Abstract
Ecosystems are fragmented by natural and anthropogenic processes that affect organism movement and ecosystem dynamics. When a fragmentation restricts predator but not prey movement, then the prey produced on one side of an ecosystem edge can subsidize predators on the other side. When prey flux is high, predator density on the receiving side increases above that possible by in situ prey productivity, and when low, the formerly subsidized predators can impose strong top-down control of in situ prey—in situ prey experience apparent competition from the subsidy. If predators feed on some evolutionary clades of in situ prey over others, then subsidy-derived apparent competition will induce phylogenetic structure in prey composition. Dams fragment the serial nature of river ecosystems by prohibiting movement of organisms and restricting flowing water. In the river tailwater just below a large central Mexican dam, fish density was high and fish gorged on reservoir-derived zooplankton. When the dam was closed, water flow and the zooplankton subsidy ceased, densely packed pools of fish formed, fish switched to feed on in situ prey, and the tailwater macroinvertebrate community was phylogenetic structured. We derived expectations of structure from trait-based community assembly models based on macroinvertebrate body size, tolerance to anthropogenic disturbance, and fish-diet selectivity. The diet-selectivity model best fit the observed tailwater phylogenetic structure. Thus, apparent competition from subsidies phylogenetically structures prey communities, and serial variation in phylogenetic community structure can be indicative of fragmentation in formerly continuous ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predators affect the composition, structure, and diversity of prey communities by selectively feeding on some prey over others and by indirectly influencing interactions among prey species and their resources (Hairston et al. 1960; Paine 1966). Predator effects are greatest when predator density is higher than that which in situ prey density and production can support. This can occur if an ex situ energy flow from another ecosystem subsidizes predators (Polis and Strong 1996). For example, the production of invertebrate predators (e.g., spiders) along river shorelines can be enhanced by aquatic insects that emerge from the river (Henschel et al. 2001). These subsidized spiders can then depress riparian invertebrate prey species that are most susceptible to high predation (Henschel 2004). Subsidies can thus strongly affect in situ prey species distributions, abundances, and community composition via apparent competition through shared predators (Holt 1977).
Predators selectively feed on prey based in part on key functional traits (e.g., body size; Cohen et al. 1993). In many cases, the traits on which predators select prey are either unknown or are difficult to determine due to trait interactions, tradeoffs, plasticity, rapid evolution or other confounding factors. Since traits and other variables that affect predator selectivity have varying degrees of phylogenetic signal, conservatism, and convergence (Blomberg et al. 2003), exploring the phylogenetic structure of prey communities—the nonrandom tendency for communities to contain distantly or closely related species—could help elucidate how predators structure prey communities and what types of prey predators select. Prey communities experiencing strong apparent competition from a subsidy should comprise species that predators generally avoid or are relatively tolerant to predation, and if there are phylogenetic patterns in this predator selectivity on prey, the prey community should exhibit phylogenetic structure.
Many specialized consumer-resource interactions exhibit strong phylogenetic patterns in prey selectivity. For example, closely related parasites tend to have closely related hosts (e.g., Gilbert and Webb 2007) and closely related plant species typically support similar communities of herbivorous insects (e.g., Weiblen et al. 2006). However, for groups of generalist predators that feed on a variety of prey, co-evolution of traits is diffuse and phylogenetic patterns may be weaker than in specialized interactions. For example, a comprehensive meta-analysis on phylogenetic patterns in species interactions looked at one generalist predator–prey data set, the diets of snakes (Thamnophis, Colubridae), which had no phylogenetic pattern in the types of prey that were selected (Gómez et al. 2010). However, taxonomy can explain much variation in food web interaction networks (Eklöf et al. 2012; Stouffer et al. 2012). For example, Naisbit et al. (2012) found that taxonomically related prey species were fed on by similar predators. This suggests that vulnerability to generalized predation may vary among clades such that predation will cause phylogenetic structure to prey communities. Here, we explore how apparent competition from subsidies to generalist predators phylogenetically structures prey communities in an ecosystem that has been artificially fragmented by humans.
Natural rivers are generally thought of as uninterrupted continua with characteristics that gradually change along the elevation gradient from source to mouth (Vannote et al. 1980). Dams fragment this continuum and cause discontinuities in abiotic and biotic parameters (Ward and Stanford 1983). For example, dams impede water flow, organism movements, and shift species distributions such that species not normally found in shallow rivers, such as plankton, are abundant. The Laja River of central Mexico is impounded by a large dam to form the Ignacio Allende reservoir (Mercado-Silva et al. 2006). In the tailwater of this reservoir (i.e., in the river segment just downstream from the dam), we previously found tailwater fishes to have low 13C to 12C stable isotope ratios (low δ13C values) compared to those from other areas of the river (Mercado-Silva et al. 2009). These values were similar to those of fish sampled within the reservoir and were much lower than those sampled in riverine areas distant from the reservoir. Consumers retain the δ13C value of their prey, and carbon assimilated by primary producers in lentic, open-water habitats have low δ13C values compared to the primary producers of benthic habitats (Vander Zanden and Rasmussen 2001). The low δ13C signature we found in Laja tailwater fish suggests that tailwater fish in the Laja River are subsidized by prey found in the dam outflow (Mercado-Silva et al. 2009).
In this work, we identify the nature of the Laja reservoir subsidy to fish (i.e., zooplankton), estimate its temporal dynamics, and explore how this subsidy affects consumer-resource interactions between fish and their typical prey, riverine benthic macroinvertebrates. We calculate the phylogenetic diversity (both alpha and beta) of Laja River macroinvertebrate communities along the river gradient, highlight how serial changes to phylogenetic structure indicate the ecosystem discontinuity caused by the dam, and then compare the observed diversity to that expected from community assembly models based on functional traits. These models test mechanistic hypotheses for observed structure, and we provide R code to facilitate the use and visualization of this simulation approach in other studies.
Materials and methods
Study system and data collected
We studied the Laja River, a tributary to the Lerma River in the state of Guanajuato, Mexico. Dams regulate water throughout the watershed (drainage area 3,476 km2, main stem length ~154 km). Two reservoirs are located on the main stem, Presa Jesus Maria (~15 km below the headwaters) and Presa Ignacio Allende (~124 km below the headwaters). At least 12 other dams are located on Laja tributaries. The river basin is periodic; flow is dependent on season and management practices of the dams. Pools regularly form along the main stem of the river during times of low water flow when the dams hold water for crop irrigation and consumption during drier times.
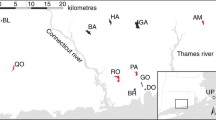
Eleven sites on the main stem were sampled between 2003 and 2004 for fish and macroinvertebrates (see our electronic supplementary material Appendix A: Site description table and Mercado-Silva et al. 2009 for a map). Sites were selected a priori to maximize variation in fish diversity, to span river order, and to adequately sample on either side of the Ignacio Allende impoundment (Mercado-Silva et al. 2009; Mercado-Silva et al. 2006). One site was in the river just below Presa Ignacio Allende, in the dam tailwater (BP), and another site, La Cieneguilla (LC), was in the reservoir where the Laja enters. Six sites were located upstream of LC and three sites downstream from BP. The highest site, Cantera (CA), was above Presa Jesus Maria. We sampled the river twice during periods of low to no water flow (June 2003, November 2003) and twice during periods with high water flow (August 2003, January 2004). We found fish and macroinvertebrates every time we sampled a site except for the site located in the town of Rio Laja (RL) which was completely dry on one sampling date.
The abiotic characteristics of each site we measured were: altitude (Garmin GPSMAP 76), dissolved oxygen, temperature, conductivity (YSI model 85 multimeter), and thalweg depth (depth at the deepest point). On ordinal scales, we measured turbidity, % riffle-pool-run, % muck-cobble, % canopy cover, % of bank eroded, bottom substrate complexity, habitat complexity, bank stability, bank protection and canopy quality following Resh et al. (1996, and references therein) who provide methods to quantitatively assess physical habitat variables known to affect river benthic macroinvertebrates. We constructed a matrix of Gower’s distances among sites (Gower distances are a combination of both ordinal and continuous data; Maechler et al. 2010), performed a principal coordinates analysis on the matrix, and retained the first two axes as measures of site abiotic environment (Appendix B: Laja river abiotic environment).
Seines and DC backpack electrofishing units were used to sample the fishes in all habitats at all sites. Each site was ca. 175 m in river length and was sampled for a period of ca. 40 min each sampling time. Total fish sampled across sites was standardized as catch per unit of effort (CPUE) by taking the natural log of the total number of fish per meter of shoreline sampled. Water level and flow fluctuated greatly due to seasonality and management, thus we regressed fish CPUE on sampling date and calculated the mean residual CPUE for each site as a standardized measure of total fish density (Appendix C: Laja Fish Community Composition).
Benthic macroinvertebrates were collected with a Surber sampler (0.02 m2 area, 500-μm mesh) placed on the benthos in habitats representative of the whole site. Three Surber samples were taken at each site each sampling time. Surber location was recorded as in a riffle, pool, or run. For our last sampling, we also used a D-net (500 μm) to sample all habitats of each site until no additional macroinvertebrate taxa were found (i.e., by eye while in the field). Invertebrates were stored in 70 % ethanol and later counted and identified to the lowest taxonomic level possible (typically genus). The Surber samples gave us estimates of total macroinvertebrate density which we regressed on sampling time and Surber location. The mean residual density of each site was used as a standardized measure of total macroinvertebrate density. Rather than analyzing the phylogenetic structure of each sample—structure is highly stochastic for small samples with few taxa (Helmus et al. 2007)—we combined all the D-net and Surber samples from each site into one site-by-taxa matrix and used this matrix to estimate the overall phylogenetic diversity of the community at each site (see below).
We sampled zooplankton and fish diets at three sites above and three sites below BP. Zooplankton was captured using an 80-μ-mesh zooplankton net. Three tows were taken at each site each sampling time, and total zooplankton density calculated as the number of individuals per liter of sampled water. The stomach contents of a representative sample of fishes at the sites were collected (ca. 15 individuals per site per date, sampled in proportion to sampled species abundances), and consumed macroinvertebrates were identified to the lowest possible taxonomic level, typically to order since the contents were digested and difficult to identify. Regardless, this coarse resolution had predictive power (see “Results”). The stomach contents were then divided into broad diet categories: aquatic macroinvertebrates, zooplankton, and other (e.g., algae, detritus); and the dry biomass for each category estimated.
For comparison with the macroinvertebrate community found in BP, in January 2005 we took three Surber samples and used a D-net to exhaustively sample macroinvertebrates again in BP and in three other dam tailwaters also in the Lerma basin located in rivers of a similar size to the Laja (sites BA, TE, MO; Appendix A). Also, at BP, BA, MO, and in another dam tailwater, SL, we sampled zooplankton and fish diets. Thus, we sampled four tailwaters in additional to BP, but were able to sample only macroinvertebrates at TE and only zooplankton and fish diets at SL.
Estimating phylogenetic community structure
We built a highly resolved phylogenetic topology of all sampled macroinvertebrate taxa based on published phylogenetic and taxonomic studies. As many nodes as possible were dated on the topology (Hedges and Kumar 2009) and branch lengths were evenly scaled between the dated nodes (Webb et al. 2008). With this tree, we estimated benthic macroinvertebrate phylogenetic community structure with the phylogenetic species variability (PSV) metric as a measure of alpha diversity, and the phylogenetic community dissimilarity (PCD) metric as a measure of beta diversity. The PSV metric measures phylogenetic alpha diversity as:
where n is community species richness, C is the community phylogenetic covariance matrix, a submatrix of the full covariance matrix of the species pool phylogeny (i.e., all the species in a data set), and trC and ΣC are the sum of the diagonal elements and all the elements of C, respectively (Helmus et al. 2007). Like all phylogenetic diversity metrics calculated from a defined species pool, PSV is bounded. Communities of distantly related species have PSV values close to one, while those comprising closely related species have PSV values closer to zero. The statistical expectation of PSV is independent of species richness, thus we were able to independently compare how both species richness and phylogenetic diversity differed among sites.
We compared the observed tailwater PSV values to those expected under a random community assembly model to test for tailwater macroinvertebrate phylogenetic community structure. The permutation model we used assumed species to randomly disperse into communities based only on their observed prevalence in the species pool and not on their phylogenetic relationships. We created 1000 null matrices by randomizing the observed presence/absence matrix (i.e., all the Laja sites plus the three additional tailwater sites) maintaining site richness and species prevalence (Helmus et al. 2007). The expectation of the PSV value for each site under random community assembly was then the average PSV value of each site across the 1,000 matrices. This approach of comparing the observed data to randomized data is typical for any study of phylogenetic community structure; however, it only allows for very simple hypotheses to be tested, none of which are mechanistic (Ives and Helmus 2011).
The PCD metric measures the dissimilarity of two communities in their phylogenetic compositions, where PCD = PCDc × PCDp (Ives and Helmus 2010). The PCD of two communities can be decomposed into the similarities of shared and unshared species. PCDc measures the dissimilarity of communities in the species they share, defined as one minus Sorensen’s similarity index with a correction for the fact that the expectation of Sørensen’s similarity increases with the species richness. PCDp measures the phylogenetic relationships among species that are not shared among communities. The full derivation and equations for PCD can be found in Ives and Helmus (2010). We calculated PCD, PCDc and PCDp for all pairwise combinations of the sites and performed a principal coordinates analysis on each of the three resulting dissimilarity matrices. We then plotted the communities along the first two axes in order to visualize phylogenetic beta diversity in units of community similarity.
To understand the variation in alpha and beta phylogenetic diversity, we calculated the overall prevalence of each taxon in tailwater versus riverine sites as taxa-occurrence ratios
where T and R are the total number of tailwater (four) and riverine (nine) sites, respectively, t indexes the four tailwater sites, r indexes the nine riverine sites, and p is whether or not (one or zero) we found taxon i at a particular tailwater or riverine site. For each taxon, g varied between −1 (all riverine sites and no tailwater sites contained a particular taxon) to +1 (all tailwater sites and no riverine sites contained a particular taxon).
Community assembly models
We developed an approach where we simulated communities based on species-level traits or attributes that we hypothesized could be mechanistically related to the cause of observed phylogenetic community composition. For a given attribute a, where species i has the value a i , the probability of species i occurring in a simulated community was defined as
where \(\phi\) was exponentially shaped and c was a scalar. The scalar c allowed us to vary how strongly the attribute a affected simulated community composition. When c is large, only those species with high values of a are found in simulated communities and on average simulated species richness is lower than the species richness of communities simulated irrespective of a (i.e., c is low). In our case, since we hypothesized that fish structure the phylogenetic composition of macroinvertebrates, c is analogous to fish density. Assembly was a binomial stochastic process where species i was present in a particular simulated community if its probability \(\phi_{i}\) was greater than a randomly drawn number between zero and one. This resulted in random variation in the composition of each simulated community. We allowed all species found at all the sites to potentially colonize a simulated community. For each prey-species attribute, a, that we studied, we incrementally varied c to simulate communities that on average had species richness values ranging between five and 29 (the minimum and maximum observed site macroinvertebrate species richness) and simulated 10,000 macroinvertebrate communities for each of the 25 richness values. To be clear, no phylogenetic information was used in the model when simulating these communities. In appendix D (Diet selectivity and assembly models) we provide the model profile across the species richness values for each attribute. Below we only provide the simulated PSV values for species richness of nine, the mean richness of the four tailwater sites. Code written in R based on Eq. 3 is provided to produce simulated communities for other data sets, and to plot species-level attributes across the tips of phylogenetic trees (Appendix E: Generalized R Code).
We built four assembly models based on Eq. 3 for three species attributes: diet selectivity, body size, and disturbance tolerance. For each attribute, we tested for phylogenetic signal with the K metric based on Brownian motion evolution (Blomberg et al. 2003). Phylogenetic signal in functional traits can result in phylogenetic community structure (Kraft et al. 2007); however, phylogenetic patterns may still emerge even if there is not clear signal as measured by metrics like K built on particular models of trait evolution.
The first simulation model was based on sampled diets from Laja River fish. It was constructed to test if fish predation could phylogenetically structure macroinvertebrate communities. Fish diet selectivity on macroinvertebrate taxon i, d i , was calculated as the deviations from the null hypothesis that fish had no selective preference on taxon i and feed on i in proportion to its relative density at a site when a particular fish diet was sampled (Jacobs 1974, Appendix D). A positive d i indicated that fish on average selected for taxon i more than expected, and a negative d i indicated an aversion. We multiplied d by −1 to create a scenario where highly selected macroinvertebrate taxa were the least likely to be found in the simulated communities (note that d replaces a in Eq. 3 for this model). We then randomly selected 300 of these communities and calculated the PCD values among these simulated communities and the observed communities in order to assess how compositionally different were the simulated and observed communities.
Our second and third models tested if a functional trait, body size, was associated with the observed macroinvertebrate phylogenetic structure. Many abiotic factors can affect the body size distribution of macroinvertebrate communities such as substrate composition, geomorphology, and water flow (e.g., Bourassa and Morin 1995; Poff et al. 1993). Fish might also select large-bodied macroinvertebrates and thus both diet selectivity and macroinvertebrate body size could explain the observed community structure. We built two body size assembly models. The first made it more likely for small macroinvertebrates to assemble into simulated communities and the second, the converse, with large taxa being more likely. Body size, b, was estimated as the mean length along the longest body dimension (i.e., excluding antennae, gills) of our macroinvertebrate voucher specimens (ca. one to ten individuals per taxon) with b standardized to z-scores. For the small size model, b was multiplied by −1.
Our final model was based on independent estimates of the tolerance of riverine macroinvertebrate taxa to anthropogenic disturbance. The United States Environmental Protection Agency designates lotic benthic macroinvertebrate taxa, mostly genera and families, as to how tolerant they are to anthropogenic disturbance—primarily based on sensitivity to organic pollution—with zero indicating no tolerance and ten maximum tolerance (Barbour 1999). The tolerance values were standardized and a simulation model constructed as previously described for diet selectivity and body size. However, this simulation model should not be considered independent of the diet simulation model since EPA tolerance values include both abiotic and biotic effects of damming and other human impacts. These values are metrics of general human disturbance irrespective of underlying mechanisms.
Results
In the tailwater of the Ignacio Allende reservoir (i.e., the river just downstream from the dam, site BP) total fish density was higher and benthic macroinvertebrate density much lower than at all other sampled sites (Fig. 1). Fishes in the Laja River typically feed on autochthonous prey such as benthic macroinvertebrates, algae and detritus (Mercado-Silva et al. 2006; Mercado-Silva et al. 2002; Miller et al. 2005), but all fishes at site BP fed on zooplankton (Copepoda and Cladocera; Fig. 2a) exported from the reservoir into the tailwater when water was released (Fig. 2b). The subsidy was also observed at tailwater sites on other rivers—fishes fed on zooplankton if dams were open and releasing water (Fig. 2b). All fish species ate zooplankton when zooplankton densities were high, and fish community composition at BP was similar to other Laja sites, except that at BP all fishes were at very high density (Appendix C).
a Fish density and b macroinvertebrate density at 11 sampling sites along the Laja River of central Mexico (standardized residuals ± SE from ANOVA). Sampling sites are identified as riverine (open circles), in inflow of a reservoir (shaded circle) or in a tailwater (solid circle) and are listed from upstream to downstream. See Appendix A for sampling site descriptions. The ANOVA for fish density included sampling date, and for macroinvertebrates included date and sampling location (riffle, pool, run). Below Presa Ignacio Allende (BP) had significantly more fish (P < 0.001) and fewer macroinvertebrates (P < 0.0001) based on post hoc contrast tests (R: Tukey honestly significant difference). CA Arroyo Cantera, LQ La Quemada, LA Las Adjuntas, SJ San Juan, RL Rio Laja, AT Atotonilco, LC La Cieneguilla, FC Ferrocarrileros, SO Soria, RR Rinconcillo de los Remedios
a Proportion of zooplankton in fish diets at sites along the Laja River (mean ± SE). Circles represent means across sampling times and triangles represent means within sampling times. b Fish diet composition (means of diet categories across all sampled fish; bars) and zooplankton density (mean number per liter ± SE based on three tows, natural log scale; open circles) in the tailwaters of four dams. Site BP was sampled multiple times, while the other tailwater sites located on different rivers were sampled only once in January 2005. BA El Barrial, MO Melchor Ocampo, SL Solis; for other abbreviations, see Fig. 1
Tailwater macroinvertebrate alpha phylogenetic diversity (PSV) at BP and in tailwaters of the three other dams was higher than that found in the riverine sites along the Laja (Fig. 3a). Tailwater phylogenetic diversity was higher than expected from random community assembly indicating phylogenetic overdispersion (i.e., tailwater communities comprise more distantly related species than expected), while riverine communities were underdispersed (i.e., comprise more closely related species than expected). On average there were nine (±2.1 SE) macroinvertebrate taxa at the tailwater sites and 20.1 (±1.8) taxa at the riverine sites, and there were fewer taxa at site BP, eight taxa, than at any other site on the Laja (riverine richness range: 12–29). Variation in the abiotic environment across sites (Appendix B) did not explain variation in alpha phylogenetic diversity (R 2 of abiotic principal coordinate axis one = 0.06, F = 0.61, P > 0.05; R 2 axis two = 0.08, F = 0.78, P > 0.05); neither did variation in fish community composition (Appendix C). Instead macroinvertebrate PSV was explained by total fish density (R 2 = 0.62, F = 14.61, P < 0.005). Even though site BP was in a dam tailwater, based on our measures of the abiotic environment, it was similar to other Laja riverine sites (Appendix B). We believe this is the case since the entire river is a seasonal ecosystem with high water flow at times during the rainy season that creates flash floods and deep, scoured channels like those found in the tailwater. However, during times when dams are closed, water flow ceases across the entire river, not just at BP, and aquatic organisms are relegated to pools at all sites.
a Phylogenetic alpha diversity (phylogenetic species variability) of macroinvertebrates sampled along the Laja River and at three dam-tailwater communities in other rivers is compared to expectations of phylodiversity from five community assembly models (indicated by x; see Appendix B). Non-capped error bars on individual sites are expected SEs (see Equation 3 in Helmus et al. 2007) and capped error bars are SEs for sampled or simulated groups of sites. b–d Principal coordinates (PC) ordinations of distance matrices of phylogenetic community dissimilarity (PCD) values measure macroinvertebrate phylogenetic beta diversity (Ives and Helmus 2010). The first two axes in b–d explain 44 %, 48 % and 31 % of the original variation, respectively. Dotted lines encompass tailwater sites and dashed lines encompass riverine sites. b Ordination of phylogenetic beta diversity, PCD = PCDc × PCDp, in relation to the fish diet selectivity simulations. c Ordination of PCDc, the proportion of shared species between community pairs. d Ordination of PCDp, community similarity based on the phylogenetic relatedness of unshared species. TE, Tepuxtepec for other abbreviations, see Figs. 1and 2
The macroinvertebrates that were underrepresented in tailwater sites were generally those that were selectively fed on by fish. The low tailwater macroinvertebrate richness and high phylogenetic diversity were mostly due to tailwaters being depauperate in insect taxa, while also having more non-insect taxa (Fig. 4a). Fish diet selectivity corresponded to this community pattern. Fishes generally selected insects, but averted taxa found more often at tailwater sites (Fig. 4b, Appendix D).
a The phylogeny of all sampled macroinvertebrate taxa is plotted next to a bar chart of taxa-occurrence ratios at either riverine (nine) or tailwater (four) sites. The ratios are from −1 (all riverine sites and no tailwater sites contained a particular taxa) to +1 (all tailwater sites and no riverine sites contained a particular taxa). b Occurrence ratios and fish diet selectivity (Appendix D) grouped to the broader phylogenetic clades used in the fish diet analyses
The fish diet assembly model best predicted the observed alpha phylogenetic diversity, while those from the body size and EPA tolerance models did not (Fig. 3a; Fig. D1, and goodness of fit statistics in Appendix D). Fish diet selectivity also explained variation in macroinvertebrate phylogenetic beta diversity across the riverine, reservoir and tailwater sites. A principal coordinates analysis of the PCD among sampled and simulated communities ordered the communities along the first axis corresponding to site type, with the simulated communities on one end, followed by the reservoir site, the four tailwater sites, and then the nine riverine communities. The fish diet model thus produced communities composed of species that were phylogenetically similar, but not identical, to species in tailwater communities than to communities at other site types. When the sites were plotted in PCD space without simulated communities, tailwater and reservoir sites still grouped together separately (Fig. 3c, d) indicating that tailwater sites were more similar in the species they share than they were to the riverine sites (Fig. 3c), and that the unshared species between pairs of tailwater sites were more closely related to each other than they were to species in the riverine sites (Fig. 3d). Phylogenetic signal in both body size (K = 0.30) and EPA tolerance (0.39) was less than expected from Brownian motion evolution and not significant based on randomization of phylogenetic tips. In contrast, diet selectivity had more phylogenetic signal than expected (K = 0.75, P < 0.05).
Discussion
An example of a subsidy causing phylogenetic structure
Predators can influence prey community composition by selectively feeding on some prey over others and by causing prey to emigrate from areas of high predation (e.g., Cooper et al. 1990). We hypothesized that if predator effects on prey are phylogenetically nonrandom, then predators may determine the phylogenetic structure of prey communities, especially when those predators are subsidized. Spatial and temporal patterns of phylogenetic community structure may thus be determined by predator–prey interactions and energy flows among ecosystems. Our results suggest that in dam tailwaters reservoir-zooplankton subsidies to fish affect the phylogenetic structure of benthic macroinvertebrate prey communities via apparent competition.
Fishes in the tailwater of the Ignacio Allende Reservoir on the Laja River of central Mexico were at very high density (Fig. 1a). These fishes, and those in other dam tailwaters, were subsidized by zooplankton exported from reservoirs when dams were open and releasing water (Fig. 2b; Mercado-Silva et al. 2009). Once a dam gate was closed, zooplankton outflows ceased and tailwater fishes were relegated to large pools (Appendix F: photos of the Ignacio Allende tailwater site BP) where they switched to feed on macroinvertebrates and other prey (Fig. 2). Dams regulate water flow along the entire river, thus similar to the tailwater site, all sites dried to pools during times when dams were closed. Total macroinvertebrate density at the site was very low compared to other sites (Fig. 1b), and communities were generally depauperate of the exact same macroinvertebrate taxa on which fishes selectively fed (Fig. 4; Appendix D). This low density is in contrast to other well-studied tailwaters where benthic macroinvertebrate densities are higher than in riverine reaches (e.g., Doi et al. 2008). We simulated communities where the probabilities of macroinvertebrate taxa occurring in communities were based only on fish diet selectivity. This model produced macroinvertebrate communities with phylogenetic diversity values that well matched those observed at the tailwater sites (Fig. 3a; Appendix D). While our data are consistent with the hypothesis that a zooplankton subsidy to tailwater fishes phylogenetically structures tailwater macroinvertebrate communities via apparent competition, below we discuss alternative hypotheses.
Is the high tailwater fish density caused by a reservoir subsidy?
High tailwater fish density can be caused by high in situ fish production enhanced by prey subsidies such as zooplankton exported from reservoirs into downstream waters (Angradi 1994; Armitage 1976; Hudson and Lorenzen 1980). We previously found that all fishes in the Ignacio Allende tailwater had low (more negative) δ13C values indicating that tailwater fish biomass was derived from reservoir prey (Mercado-Silva et al. 2009), and here we identified zooplankton as that prey. The observed high tailwater fish density was thus likely due to a reservoir prey subsidy. However, there are two alternative scenarios to explain our observation. First, dams may impede upstream movement of migratory fishes to cause high tailwater density (Larinier 2000). However, in the Laja no fishes have long distance migratory behaviors (Miller et al. 2005) and small fishes, like those we captured, spend most of their lives in restricted areas (e.g., Gerking 1959). Tailwater fish density was also consistently high and did not vary greatly as it can in rivers with fishes that migrate (e.g., salmonids in temperate rivers). Second, fish, particularly larval fish, inside reservoirs can be exported downstream when water is released (Bednarek 2001). On the reservoir side of the Ignacio Allende impoundment is a deep channel with little littoral zone. Of the fish species found in the tailwater, only two, Chirostoma jordani (Atherinidae) and Lepomis macrochirus (Centrarchidae, but only one individual was captured of this species), are found both in rivers and in the pelagic zones of Mexican reservoirs and lakes (Miller et al. 2005). Thus, it is likely only C. jordani that could be exported from the pelagic zone of the reservoir in such an amount as to subsidize the tailwater with fish. However, we believe fish export an unlikely major factor here because the Ignacio Allende impoundment is tall and water flows down a spillway until it hits a concrete flip lip which creates a hydraulic jump so that water is violently sprayed high into the air (Appendix F). This would induce severe trauma to fish. In our experience, C. jordani the most likely candidate for export, is not a robust fish and would not survive this ordeal. Also, if tailwater density was caused by fish expelled from the reservoir, then there should have been high CPUE when the dam was open and low CPUE when it was closed. This was not the case. During the June 2003 and November 2003 samplings when the dam was closed, total fish CPUE was 2.57 and 0.98, respectively, and during August 2003 and January 2004 when the dam was open, CPUE was 1.27 and 0.93, respectively.
Do fish phylogenetically structure tailwater macroinvertebrate communities?
The ability of fish to regulate benthic macroinvertebrate community composition is dependent on fish density, fish community composition, and the degree to which fish and macroinvertebrates can move. Higher fish densities have larger impacts, benthic-feeding fishes have larger impacts than drift-feeding fishes, and if fish and macroinvertebrate movement is restricted, such as in experimental mesocosms, fish impact is greater (Cooper et al. 1990; Dahl and Greenberg 1996; Wooster 1994). The Laja River system periodically dries to a discontinuous network of pools due to extensive damming of its watershed. In the Laja River, tailwater fish density was very high, all fishes were facultative benthic feeders, and when water was not released, fish and macroinvertebrates were restricted to unconnected pools (Appendix F). Similarly, fishes at the other tailwater site with a closed dam were relegated to unconnected pools (El Barrial, BA). The tailwaters we studied are thus ideal systems for fish to strongly regulate macroinvertebrate community composition. Furthermore, previous work has found macroinvertebrate density in the tailwaters of surface-release dams, like those we studied here, to be very high and dominated by filter-feeding macroinvertebrates that are subsidized by reservoir-produced detritus, algae and zooplankton (Doi et al. 2008; Herlong and Mallin 1985; Richardson 1991). However, in our study, tailwater macroinvertebrate density was lower than anywhere else on the Laja, but it did contain filter-feeding invertebrates from those clades found in other tailwater studies (e.g., Vinson 2001).
Even though alpha phylogenetic diversity, PSV, along the Laja was well explained by fish density and not site environmental characteristics, abiotic variables certainly affect Laja River macroinvertebrate community composition. This can be seen in the PCD ordination. In Fig. 3b along the first axis, the simulated communities are more similar to the tailwater sites than to the riverine sites, but the tailwater sites are more similar to the riverine sites than to the simulated communities. This is expected since the simulated communities are by definition structured only by fish diet selectivity, while both tailwater and riverine sites are affected by other variables besides fish. Regardless of other factors, however, the low macroinvertebrate density and high fish density, the observed diet switching and periodicity of the subsidy to fish, and the fit of the diet-selectivity simulation model all strongly support the hypothesis that it is top-down control of subsidized fish that is the main cause of the observed tailwater macroinvertebrate phylogenetic community structure. Fish likely affect macroinvertebrates along the entire river, but at the tailwater site where fish density is highest, the effect is most evident.
Ecosystem discontinuities, disturbance and phylogenetic community structure
Dams fragment upstream and downstream reaches and create discontinuities in the serial nature of river ecosystems (Ward and Stanford 1983). They impose disturbances to natural river flow and biotic composition (e.g., Baxter 1977; Vinson 2001). Disturbance to communities or ecosystem is generally thought to reduce phylogenetic diversity and cause underdispersed phylogenetic community structure (Helmus et al. 2010; Warwick and Clarke 1998). This can occur when closely related species have similar sensitivities to disturbance and thus entire clades of sensitive species are lost during disturbance events. However, our study does not support the general hypothesis that communities contain closely related species during ecosystem disturbance. Our work instead highlights how the phylogenetic structure of disturbed communities depends on patterns of species disturbance sensitivities and the phylogenetic scale of analyses. Fishes selectively fed upon and tailwaters were depauperate in taxa from a clade that was nested within the phylogeny (i.e., insects). Fishes avoided taxa and tailwaters were more abundant in taxa from clades distantly related to insects (e.g., mollusks). The relative paucity of insect species, likely caused by the selective feeding of fish, determined the observed tailwater phylogenetic overdispersion. Careful consideration of phylogenetic scale (Swenson et al. 2006) must be considered in any study of disturbance and phylogenetic community structure so that the phylogenetic scale of analyses is relevant to the mechanisms under study. If we had focused our study on a smaller phylogenetic scale, such as only insects, the observed structure would have been obscured. Instead, the broad phylogenetic scale at which we defined our prey communities was reasonable since fish feed on the entire benthic macroinvertebrate community with varying preference across species. Spatial discontinuities within ecosystems might therefore be indicated by changes in phylogenetic community structure, but only at relevant phylogenetic scales.
References
Angradi TR (1994) Trophic linkages in the lower colorado river––multiple stable-isotope evidence. J North Am Benthol Soc 13:479–495
Armitage PD, Capper MH (1976) The numbers, biomass and transport downstream of micro-crustaceans and hydra from cow green reservoir (Upper Teesdale). Freshwater Biol 6:425–432
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, Second Edition. EPA 841-B-99-002. U.S. Environmental Protection Agency Office of Water; Washington, DC
Baxter RM (1977) Environmental effects of dams and impoundments. Annu Rev Ecol Syst 8:255–283
Bednarek AT (2001) Undamming rivers: a review of the ecological impacts of dam removal. Environ Manage 27:803–814
Blomberg SP, Garland TJ, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Bourassa N, Morin A (1995) Relationships between size structure of invertebrate assemblages and trophy and substrate composition in streams. J North Am Benthological Soc 14:393–403
Cohen JE, Pimm SL, Yodzis P, Saldana J (1993) Body sizes of animal predators and animal prey in food webs. J Anim Ecol 62:67–78
Cooper SD, Walde SJ, Peckarsky BL (1990) Prey exchange rates and the impact of predators on prey populations in streams. Ecology 71:1503–1514
Dahl J, Greenberg L (1996) Impact on stream benthic prey by benthic versus drift feeding predators: a meta-analysis. Oikos 77:177–181
Doi H et al (2008) Drifting plankton from a reservoir subsidize downstream food webs and alter community structure. Oecologia 156:363–371
Eklöf A, Helmus MR, Moore M, Allesina S (2012) Relevance of evolutionary history for food web structure. Proc Biol Sci 279:1588–1596
Gerking SD (1959) The restricted movement of fish populations. Biol Rev 34:221–242
Gilbert GS, Webb CO (2007) Phylogenetic signal in plant pathogen–host range. Proc Natl Acad Sci 104:4979–4983
Gómez JM, Verdu M, Perfectti F (2010) Ecological interactions are evolutionarily conserved across the entire tree of life. Nature 465:918–921
Hairston NG, Smith FE, Slobodkin LB (1960) Community structure, population control, and competition. Am Nat 94:421–425
Hedges SB, Kumar S (eds) (2009) The time tree of life. Oxford University Press, New York
Helmus MR, Bland TJ, Williams CK, Ives AR (2007) Phylogenetic measures of biodiversity. Am Nat 169:E68–E83
Helmus MR, Keller W, Paterson MJ, Yan ND, Cannon CH, Rusak JA (2010) Communities contain closely related species during ecosystem disturbance. Ecol Lett 13:162–174
Henschel JR (2004) Subsidized predation along river shores affects terrestrial herbivore and plant success. In: Polis GA, Power ME, Huxel GR (eds) Food webs at the landscape scale. The University of Chicago Press, Chicago, pp 189–199
Henschel JR, Mahsberg D, Stumpf H (2001) Allochthonous aquatic insects increase predation and decrease herbivory in river shore food webs. Oikos 93:429–438
Herlong DD, Mallin MA (1985) The benthos-plankton relationship upstream and downstream of a blackwater impoundment. J Freshwater Ecol 3:47–59
Holt RD (1977) Predation, apparent competition, and the structure of prey communities. Theor Popul Biol 12:197–229
Hudson PL, Lorenzen WE (1980) Manipulation of reservoir discharge to enhance tailwater fisheries. In: North RM, Dwoesky LB, Allee DJ (eds) Symposium proceedings: unified river basin management. American Water Resources Association, Minneapolis
Ives AR, Helmus MR (2010) Phylogenetic metrics of community similarity. Am Nat 176:E128–E142
Ives AR, Helmus MR (2011) Generalized linear mixed models for phylogenetic analyses of community structure. Ecol Monogr 81:511–525
Jacobs J (1974) Quantitative measurement of food selection. Oecologia 14:413–417
Kraft NJB, Cornwell WK, Webb CO, Ackerly DD (2007) Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am Nat 170:271–283
Larinier M (2000) Dams and fish migration. In: Berkamp G, McCartney M, Dugan P, McNeely J, Acreman M (eds) Dams, ecosystem functions and environmental restoration, thematic review 2.1 prepared as an input to the world commission on dams, Cape Town
Maechler M, Rousseeuw P, Struyf A, Hubert M (2010) Cluster analysis basics and extensions, 1.13.1 edn
Mercado-Silva N, Lyons JD, Maldonado GS, Nava MM (2002) Validation of a fish-based index of biotic integrity for streams and rivers of central Mexico. Rev Fish Biol Fisheries 12:179–191
Mercado-Silva N et al (2006) Long-term changes in the fish assemblace of the Laja river, guanajuato, central mexico. Marine and Freshwater Ecosystems, Aquatic Conservation
Mercado-Silva N, Helmus MR, Vander Zanden MJ (2009) The effects of impoundment and non-native species on a river food web in mexico’s central plateau. River Res Applications 25:1090–1108
Miller RR, Minckley WL, Norris SM (2005) Freshwater fishes of Mexico. University of Chicago Press, Chicago
Naisbit RE, Rohr RP, Rossberg AG, Kehrli P, Bersier L-F (2012) Phylogeny versus body size as determinants of food web structure. Proc Biol Sci 279:3291–3297
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:65–75
Poff NLR, et al. (1993) Size structure of the metazoan community in a piedmont stream. Oecologia 95:202–209
Polis GA, Strong DR (1996) Food web complexity and community dynamics. Am Nat 147:813–846
Resh VH, Myers MJ, Hannaford MJ (1996) Macroinvertebrates as biotic indicators of environmental quality. In: Hauer RF, Lamberti GA (eds) Methods in stream ecology. Academic Press, San Diego, pp 647–667
Richardson JS, Mackay Rosemary J (1991) Lake outlets and the distribution of filter feeders: an assessment of hypotheses. Oikos 62:370–380
Stouffer DB, Sales-Pardo M, Sirer MI, Bascompte J (2012) Evolutionary conservation of species roles in food webs. Science 335:1489–1492
Swenson NG, Enquist BJ, Pither J, Thompson J, Zimmerman JK (2006) The problem and promise of scale dependency in community phylogenetics. Ecology 87:2418–2424
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) River continuum concept. Can J Fish Aquat Sci 37:130–137
Vinson MR (2001) Long-term dynamics of an invertebrate assemblage downstream from a large dam. Ecol Appl 11:711–730
Ward JV, Stanford JA (1983) The serial discontinuity concept of lotic ecosystems. In: Bartel S, Fontaine T (eds) Dynamics of lotic ecosystems. Scientific Publishers, Ann Arbor, pp 29–42
Warwick RM, Clarke KR (1998) Taxonomic distinctness and environmental assessment. J Appl Ecol 35:532–543
Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics Appl Note 24:2098–2100
Weiblen GD, Webb CO, Novotny V, Basset Y, Miller SE (2006) Phylogenetic dispersion of host use in a tropical insect herbivore community. Ecology 87:S62–S75
Wooster D (1994) Predator impacts on stream benthic prey. Oecologia 99:7–15
Acknowledgments
We thank E. Diaz Pardo, V. Campos, T. Helmus, G. Motomi Kato, P. Ornelas García, C. Pedraza Lara, and J. Behm for help with sampling, and members of the Ives, Wootton, and Pfister labs for general comments and help. This work was supported in part by the UCMEXUS program with collaborative grants to M. R. H. and N. M. S., the University of Wisconsin Latin American and Iberian Studies Program and National Science Foundation DBI-0906011 to M. R. H. (Bioinformatics post-doctoral fellowship).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Robert Hall.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Helmus, M.R., Mercado-Silva, N. & Vander Zanden, M.J. Subsidies to predators, apparent competition and the phylogenetic structure of prey communities. Oecologia 173, 997–1007 (2013). https://doi.org/10.1007/s00442-013-2661-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2661-4