cold weather. The movement areas were the same for both sexes and both fragmentation treatments, but weasels of both sexes were more prone to take risks in crossing the open matrix in the fragmented treatment. Our results support the hypothesis that habitat fragmentation may increase the persistence of specialist predator and prey populations if predators are limited in the same habitat as their prey and they share the same risk from avian predation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and fragmentation increases the proportion of inhospitable and risky area in the landscape, increases the ratio of edges to interior habitats, and decreases habitat connectivity (reviewed by Saunders et al. 1991). Habitat fragmentation is predicted to change individual behavior and species interactions (Lindenmayer and Fischer 2006). Specifically, habitat fragmentation is thought to change predator–prey relationships, prey searching by the predator, and predator avoidance behaviors of prey individuals (Gorini et al. 2012). However, the directions and effects of these changes are still unclear (Ryall and Fahrig 2006). The common prediction is that the more specialized species, both in terms of habitat use and food requirements, suffer more from habitat loss. However, mere habitat fragmentation effects per se affecting predator–prey relationships are not clear (Fahrig 2003).
A special case of predator–prey relationship in a fragmented landscape exists between species of about the same size where the predator is also prey to other, often larger predators. This kind of predator–prey interaction between two or several predators is called intraguild predation (Hoset et al. 2009). Our study system provides an example of an intraguild predator–prey system in the form of small rodents, boreal voles as prey, small mustelids as mesopredators and avian predators, raptors and owls at the top (Sundell and Ylönen 2008; Hoset et al. 2009). This relationship should strongly be affected by habitat configuration and protectiveness, as both small mammal prey and small mustelid predators are ground-dwelling animals sharing the same risks of owl and raptor predation in an open matrix. This could mean that, in a fragmented landscape, the isolated small patches are less likely to be reached by the weasels because they may be reluctant to cross the open matrix with higher predation risk. In this case, some patches in the fragmented habitat that provide cover and food can serve as a kind of refuge for at least a few of the prey inhabitants of the patch (Ylönen et al. 2003), if the predator neglects to visit the patch. From the predator point of view, non-fragmented habitat is safe and easy for searching and hunting due to the shelter that vegetation provides compared to fragmented habitat where there is a need to cross the inter-patch areas.
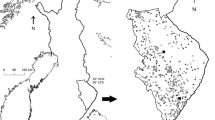
We conducted an experiment on the effects of habitat fragmentation on behavior and hunting efficiency of the specialist vole predator, the least weasel (Mustela nivalis nivalis) in a large experimental enclosure system (Haapakoski and Ylönen 2010). Our 2,500-m2 enclosures provide a configuration with the same proportion of covered and open habitat with protective tall-grass habitat for both the prey voles and weasel predators, but differ in fragmentation type (Fig. 1). Populations living in either one large continuous patch or four small separate patches, and thus experiencing different risk levels, were exposed to 2-day radio-collared weasel hunting periods. Our experiment consists of two major replicates with 12 populations in summer and in autumn. To our knowledge, our system that employs large outdoor enclosures with different habitat configurations for experimentation with both free-ranging mammalian prey and predators, is a unique one in studies of predator–prey relationships in vertebrates. With larger carnivores than small mustelids, often the only experimental approach is predator removal and subsequent comparison of predator-free/low predation areas to high predation areas (see Salo et al. 2010 for review).
Ryall and Fahrig (2006) reviewed existing population scale modeling literature of predator–prey interaction in fragmented landscape. Based on their review, specialist predators restricted to the same habitat as their prey species can increase or decrease prey extinction risk depending on whether the prey is dispersing more from patches also occupied by specialist predators than prey-only patches. If the predator enters a small patch which contains only a few prey individuals, the prey is more prone to disperse, even with their own risk of crossing the matrix, than from a patch without a sudden increase in predation risk. Thus, the key mechanism responsible for decreasing the predator and prey extinction risk in fragmented habitat is the rapid dilution of the predator food resource leading to risky movements to seek for new resources (Prakash and de Roos 2002; Dalkvist et al. 2011; Ryall and Fahrig 2006). If prey dispersal from the predator patch is low, predators can easily deplete the food resource which inevitably decreases persistence of both predator and prey populations in a fragmented landscape (Nakagiri et al. 2001; Ryall and Fahrig 2006).
We predicted that our experimental habitat fragmentation affects weasel hunting behavior so that the weasels prefer the covered habitat as long as there is prey available. As the prey voles also prefer the same habitat, this means that the weasel would kill more voles in the non-fragmented habitat where it can move and hunt without exposure to avian predation, as suggested in a model simulation by Dalkvist et al. (2011). On the other hand, this means that survival of prey voles in fragmented habitat could be improved by fragmentation if the weasel avoids crossing the open matrix between patches due to avian predation risk, as suggested by Prakash and de Roos (2002). Due to energy demands of the predator, we predicted a more intensive hunting effort by weasels during the colder autumn period. Due to colder temperatures, the number of prey killed doubles during autumn (Jedrzejewska and Jedrzejewski 1989), a season during which weasels are known to kill more for hoarding.
Materials and methods
Study enclosures and habitat manipulation
All the experiments were carried out in the Sukeva enclosure area (62°N, 26°E) near Konnevesi Research Station in Central Finland. Six 50 m × 50 m (=2,500 m2) enclosures made of galvanized steel sheet were used. Enclosures were built on an old bog meadow. Before erecting the fences, the field was ploughed, smoothed, and tall-grass meadow seed mixture sown to make the vegetation homogenous and equal between enclosures. The fence reached 0.5 m below ground and 0.75 m above ground and prevented voles and weasels from escaping the enclosures. Avian predators had free access to the enclosures as there was no netting covering the enclosures.
All enclosures consisted of two habitats: protective intact tall-grass and open matrix almost totally lacking vegetation. This was done to manipulate the area representing safe and risky movement or foraging areas for ground-dwelling animals like the voles and weasels in our study. The matrix area with high avian predation risk was created by mowing the vegetation in the enclosures in two configurations so that the protective tall-grass habitat formed one (non-fragmented) or four habitat (fragmented) patches of equal total area of 900 m2 (Fig. 1). The matrix area was a total of 1,400 m2, wherein vegetation was kept absent or low by frequent mowing to the ground level. Subsequent vegetation growth was prevented with the herbicide Round-Up®. The matrix area was considered risky and inhospitable for both voles and weasels as, practically, it did not provide any food or shelter against avian predators.
Study species and experimental animals
We used four females and four males in each experimental enclosure, and thus had an equal sex ratio as reported for natural populations of bank vole during the summer (Bujalska 1986). Naturally, due to our habitat configuration, we can expect a more even distribution of females within the fragmented enclosures but we also know that four females are able to breed in a habitat area of 900 m2 (Haapakoski and Ylönen 2010, 2013). Occupancy of breeding territory in females and breeding condition affects spacing and movements of males (Ims 1987). Experimental bank voles were born during spring (for the 1st replicate) and summer (for the 2nd replicate) in enclosures or in the laboratory at the Konnevesi Research Station. Prior to the experiments, all animals were housed singly in standard laboratory cages of (43 × 26 × 15 cm3), or with same sex individuals if they were young immature voles. Wood shavings were used to keep the cages dry, hay was provided as bedding material, rodent food pellets and fresh water were available ad lib. Light:dark period was 18:6 h, which corresponds roughly to the natural light:dark regime during the experimental period in summer. Voles for each experiment were chosen so that there were no relatives in the same enclosure and age and weight distribution of the animals were always as similar as possible in every enclosure. Animals were marked individually with ear tags.
We used 24 weasels (12 females and 12 males) which were either trapped from the wild (n = 15) or were captive born (n = 9). Six females and six males were used per season so that half of the individuals from each sex were released into the fragmented treatment and the other half into the non-fragmented treatment. The mean weight of weasel males was 61.7 g (±1.6 SE, n = 12) and that of females 46.6 g (±2.6 SE, n = 12). Prior to the experiments, weasels were housed singly in 60 × 80 × 60 cm3 cages in an outdoor shelter at the Konnevesi Research Station. Each cage had a nest box and bedding was provided in the form of wood shavings and hay. Weasels were radio-collared 1 day before release into enclosures to get used to the 1.8 g radio-collars (Biotrack, Wareham, UK). These were lighter than normal weasel collars and were originally made for voles and therefore affected weasel movements as little as possible.
We assumed that weasels and voles would avoid the open matrix area independent of the presence/absence of predators, as weasels and voles cannot purely rely on sightings of avian predators or other cues in their decision making. Normally, these small prey species live cryptically under vegetation cover from which sightings are almost impossible to make. In addition, avian predators have large territories and they can quickly arrive in the study area having been only recently absent. However, we have many anecdotal observations of avian predators during weasel radio-tracking, although they were clearly avoiding humans in the enclosure area and left the area after our arrival. Avian predators, such as long-eared owls (Asio otus, one sighting in summer and autumn), common buzzards (Buteo buteo, two observations in autumn), hen harrier (Circus cyaneus, one sighting in autumn) and kestrels (Falco tinnunculus, resident throughout the summer) were observed in the study area. These species were also regionally numerous during the year of the experiment (Valkama et al. 2011). Also, owls like ural owls (Strix uralensis) and pygmy owls (Claucidium passerinum) were resident in the area throughout the year, but were not seen during the study period. Vole density in the forests around the enclosure area was extremely high in spring and early summer and crashed towards autumn (Kallio et al. 2009). Avian predators rapidly track high vole densities (Korpimäki 1994) and high vole densities are known to lead to high avian predator nesting success (Sundell et al. 2004). Strength of avian predation pressure between summer and autumn was measured by the number of voles replaced before release of the weasels (for more in detail, see below).
Study replicates
We conducted our experiment in four similar replicates in 2006. The first two were conducted under favorable summer conditions during mid-summer (summer = start dates 5 and 17 July) and the second two in autumn (autumn = start dates 4 September and 1 October). During the summer replicates, the average day temperature was around +20° C and nights were mild. The autumn replicate day temperatures were +10° C or below and nights were cold, often with temperatures below zero.
On day 1 of the each experimental replication, four female and four male bank voles were released in female–male pairs into random corners of the habitat patch in each enclosure with different habitat configurations (Fig. 1) to get voles equally distributed. Between days 3 and 5, we conducted live-trapping to see which voles were alive. In the evening of day 3, we set multiple-capture traps (Ugglan special; Grahnab, Hillerstorp, Sweden) and checked them three times every 12 h, and traps were left unset in the morning of day 5. Voles that were not captured during this period were regarded to be dead, most probably due to avian predation, and they were replaced with new individuals with the same sex and weight as the original ones.
One weasel per enclosure was released to a random corner of the enclosure in the afternoon of day 5. Radio-tracking of the weasel started approximately 1 h after release. Weasels were located once per hour by triangulation during the next 48 h. After 48 h in the enclosure, we located the weasel the last time and surrounded its location with traps, and within a short time the weasels from all enclosures were removed and all traps were set for trapping voles to estimate their survival rate in different fragmentation treatments across seasons. We continued vole trapping until there were two consecutive trap checks without any voles in the enclosure. In the final analyses, we used data of 21 out of 24 enclosures as 3 (1 fragmented and 2 non-fragmented) needed to be left out from the analysis, because two of the weasels escaped and one was killed by a buzzard.
Statistical analysis
We analyzed weasel movement with 100 % minimum convex polygons using Ranges VI program (Anatrack, UK). Habitat content of the weasel movement area was counted from 100 % minimum convex polygons with Ranges VI, which measured how much habitat patch area the weasel movement area included. Weasel activity was analyzed as a proportion of active fixes from all the fixes that we got. For the movement and activity analysis, we took into account all weasels with more than 20 location fixes (n = 19). Two individuals lost their collars at the beginning of the tracking session and we excluded those from movement and activity analysis, but as they were captured alive after the tracking session, the enclosures could be used for vole survival/weasel hunting success analysis. Weasel hunting success was measured from the number of surviving voles after weasel removal. Sex ratio of killed voles was analyzed by counting the proportion of females in the weasel catch. Avian predation risk was measured as the number of voles replaced before releasing weasels. We calculated trappability before weasel release as a proportion of the times that a vole was trapped out of all trap checks in a given replicate. Trappability after weasel removal was analyzed based on the number of the trap checks before an animal was captured and removed from an enclosure.
All statistical analysis was conducted with R 2.15.0 (R Development Core Team 2010). Mixed effects models with library nlme (Pinheiro et al. 2010) were used. Model selection was done by choosing the best model from the set of predefined models based on the lowest AIC value (see all the models from electronic supplemental material ESM). Fragmentation (fragmented, non-fragmented) and season (summer, autumn) interaction addition with weasel sex without interaction was used as a full model in all weasel-related analysis. Weasel sex was included in the model to take into account the sexual dimorphism and possible differences in the energy need and kill-rate due to body size. All possible combinations of models from full to simple up until the null model including only constant were included as a set of candidate models. In the analysis on the strength of avian predation risk, a fragmentation and season interaction model was used as a full model. Maximum likelihood method was used for comparing models and restricted maximum likelihood (REML) in the final model to obtain the model estimates. To account for pseudoreplication, enclosure within replicate was used as a random factor in all analyses (Crawley 2007).
Results
Weasel hunting success and fragmentation
The full model with a fragmentation season interaction and weasel sex best explained the number of voles caught by weasels. Male weasels killed on average more voles (2.2 ± 0.3 SE) than females (1.3 ± 0.3) during the 2-day study periods (GLM; sex: F 1,12 = 5.74, P = 0.034). There was a clear interaction between season and fragmentation in weasel hunting success (GLM; fragmentation × season: F 1,12 = 8.92, P = 0.011; Fig. 2; main effects of fragmentation and season: P > 0.100). There were no differences in the sex of the voles killed (null model best according to AIC).
Weasel movements and activity
The best model describing movement included only sex although there was no significant differences between sexes in the size of the area that weasels moved (GLM; sex: F 1,12 = 2.30, P = 0.156, Fig. 3a). Habitat content of the home ranges were fitted best by a fragmentation* season model. In fragmented enclosures, weasel home ranges included less habitat area (69.8 ± 6.4 SE %) compared to non-fragmented treatment (96.0 ± 2.5 SE %), but this result was marginally non-significant (GLM; fragmentation: F 1,4 = 5.62, P = 0.077, season effect and interaction P > 0.112).
a Weasel movement areas (m2) in summer and in autumn in non-fragmented habitat (filled squares) and in the fragmented treatment (open symbols), mean ± SE in different treatments. Sample sizes above x-axis. b Weasel activity during summer and in autumn in non-fragmented habitat (filled squares) and in the fragmented treatment (open symbols), mean ± SE in different treatments. Activity is measures as a proportion of active fixes out of all location fixes. Sample size above x-axis
According to AIC, a model with season as a main effect best described weasel activity which was higher in summer but not significantly so (Fig. 3b; GLM; season: F 1,12 = 3.30, P = 0.094). Males tended to have longer interfix distances (8.2 ± 1.4 SE m) than females (4.8 ± 0.7 SE m), i.e. distance between two consecutive locations (GLM; sex: F 1,12 = 3.60, P = 0.082) based on the best model which included only the main effect of sex.
Avian predation risk
In summer, we replaced (0.72 ± 0.3 SE) and in autumn (2.2 ± 0.5 SE) voles per enclosure. There was no difference between fragmentation treatments (1.2 ± 0.4 SE) and (1.63 ± 0.5 SE), and voles were replaced per enclosure in non-fragmented and in fragmented enclosures, respectively. Only the season effect was statistically significant (Fig. 4; GLM; season: F 1,14 = 6.65, P = 0.022). One female weasel was killed by a buzzard in the edge of the non-fragmented patch during the fourth replicate.
Trappability before weasel introduction was slightly higher in summer (mean 77 ± 4 SE %) for both fragmentation treatments than in autumn (64 ± 5 SE % for fragmented and 74 ± 5 SE % for the non-fragmented treatment; GLM season: F 1,148 = 3.24, P = 0.074). There were no differences in trappability between seasons or fragmentation treatments after weasel removal from the enclosures (summer non-fragmented 84 ± 5 SE %, fragmented 76 ± 5 SE %; autumn non-fragmented 82 ± SE 5 %, fragmented 82 ± SE 5 %; P > 0.399 in all variables).
Discussion
We found evidence that habitat fragmentation affects predator–prey relationships through movements and hunting behavior of the least weasel in the way we predicted. Vole mortality risk was higher in the non-fragmented habitat than in fragmented habitat. Our result supports the hypothesis that more fragmented habitat may, at least in the short term, protect prey individuals occupying small patches in the fragmented landscape. In the long term, this may lower the local extinction risk of prey caused by the specialist predator probably because prey individuals are more prone to leave or escape predator–prey patches than patches without predators (Prakash and de Roos 2002; Dalkvist et al. 2011). This is supported by the fact that weasel hunting efficiency was lower in the fragmented than in the non-fragmented habitat. They killed less than two voles per capita in the fragmented habitat which is less than expected based on vole distribution of an average two voles per patch in our fragmented treatment.
Intraguild predation risk by diurnal raptors and nocturnal owls was high for hunting weasels throughout the study period in summer and autumn. The enclosure area was surrounded by large contiguous forest areas with small clear-cuts supporting high densities of bank voles and also field voles (Microtus agrestis) around the enclosures. This was particularly true for the cyclic vole dynamics of the study year with very high densities still in spring and early summer (Kallio et al. 2009) followed by the typical summer decline of vole populations towards the autumn (Henttonen et al. 1987). Raptor and owl populations are known to closely track high prey vole densities (Korpimäki 1994) and, accordingly, observations of raptors and owls were common during the study summer. The avian predation risk was high through the summer, and, due to decline of surrounding populations, the risk for enclosed populations increased during late summer and autumn. High avian predation risk may have made weasels more reluctant to cross the risky open matrix and to reach all small patches and the voles living in them. One of our radio-collared weasels was killed by a buzzard and, during the pre-phase of each experimental run, on average less than one vole per enclosure in summer and more than two per enclosure in autumn were estimated to have been killed by raptors or owls. This indicates high risk for both voles and weasels by avian top-predators which increased significantly towards autumn, when, in addition to the decline of vole populations, raptors with their fledged young were preparing for migration and the energy need of resident owls was also increasing (Norrdahl and Korpimäki 1995, 2002).
Weasel hunting and vole mortality in summer and autumn in fragmented landscape
Mortality caused by male weasels was higher than that of smaller females probably due to larger body size and the higher energy demands of males. This was pronounced in the autumn. Females on average killed less than one vole per day, which is known to be the average daily consumption of food in weasels (Sundell et al. 2003). Males’ average killing rate was the daily need of one vole per day. Mortality caused by the weasels increased only in the non-fragmented habitat towards autumn, while in enclosures with fragmentation it remained similar throughout the study. The season effect on weasel hunting was strong, especially in males, as predicted. Towards the colder season, weasels are known to start to intensify their hunting for collecting caches for energetically demanding colder autumn and freezing winter (Oksanen 1983).
The habitat fragmentation treatment had significant effects on weasel hunting and kill-rate in autumn, as suggested by the model of Prakash and de Roos (2002). Similarly, Dalkvist et al. (2011) found that simulated predation by the specialist predator was higher in the non-fragmented habitat than in the fragmented one. Our study was slightly different compared to Dalkvist et al.’s (2011) simulation because weasels in the matrix were also at risk of being predated. It has been documented that, as vole populations are in decline, as was the case in this study, about 80 % of weasels are killed by avian predators (Korpimäki and Norrdahl 1989). The intraguild avian predation risk was increasing in autumn due to the fledged new cohort of avian predators and influx of migrating birds of prey from the north (Norrdahl and Korpimäki 1995, 2002). Due to the increased avian predation risk in the matrix, weasels probably preferred to stay inside the protective tall-grass habitat where supposedly the majority of prey voles were also living. This inevitably led to higher hunting success and higher vole mortality in the non-fragmented habitat. It is known that several weasel species’ movements follow habitat edges where the highest prey densities are found, while at the same time providing cover during prey search (Gehring and Swihart 2004; Brandt and Lambin 2007; Magrini et al. 2009). In the fragmented habitat, certainly a proportion of voles took the risk of getting caught by an avian predator in the open matrix rather than sharing a small patch with a specialist vole predator like the weasel. Thus, voles at constant risk from a multi-species predator community, traded-off the talons of raptors and owls over the teeth of weasels (cf. Kotler et al. 1992). This risk taking might have been beneficial for voles in fragmented enclosures especially during autumn, because weasels were able to hunt less than one vole per day. Voles may not have functional hiding places inside a habitat patch regardless of patch size against the world’s smallest member of the order Carnivora, the least weasel, as it takes advantage of its elongated small size when actively hunting in tunnels and burrows (Sundell and Ylönen 2004). We can be relatively confident that vole mortality differences were a result of weasel predation, as, in previous studies, we found no difference in vole survival between fragmented and non-fragmented treatment without weasel predation (Haapakoski and Ylönen 2010, 2013).
Weasel spacing behavior and habitat fragmentation
The high hunting success of weasels in autumn in the non-fragmented habitat was, somewhat counterintuitively, associated with reduced movement. In summer, weasels were, contrary to our predictions, slightly more active, as shown in the proportion of active fixes out of the total fixes over the 2-day radio-tracking. Especially in the summer, weasel movement areas also contained more crossings of the matrix area. The slightly higher activity during summer may be affected by mating related behaviors and the innate need for finding a mating partner as weasels reproduce during summer (Erlinge 1974).
When measured according to area used, there was no difference in the space use of females or males though male interfix distances were slightly longer. This may indicate that a confined area of 0.25 ha is not large enough to manifest sex differences in movement radiuses, especially for larger males. In the fragmented enclosures with four patches, movement included the more risky matrix areas, as predicted. The most plausible explanation is that the small patches in the fragmented habitat which contained 1–2 voles quickly became devoid of prey, or the search costs increased (Brown 1988) and hunting reward compared to hunting effort decreased. Hunger forces increased risk taking in any system with a depleting food patch (Brown et al. 1997; Ylönen et al. 2002).
The spatial scale of our study was proper for a short-term experiment for the predator–prey pair we used, as shown in previous studies of ours with weasel–vole interactions (Sundell et al. 2003, 2008). Our scale and design were explicitly focused on fragmentation and not on habitat loss effects, which would have required far larger study areas. In this study, the focus was behavior of a freely-hunting small carnivore, the least weasel, facing the same risks as its prey from avian top-predators. Even the smallest avian predator of mammals in the boreal landscapes, the pygmy owl (Glaucidium passerinum), is able to kill weasels especially in autumn as this small owl starts to collect winter caches (Solheim 1984).
Conclusions
Our study did not provide as clear of a pattern as we expected in the effects of habitat fragmentation on weasel hunting behavior and its expression as kill-rate of their prey, the bank vole. But it was clear that, when hungry, weasels are prone to take risk in a fragmented landscape as soon as hunting reward in a selected patch decreases. When the energy need of weasels increased due to colder temperatures in autumn, their hunting success increased, especially for larger males and in non-fragmented habitat providing shelter against higher avian predation risk. Hunting in autumn must have been more cautious due to increased predation risk, but also more effective than in summer as the weasels killed more voles despite their lower activity.
With our experimental system, we were able to test in the field the theoretical predictions of Ryall and Fahrig (2006), which suggest negative effects for a specialist predator dependent on one prey type and increased persistence of both predator and prey population in fragmented habitat. These predictions gained support from our field study and matched the outcome of the simulations of Dalkvist et al. (2011) and Prakash and De Roos (2002), i.e. prey have higher dispersal rates out of patches occupied by both predator and prey than prey-only patches.
References
Brandt MJ, Lambin X (2007) Movement patterns of a specialist predator, the weasel Mustela nivalis exploiting asynchronous cyclic field vole Microtus agrestis populations. Acta Theriol 52:13–25
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Brown JS, Kotler BP, Mitchell WA (1997) Competition between birds and mammals: a comparison of givingup densities between crested larks and gerbils. Evol Ecol 11:757–771
Bujalska G (1986) Sex ratio in an island population of Clethrionomys glareolus. Acta Theriol 31:71–78
Crawley MJ (2007) The R book. Wiley, New York
Dalkvist T, Sibly RM, Topping CJ (2011) How predation and landscape fragmentation affect vole population dynamics. PLoS ONE 6:e22834
Erlinge S (1974) Distribution, territoriality and numbers of the weasel Mustela nivalis in relation to prey abundance. Oikos 25:308–314
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Syst 34:487–515
Gehring TM, Swihart RK (2004) Home range and movements of long-tailed weasels in a landscape fragmented by agriculture. J Mammal 85:79–86
Gorini L, Linnell JDC, May R, Panzacci M, Boitani L, Odden M, Nilsen EB (2012) Habitat heterogeneity and mammalian predator–prey interactions. Mammal Rev 42:55–77
Haapakoski M, Ylönen H (2010) Effect of fragmented breeding habitat and resource distribution on behaviour and survival of the bank vole (Myodes glareolus). Popul Ecol 52:427–435
Haapakoski M, Ylönen H (2013) Snow evens fragmentation effects and food determines overwintering success in ground-dwelling voles. Ecol Res. doi:10.1007/s11284-012-1020-y (in press)
Henttonen H, Oksanen T, Jortikka A, Haukisalmi V (1987) How much do weasels shape microtine cycles in the northern Fennoscandian taiga? Oikos 50:353–365
Hoset KS, Koivisto E, Huitu O, Ylönen H, Korpimäki E (2009) Multiple predators induce risk reduction in coexisting vole species. Oikos 118:1421–1429
Ims RA (1987) Responses in spatial organization and behaviour to manipulations of the food resource in the vole Clethrionomys rufocanus. J Anim Ecol 56:585–596
Jedrzejewska B, Jedrzejewski W (1989) Seasonal surplus killing as hunting strategy of the weasel Mustela nivalis—test of the hypothesis. Acta Theriol 34:347–359
Kallio ER, Begon M, Henttonen H, Koskela E, Mappes T, Vaheri A, Vapalahti O (2009) Cyclic hantavirus epidemics in humans–predicted by rodent host dynamics. Epidemics 1:101–107
Korpimäki E (1994) Rapid or delayed tracking of multiannual vole cycles by avian predators. J Anim Ecol 63:619–628
Korpimäki E, Norrdahl K (1989) Avian predation on mustelids in Europe 1: occurrence and effects on body size variation and life traits. Oikos 55:205–215
Kotler BP, Blaustein L, Brown JS (1992) Predator facilitation: the combined effect of snakes and owls on the foraging behaviour of gerbils. Ann Zool Fenn 29:199–206
Lindenmayer DB, Fischer J (2006) Habitat fragmentation and landscape change: an ecological and conservation synthesis. Island Press, Washington, DC
Magrini C, Manzo E, Zapponi L, Angelici FM, Boitani L, Cento M (2009) Weasel Mustela nivalis spatial ranging behaviour and habitat selection in agricultural landscape. Acta Theriol 54:137–146
Nakagiri N, Tainaka KI, Tao T (2001) Indirect relation between species extinction and habitat destruction. Ecol Model 137:109–118
Norrdahl K, Korpimäki E (1995) Effects of predator removal on vertebrate prey populations: birds of prey and small mammals. Oecologia 103:241–248
Norrdahl K, Korpimäki E (2002) Seasonal changes in the numerical responses of predators to cyclic vole populations. Ecography 25:428–438
Oksanen T (1983) Prey caching in the hunting strategy of small mustelids. Acta Zool Fenn 174:197–199
Pinheiro J, Bates D, DebRoy S, Sarkar D, (2010) The R Development Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–97
Prakash S, De Roos AM (2002) Habitat destruction in a simple predator–prey patch model: how predators enhance prey persistence and abundance. Theor Popul Biol 62:231–249
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, http://www.R-project.org/
Ryall KL, Fahrig L (2006) Response of predators to loss and fragmentation of prey habitat: a review of theory. Ecology 87:1086–1093
Salo P, Banks PB, Dickman CR, Korpimäki E (2010) Predator manipulation experiments: impact on populations of vertebrate prey. Ecol Monogr 80:531–546
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Solheim R (1984) Caching behaviour, prey choice and surplus killing by Pygmy owl Claucidium passerinum during winter, a functional response of generalist predator. Ann Zool Fenn 21:301–308
Sundell J, Ylönen H (2004) Behaviour and choice of refuge by voles under predation risk. Behav Ecol Sociobiol 56:263–269
Sundell J, Ylönen H (2008) Specialist predator in a multi-species prey community: boreal voles and weasels. J Integr Zool 3:51–63
Sundell J, Eccard JA, Tiilikainen R, Ylönen H (2003) Predation rate, prey preference and predator switching: experiments on voles and weasels. Oikos 101:615–623
Sundell J, Huitu O, Henttonen H, Kaikusalo A, Korpimäki E, Pietiäinen H, Saurola P, Hanski IS (2004) Large-scale spatial dynamics of vole populations in Finland revealed by the breeding success of vole-eating avian predators. J Anim Ecol 73:167–178
Sundell J, Trebatická L, Oksanen T, Ovaskainen O, Haapakoski M, Ylönen H (2008) Antipredatory response and survival of two vole species with a shared predator. Popul Ecol 50:257–266
Valkama J, Vepsäläinen V, Lehikoinen A (2011) The third Finnish breeding bird Atlas. Finnish Museum of Natural History and Ministry of Environment. http://atlas3.lintuatlas.fi/english (cited 19.3.2013) ISBN 978-952-10-7145-4
Ylönen H, Jacob J, Davis M, Singleton GR (2002) Predation risk and habitat selection of Australian house mice (Mus domesticus) during an incipient plague: desperate behaviour due to food depletion. Oikos 99:285–290
Ylönen H, Pech R, Davis S (2003) Heterogeneous landscapes and the role of refuge on the population dynamics of a specialist predator and its prey. Evol Ecol 17:349–369
Acknowledgments
We thank all the helpers in the field during radio-tracking, Nelika Hughes, Hanne Vihervaara, Janika Aaltonen, Audrey Proust, Netta Seppälä, Sirke Piirainen and Lenka Trebaticka. We thank technicians of the Konnevesi Research Station for maintenance of the enclosures. John Loehr commented manuscript and corrected the language. The study was supported by the Finnish Academy to the CoE in Evolutionary Ecology at University of Jyväskylä. The authors declare that there is no conflict of interest. Experiment and animal handling was conducted with animal experimentation permission at Jyväskylä University no. 35/31.5.2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haapakoski, M., Sundell, J. & Ylönen, H. Mammalian predator–prey interaction in a fragmented landscape: weasels and voles. Oecologia 173, 1227–1235 (2013). https://doi.org/10.1007/s00442-013-2691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2691-y