Abstract
Food availability and pond desiccation are two of the most studied factors that condition amphibian metamorphosis. It is well known that, when food is abundant, organisms undergo metamorphosis early and when they are relatively large. The capability of anurans to accelerate their developmental rate in response to desiccation is also common knowledge. These two variables must act together in nature, since we know that, as a pond dries, the per capita resources decrease. We conduct an experiment to evaluate the effects of desiccation and food availability separately and in combination in tadpoles of the painted frog (Discoglossus pictus). We demonstrate that food deprivation leads to slow growth rates, which delay metamorphosis and produce smaller size and weight. The capability to accelerate metamorphosis when facing a drying pond is also confirmed, but, nevertheless, with factor interaction (when the pool is drying and resources are scarce) the capacity to respond to desiccation is lost. In addition, slow drying rates are shown to be stressful situations, but not enough to provoke a shortening of the larval period; in fact, the larval period becomes longer. We also demonstrate that the interaction of these factors changes the allometric relationship of different parts of the hind limb, which has implications for the biomechanics of jumping. Due to low mortality rates and an adequate response to both environmental factors, we expect D. pictus to have a great invasive potential in its new Mediterranean distribution area, where lots of temporary and ephemeral ponds are present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphibians are unique among tetrapods in the complexity of their lifecycles and the degree to which their lifecycles vary in response to environmental conditions. The life history of most anurans is characterized by an abrupt ontogenetic change in morphology, physiology and behavior (Wilbur 1980; Wells 2007). Even so, many studies have shown that the morphology of metamorphs is causally affected by the tadpole environment. The connection between larval and juvenile stages limits the range of adaptive larval phenotypes that can be expressed even though it has a low impact on adult fitness (Van Buskirk and Saxer 2001; Watkins 2001; Richter-Boix et al. 2006).
Although there has long been an interest in the influence of environmental factors on the growth and developmental rates of amphibian larvae (Savage 1952, 1962), the first synthetic theory of the timing of metamorphosis was not postulated until 1973 (Wilbur and Collins 1973; mathematically formalized by Day and Rowe 2002). The Wilbur–Collins model is based in the hypothesis that there is a negative relationship between growth and developmental rate, and the model thus predicts that, if the growth rate of a larva increases, development is retarded to exploit the new growing conditions to the maximum. In contrast, if the growth rate decreases, development should increase in an attempt to escape a deteriorating environment. The model also establishes that there is a minimum and a maximum body size at which metamorphosis will occur, and the range of possible body sizes will depend on the phenotypic plasticity of the species (Wilbur and Collins 1973). Day and Rowe (2002) mathematically formalized the developmental threshold below which larvae are expected to respond to slow growth rates by lengthening the larval period and above which larvae are thought to accelerate development and expedite metamorphosis. As a consequence, different food levels will produce an L-shaped reaction norm (Plaistow et al. 2004; Lind et al. 2008). Even though the exact mechanisms underlying developmental thresholds remain unclear, endocrinological mechanisms have been suggested (Wilbur and Collins 1973; Hensley 1993). Since the threshold has been demonstrated to evolve (Morey and Reznick 2000, 2004; Lind et al. 2008), it is not surprising to find differences between populations (Lind et al. 2008) and species (Morey and Reznick 2000, 2004). As an alternative to the Wilbur–Collins model, Travis (1984) proposed the “fixed-rate model”. Travis stated that the developmental rate may lose its responsiveness to food supply, metabolic state and growth rate, although remaining responsive to temperature, water level and predators (Travis 1984; Leips and Travis 1994; Rose 2005).
As a very important part of the growth of most amphibians occurs in the terrestrial habitat, Werner (1986) developed a model that includes the terrestrial phase. The predictions of that model were based on an optimal balance between maximizing growth and minimizing mortality in both aquatic and terrestrial habitats. In water, amphibian potential for changes in growth and mortality rates are reflected by phenotypic plasticity (Rudolf and Rödel 2007).
The three models have been extended by many authors in order to include more factors such as time constraints or seasonality (Rowe and Ludwig 1991; Rudolf and Rödel 2007; for more information, see Harris 1999 and Wells 2007), but they are still the main conceptual framework.
A number of biotic and abiotic factors can determine the growing conditions that larvae experience. These factors include changes in larval density (Wilbur and Collins 1973; Tejedo and Reques 1994; Richter-Boix et al. 2004), aquatic predators (Laurila et al. 2002; Benard 2004; Pujol-Buxó et al. 2012), and food availability (Alford and Harris 1988; Morey and Reznick 2000; Lind et al. 2008) as well as a variety of abiotic parameters such as pool temperature (Newman 1998) and desiccation (Richter-Boix et al. 2006; Lind et al. 2008; Márquez-García et al. 2009).
One of the factors that conditions the timing of metamorphosis that has been most studied is food availability. It is known that, if food is abundant, organisms undergo metamorphosis earlier and when they are larger than they do if food is scarce (Gotthard and Sören 1995; Morey and Reznick 2000; Lind et al. 2008). This could be explained by them reaching the development threshold earlier under abundant food conditions, as they have more energy available to allocate to growth. Furthermore, only a slight delay in maturity after the developmental threshold has been reached will produce a large increase in weight in animals exposed to an abundance of food (Lind et al. 2008).
A major threat for developing tadpoles that has been well studied is pool desiccation (Newman 1992). Many amphibians breed in temporary ponds that are sporadically filled by rain and then dry out at different rates. Such species have evolved adaptive plasticity, including the capacity to accelerate development in response to pond drying; these traits allow successful development (Denver et al. 1998; Leips et al. 2000; Loman and Claesson 2003; Merilä et al. 2004). When the risk of death in the larval environment increases as a function of time, metamorphosis may be favored at a younger age, in spite of the costs associated with a smaller body size (Loman and Claesson 2003; Rudolf and Rödel 2007; Márquez-García et al. 2009).
As ponds dry, tadpole density increases, thus reducing the per capita resources through the reduction of the available foraging range (Leips et al. 2000). Therefore, the two variables affecting the timing of metamorphosis (food availability and pool desiccation) must act together in nature. The effects of each of them are well known on their own and there have been several attempts to unravel the effects of their interaction (Semlitsch 1987; Newman 1989). Nevertheless, we consider the experimental design in the papers addressing the interaction not to be entirely appropriate as the results could have been influenced by other unmeasured factors (e.g., temperature or changes in water chemistry). For instance, Semlitsch (1987) stated that “water temperature in the drying ponds becomes more variable (increase in range during a 24-h period) at the end of the experiment and probably contributed to the overall ‘drying effect’.”
In this study, we evaluate the effects of food availability and pool desiccation, separately and acting together, on the painted frog Discoglossus pictus (Otth 1837). The species is native to North Africa, and was introduced into Europe where it is currently expanding (Geniez and Cheylan 1987; Llorente et al. 1995, 2001; Barbadillo et al. 1999; Salvador and García París 2001; Franch et al. 2007; Martínez-Solano 2009). We consider the adaptability that this invasive species shows in its new Mediterranean distribution area, where pond drying is a normal event, to be particularly interesting. We also analyze the plasticity of the life-history traits of this species. Furthermore, we evaluate which metamorphosis timing model best fits our data.
Materials and methods
Study species and populations
Discoglossus pictus is a North African anuran species that was first introduced into Europe in the late nineteenth century, but the introduction was not successful until the early twentieth century (Martínez-Solano 2009). Since then, D. pictus has expanded its area of distribution and is now present in the northeast of Spain and the south of France (Geniez and Cheylan 1987; Llorente et al. 1995, 2001; Barbadillo et al. 1999; Salvador and García París 2001). The species breeds in irrigation canals, streams, natural ponds and even in pools of seawater with high salinity (Llorente et al. 1997). D. pictus shows a clear preference for ephemeral and temporary ponds (Richter-Boix et al. 2012), representing 70 % of the ponds used (personal data). They usually lay their egg clutches in small ponds (from 20 × 20 cm to 10 × 20 m) and therefore often suffer from high mortality rates owing to pool desiccation (Martínez-Solano 2009).
Experimental procedures
We collected three clutches of eggs from Riudarenes (Girona, Spain) to try to include some variability in the sample, but the specific genetic make-up was not within the scope of the study. The eggs were transported to the laboratory where the experiment was carried out, and each clutch was kept in individual plastic containers with dechlorinated tap water until the larvae reached developmental stage 25 (Gosner 1960).
In order to evaluate the effects of both food availability and desiccation, we designed a 2 × 4 factorial experiment. A “food availability” factor with two levels [ad libitum (A) and restricted (R) food supply] and a “hydroperiod” factor with four levels [constant (C), fast drying (F), variable (V) and slow drying (S)]. Each treatment consisted of 10 randomly selected tadpoles from each of the three egg clutches (240 tadpoles in total) being placed individually in 1-L plastic containers, and all 240 containers were initially filled with 700 mL of dechlorinated tap water. The water was changed every 3 days and the tadpoles were fed with lightly boiled spinach (ad libitum; A) or once every two water changes (restricted food supply; R). The fast drying hydroperiod (F) lasted 30 days, the variable level (V) dried for 23 days at the fast drying rate and then the water column was restored and the slow drying (S) lasted for 60 days. For each water regime, the level was decreased following the curve defined by Wilbur (1987). When the water depth reached the zero level, the bottoms of the plastic containers were covered with wet filter paper, which simulated moist soil. All the treatments were conducted at the same time, under the same conditions of photoperiod (12L:12D) and at 20–22 °C.
Response variables
The containers were checked daily and the larval period (Gosner stage 25–42) and the time to the end of metamorphosis (Gosner stage 25–46) of each animal were recorded. The time from stage 42 to 46 (tail resorption) was also noted. The tadpoles that did not accomplish metamorphosis before the pond dried were considered dead, and emergence and survival rates were calculated accordingly.
When the toadlets reached the climax of metamorphosis, we measured their hopping performance by placing them into a cage covered with wet filter paper and marking the successive landing points of their jumps until they showed signs of fatigue (Richter-Boix et al. 2006). After that, we analyzed the total distance covered, the length of the longest jump, the average of all the jumps, and the average of the 5 longest jumps. The jumps were measured in centimetres with a ruler.
After jumping performance, the toadlets were sedated using tricaine methane sulphonate (MS-222) and weighed to a precision of 0.001 g. We also took photographs of each specimen and made eight measurements of the body and hind limbs (body length, head width, femur length, femur width, tibio-fibula length, tibio-fibula width, foot length, and phalanx length) using SigmaScan Pro 5.0. All the morphological measurements were taken in millimetres.
The metamorphs were released to their original location after they had been photographed.
Statistical analysis
We analyzed the effects of food availability (FA) and different hydroperiods (H) on life-history traits, morphology and jumping performance using mixed linear models for each of the variables measured. Water regime and food availability were treated as fixed factors and the clutch (Cl) as a random effect. We constructed a full factorial model in order to study the combined effect of food availability and hydroperiod (FA × H), among clutch variation in plastic responses to food availability and hydroperiod (FA × Cl and H × Cl) and all the factors together (FA × H × Cl). Tukey post hoc tests were performed to test for differences among treatments. Tests were conducted using the SPSS 16 for mac statistical package.
In order to test whether the L-shaped reaction norm predicted by the threshold model (Day and Rowe 2002) was indeed produced in D. pictus by different food supplies, we plotted the weight at metamorphosis as a function of developmental time. The same was done for the tadpoles fed ad libitum in order to see what was the reaction norm of the weight in front of distinct hydroperiods. We then adjusted different regression lines to both plots and we chose the quadratic as it showed the highest R 2 value.
To look for differences in metamorph phenotypes in response to food availability and desiccation, we first tested for shared allometry between treatments through common principal component analysis (CPCA) using the cpcbp v.0.3.2.1. package (Bolker and Phillips 2011) in R v.2.11.1. (R Development Core Team 2007). This approach allows us to compare the phenotypic covariance matrices of the log-transformed morphological measures between treatments (Flury 1988; McCoy et al. 2006). If a common size axis is present (i.e. if they share their first CPC), the data can be size-corrected and analyzed to observe the size-independent variation in shape. If no common size axis is present, the size correction is not possible (McCoy et al. 2006). We conducted independent CPCA in order to study the effects of food availability (AC vs. RC treatment), desiccation (AC vs. AF vs. AV vs. AS) and the interaction of both (all treatments).
Because CPC1 differed for some of our treatments, we analyzed the allometric growth relationships between morphological measurements in a pairwise fashion (McCoy 2007; Touchon and Warkentin 2011). This kind of relationship is defined by the equation y = bx a, where y and x are the aspects of body shape of interest (log-transformed), b the intercept and a the slope (allometric scaling component) of the regression line (Huxley 1932). We compared the variation of the allometric scaling component as it is known to reflect the relative size of a body measurement versus another, regardless of overall size differences (Touchon and Warkentin 2011). The allometric scaling component was calculated via ordinary least squares regressions.
As the size correction was not possible, we analyzed the allometric scaling component of the regression between the log-transformed longest jump and tibio-fibula length. Although we chose the log-tibio-fibula length (as it has been used in previous studies related to locomotor speed; Llewelyn et al. 2010), similar results were obtained using the femur length and body length (results not shown). This technique was used to help us understand the change of this relationship between treatments, not to provide statistical inference.
Results
Percentage of emergence and survival
The percentage of individuals that survived to metamorphosis ranged from 56.67 to 100 % depending on the treatment. The treatment in which the fewest tadpoles metamorphosed was the RF (56.67 %), while, in the AF treatment, 86.67 % of tadpoles survived and emerged. All that mortality was due to the failure of tadpoles to metamorphose before complete desiccation. Only one other tadpole, from the AV treatment, died, but that was due to external factors (probably a fungal infection). The other treatments showed 100 % survival.
Life-history traits
The larval period was influenced by food availability (F 1, 225 = 2,166.208, P < 0.001), hydroperiod (F 3, 225 = 5.934, P = 0.031), and the interaction between them (F 3, 225 = 5.498, P = 0.037). No among clutch differences were detected (F 2, 225 = 0.3, P = 0.766).
In all the different hydroperiods, the tadpoles fed ad libitum started metamorphosing earlier than those subjected to food restriction. The individuals fed ad libitum responded to pond desiccation by shortening the larval period in the two fast drying treatments (F and V: P < 0.001) but not when the desiccation rate was slow (P = 0.436). All the tadpoles under food restriction reached Gosner stage 42 at the same time, with the exception of individuals under the slow drying treatment (P < 0.001), which metamorphosed later (Fig. 1a; Table 1).
a Larval period and b body length (mean ± 1SE) in Discoglossus pictus tadpoles fed ad libitum (filled circle) and undergoing food restriction (empty circles) in four different hydroperiod treatments, control (C), fast drying (F), variable (V) and slow drying (S). Different letters above datapoints (z, y, x, w) indicate statistical differences in the post hoc analysis between treatments
The tadpoles that experienced abundant food completed tail resorption sooner than individuals with reduced food availability (F 1, 220 = 117.778, P = 0.007), except in the case of the slow desiccation treatment, where there was no difference between the two food availabilities (AS = 4.733 ± 0.166; RS = 4.633 ± 0.337) (F 1, 58 = 0.803, P = 0.436). Pool desiccation had no effect on this life-history trait (F 3, 220 = 1.671, P = 0.268).
The time until the end of metamorphosis was also affected by food availability (F 1, 220 = 8,095.17, P < 0.001), hydroperiod (F 3, 220 = 6.334, P = 0.027) and their interaction (F 3, 220 = 5.969, P = 0.03). We observed a significant H × Cl interaction, which means there were among clutch differences for the age at stage 46 due to distinct desiccation levels (F 6, 220 = 5.475, P = 0.029). The pattern observed was almost the same as that of the larval period. The only difference is that, as the individuals in the AS treatment took longer to resorb their tails than the rest of the ad libitum treatments, they took longer from the start of the experiment to complete metamorphosis (AS = 29.033 ± 0.277 vs. AC = 27.6 ± 0.459) (see Table 1).
Size and weight at metamorphosis
Size (total body length) and weight at metamorphosis were both significantly lower with the restricted food supply in all water regimes (Tables 1, 2, 3; Fig. 2). Although the hydroperiod did not affect these two traits, we observed that in combination with food availability it had a significant influence. Size was influenced by a clutch factor and the response of the different clutches to the different desiccation treatments turned out to be distinct (Table 2). In the case of weight, we detected a clutch effect, which translated to different responses to food availability (FA × Cl) and pool desiccation (H × Cl) (Table 3).
All the tadpoles under the ad libitum treatments reached the same weight, except the AV which passed to the terrestrial stage at a lower mass. In contrast, the RC and RV tadpoles reached the same size and were larger than the RF and RS treatments. We obtained the same results for size (body length) at the end of metamorphosis (Fig. 1b; Table 1).
There is an inverse relationship between age and weight at the end of metamorphosis. Regardless of hydroperiod, individuals with abundant food reached the juvenile state earlier and bigger than those with less food (Fig. 2a; F 2, 219 = 94.930, P < 0.001, R 2 = 0.4644). The influence of pool desiccation on this trade-off was evaluated by plotting only the tadpoles fed ad libitum, as these animals only responded to pool desiccation. We observed that the individuals that accelerated or delayed metamorphosis had lower body weights, although the correlation was non-significant (Fig. 2b; F 2, 212 = 1.125, P = 0.328, R 2 = 0.0197).
Age and size at the end of the metamorphosis. a The typical L-shaped reaction norm produced by the differential food supply. Filled circles represent tadpoles fed ad libitum and empty circles larvae undergoing food restriction. b The reaction norm of tadpoles fed ad libitum with four hydroperiod treatments: control (filled circles), fast drying (empty circles), variable (filled triangles) and slow drying (gray triangles). Quadratic regression is shown by the solid line in each panel
Morphology of post-metamorphic individuals
We obtained the same results for all the absolute morphological measurements, so only the results for the absolute femur length are commented on (Table 1). The food availability influenced the absolute length of the femur (F 1, 220 = 134.229, P = 0.007) and tadpoles fed ad libitum resulted in metamorphs with longer femurs (Fig. 3a). The influence of the hydroperiod alone on this trait was not significant (F 3, 220 = 2.045, P = 0.209), but we detected a significant effect of the interaction of both factors (F 3, 220 = 25.234, P = 0.001). A clutch component that determined the length of the femur in response to distinct hydroperiods was also detected (F 6, 220 = 4.803, P = 0.039). The pattern observed was the same as that of the weight and size at metamorphosis (Fig. 3a; compare with Fig. 1b).
Food availability and desiccation, as well as their interaction had effects on metamorph morphology. a Femure length (mean ± SE) at the end of metamorphosis for four different hydroperiods, control (C), fast drying (F), variable (V) and slow drying (S). Pairwaise comparisons of b femur length and body length, c femur width and femur length, and d tibio-fibula length and femure length (slope ± SE of log–log plots, with log base 10). See Fig. 1 for symbols and abbreviations
The CPCA revealed that neither food availability (P = 0.377) nor desiccation (P = 0.343) produced differences in the body size axis. Interestingly, metamorphs did not share common allometry due to the interaction of factors (P = 0.021). Therefore, morphology could not be size-corrected for shape comparisons and we studied the allometric scaling relationships between different biomechanically important aspects (Zug 1972; Choi and Park 1996; Choi et al. 2003; Handrigan and Wassersug 2007). Food restriction resulted in a less steep slope between the femur and body length in the absence of desiccation. However, when any rate of desiccation was present, the differences produced by food availability disappeared and the slope approached 1 (isometry between femur and body length) (Fig. 3b), suggesting an interactive effect of food availability and pond duration. The allometric scaling component of the femur width and femur length was higher in the treatments where tadpoles had been subjected to a slow desiccation rate (a > 1). This was true for the tadpoles fed ad libitum, but not for the tadpoles with restricted food supply, as the standard errors between RC and RS overlapped. Therefore, it can be said that, when tadpoles had enough food available and were subjected to slow desiccation rate, they allocated more to femoral musculature relative to femur length. At higher desiccation rates, the scaling component remained near or below isometry (Fig. 3c). The lack of desiccation allowed tadpoles to display near or higher than 1 slope values for the tibio-fibula and femur length regression. This indicates a greater allocation to the distal part of the leg. In the ad libitum treatments, no differences produced by desiccation were detected, with the exception of AV, in which the gradient was lower. With food restriction, in contrast, the fast desiccation rates (RF and RV) resulted in shorter tibio-fibula lengths relative to femur length (a < 1) (Fig. 3d).
Hopping performance
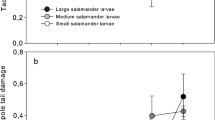
Food availability had a significant effect on the total distance covered by metamorphs, but pool desiccation did not. The interaction between the two factors also produced significant differences. Furthermore, a clutch effect was observed that affected capabilities when faced with pond drying (Table 4). Tadpoles fed ad libitum jumped a larger total distance than those with restricted feeding (see Table 1). Feeding, but not desiccation, had a significant effect on the longest jump (F 1, 220 = 99.574, P = 0.01), the average of all the jumps (F 1, 220 = 52.144, P = 0.018) and the average of the 5 longest jumps (F 1, 220 = 92.543, P = 0.01). The FA × H × Cl interaction also influenced the average of the 5 longest jumps (F 6, 220 = 2.158, P = 0.049) and the longest jump (F 6, 220 = 2.495, P = 0.024). Although the longest jump was always bigger for the tadpoles fed ad libitum than for those undergoing feeding restriction (see Table 1), the slope between the log-transformed longest jump against tibio-fibula length was higher for the animals with a restricted food supply (Fig. 4). Moreover, the differences became bigger due to the effect of desiccation, as the AC versus RC and AF versus RF treatment comparisons suggest. The feeding treatment did not influence that relationship either in the V or S treatments. The slow drying treatment on its own provoked an increase in the steepness of that relationship slope.
Comparison between the longest jump and the tibio-fibula length at the end of metamorphosis (slope ± SE of log–log plots, with log base 10) for four different hydroperiod treatments. See Fig. 1 for symbols and abbreviations
Discussion
As previously reported in other work (Morey and Reznick 2000; Lind et al. 2008), we found an inverse relationship between age and size at metamorphosis under different food availabilities. This relationship is predicted by the threshold proposed in the Wilbur–Collins model (Wilbur and Collins 1973; Day and Rowe 2002), and the predicted L-shaped reaction norm is also confirmed by our data (Fig. 2a). These results support the thesis that slow growth delays metamorphosis as the tadpoles take longer to reach the threshold weight and size. In contrast, fast growth has opposite consequences (Day and Rowe 2002). Unfortunately, our experimental design did not allow us to test whether the growth rate became fixed at some point (Travis 1984) or it continued to be susceptible to change at every moment (Alford and Harris 1988; Leips and Travis 1994).
The capacity to accelerate metamorphosis when faced with a drying pond (Leips et al. 2000; Merilä et al. 2004; Richter-Boix et al. 2006; Márquez-García et al. 2009) is also confirmed by our results, which demonstrate that pond desiccation provokes a shortening of the larval period. However, it does not influence the time needed to resorb the tail, so the differences detected in the time elapsed until the end of metamorphosis are fully due to the shortened larval period of the tadpoles under drying treatments. Surprisingly, tadpoles under the AS treatment needed more time to resorb the tail than the control tadpoles (AC), so they metamorphosed later, which is in disagreement with the published literature (Márquez-García et al. 2009). We postulate that a slowly drying pond represents stress but not enough to accelerate metamorphosis. The animals under these conditions experienced a cost associated with living in a stressful environment but they were not induced to metamorphose earlier. These specimens slowed tail resorption. This extended time of tail resorption could be hormonally mediated by prolactin secretion (Brodeur et al. 2009), or merely a consequence of reduced energy intake during the larval period (Hourdry and Beaumont 1985). The observed lower weight of the AS metamorphs seems to support the latter explanation.
Although both environmental cues are important in determining the timing of metamorphosis (Wilbur and Collins 1973; Werner 1986; Morey and Reznick 2000; Márquez-García et al. 2009), we think that food availability is probably the more important factor and abundant food is needed to respond to pool desiccation. When well-fed tadpoles are exposed to pond desiccation, they are capable of accelerating metamorphosis, but when food resources are limited, they cannot respond to pond desiccation by increasing their rate of development. In the latter situation, tadpoles receive two cues that work in opposite directions: pond drying pushes them to metamorphose earlier, but food deprivation incapacitates them from doing so. Recently, Kulkarni et al. (2011) have demonstrated that tadpoles invest most of their body fat in order to accelerate metamorphosis when faced with pond drying, supporting our results. Amazingly, the RS tadpoles took longer than the RC tadpoles to start and end metamorphosis. In this case, pond desiccation was not enough to trigger the tadpoles to metamorphose earlier (as in Gervasi and Foufopoulos 2008) but it constituted a stressful situation. So, the animals had lower developmental and growth rates related to food deprivation and, furthermore, they had to pay the energetic cost associated with the stressful situation.
Hence, our results support Werner’s model (Werner 1986), but indicate that growth conditions and mortality rates are not equally important. Instead, growth conditions seem to determine the response to increasing mortality risk. Therefore, only the most competitive tadpoles, those that best exploit the resources, are able to respond to a drying pond and successfully metamorphose.
The size and weight at metamorphosis were influenced by food availability but not by the distinct water regimes, as previously indicated by Gervasi and Foufopoulos (2008) (but see Denver et al. 1998; Morey and Reznick 2000; Merilä et al. 2004). However, the AV tadpoles appeared to be smaller and lighter. All this evidence indicates that D. pictus is able to increase its rate of development without decreasing its growth rate (Gervasi and Foufopoulos 2008) except in the case of the variable water regime. In that case, the tadpoles accomplished metamorphosis earlier with smaller size and weight. Rudolf and Rödel (2007) underlined the importance of the unpredictability of the environment where the tadpoles grow, and that could be an explanation of our results. When the water volume was restored, the tadpoles did not readjust their accelerated development, but probably suffered from a reduced energy intake due to an unexpectedly high water level. Even though they paid that cost, they had a greater survival rate than those exposed to the fast drying treatment owing to the water level recovery.
The reaction norm that the tadpoles fed ad libitum and subjected to distinct water regimes followed indicates that, although there are no statistically significant differences, the acceleration of metamorphosis resulted in slightly lighter metamorphs (either because of the energetic cost of accelerating metamorphosis or because of the shortened growth period), and that some tadpoles experienced worse growth conditions and, because of that, they delayed the metamorphosis (Fig. 3b).
Size and weight at metamorphosis were also influenced by the interaction between food availability and desiccation. We observed that tadpoles exposed to food restriction and desiccation (either fast or slow) had reduced size and weight (as previously reported in Denver et al. 1998; Morey and Reznick 2000; Merilä et al. 2004). However, although the tadpoles under the variable water regime were subjected to fast drying, the restoration of the water level meant they did not suffer in terms of reduced size and weight. As small metamorphs show reduced survival to maturity (Goater 1994; Newman and Dunham 1994), smaller size at maturity (Smith 1987), and smaller clutch size (Wilbur 1977; Berven 1981; Smith 1987), the costs of living in a pond (drying or not) with low food availability are clear.
Food availability and the interaction of both environmental factors were responsible for differences in the absolute morphological measurements. As suggested by the threshold model of Day and Rowe (2002), the entire toadlets were smaller due to food restriction. The CPCA revealed that metamorphs shared common allometry when they had faced only one of the stimuli in their larval stage, but that the combination of them resulted in differences in the growth axis. Both desiccation and resource availability had effects on the allometric scaling components of the pairwise comparisons studied. Tadpoles in the AC treatment showed the highest increase in femur length with increasing body size and also higher tibio-fibula length with augmenting femur length (the last statement was also true for the RC treatment). These elements are the most relevant ones involved in the jumping biomechanics; the bigger the hind limb, the higher the jumping capacity. Moreover, it has been demonstrated that strong jumpers have a longer tibio-fibula than femur (Zug 1972; James and Wilson 2008), and therefore we could say that metamorphs from the AC treatment had the most effective morphology for jumping. This, however, was counteracted by desiccation. At any desiccation rate, the slopes of the log–log plots overlapped, and the relationship between femur and body length became more or less isometric, while the relationship between tibio-fibula length and femur length turned to below isometry. Surprisingly, when tadpoles had been subjected to a slow desiccation rate but with enough resource availability, the allocation in femoral musculature (measured as femur width) relative to femur length was higher than in any other treatment (with restricted food supply standard errors overlapped between RC and RS). We hypothesize that, as mentioned above, slow drying represents a stressful situation, which leads to a higher allocation in the musculature involved in jumping at the expense of lower size and weight. Nevertheless, this hypothesis needs to be addressed in more detail.
All the hopping performance variables were affected absolutely by food abundance. This means that as the metamorphs were bigger due to abundant food, their jumping capacity was enhanced (Gomez-Mestre et al. 2010). The interaction of food availability and pond desiccation only affected the total distance covered among the hopping performance variables. This result suggests that, although the average length of the jumps and the longest jump are only determined by the food supply, the total distance covered is somehow dependent on the hydroperiod of the pond where the tadpole grew. We can say that, while the one-off ability of D. pictus to escape is not influenced by the hydroperiod, this environmental factor in combination with food availability determines the total distance that can be covered. Since jumping ability is known to have a positive influence on food acquisition (Walton 1988) and predator avoidance (Wassersug and Sperry 1977), underfed tadpoles would have reduced fitness in nature. We also found differences in the slope of the regression between the log-transformed longest jump and log-transformed tibio-fibula length due to food availability. Besides, when combined with desiccation, these differences became greater. Similarly to the femur length, the tibio-fibula was shorter in the tadpoles undergoing feeding restriction, and the greatest difference was observed in the AF versus RF treatments. Consequently, we argue that the same increase in length for a shorter tibio-fibulas leads to greater improvement in the distance of the longest jump than for longer tibio-fibulas. As we pointed out previously, the animals in the AS treatment showed more femoral musculature relative to femur length, and this seems to be the reason why their increase in longest jump relative to tibio-fibula length was higher.
Finally, we have likewise shown that, within the same population, there are among clutch differences that provide distinct capacities to respond to environmental cues, as previously reported for different populations (Merilä et al. 2004; Lind et al. 2008). However, although the level to which they responded to the factors was not the same, we have to say that all of them did respond to the different environmental conditions and that the direction of their responses was the same.
Our data show that this species is highly adapted to drying water ponds because no tadpoles died when facing only desiccation. However, when food was restricted and the pond dried, the mortality rate increased. Nevertheless, survival continued to be high, which suggests that this species would be a good competitor in nature. So, we think that the invasive strength of this species comes from its capacity to survive even in the most stressful ponds. Therefore, this species is preadapted for the conditions of its new Mediterranean distribution area, which is known to have a lot of temporary ponds.
References
Alford RA, Harris RN (1988) Effects of larval growth history on anuran metamorphosis. Am Nat 131(1):91–106
Barbadillo LJ, Lacomba JI, Pérez-Mellado V, Sancho V, López-Jurado LF (1999) Anfibios y reptiles de la Peninsula Iberica, Baleares y Canarias. Planeta, Barcelona
Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Evol Syst 35:651–673
Berven KA (1981) Mate choice in the wood frog Rana sylvatica. Evolution 35:707–722
Bolker B, Phillips C (2011) Package “cpcbp”. Available from http://www.math.mcmaster.ca/~bolker/R/src/contrib/cpcbp_0.3.2.1.tar.gz
Brodeur JC, Svartz G, Perez-Coll CS, Marino DJG, Herkovits J (2009) Comparative susceptibility to atrazine of three developmental stages of Rhinella arenarum and influence on metamorphosis: non-monotonous acceleration of the time to climax and delayed tail resorption. Aquat Toxicol 91:161–170
Choi IH, Park K (1996) Variations in the take-off velocity of anuran amphibians: relation to morphology, muscle contractile function and enzyme activity. Comp Biochem Physiol 113A:393–400
Choi IH, Shim JH, Ricklefts RE (2003) Morphometric relationships of take-off speed in anuran amphibians. J Exp Zool A Comp Exp Biol 299:99–102
Day T, Rowe L (2002) Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am Nat 159:338–350
Denver RJ, Mirhadi N, Phillips M (1998) Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 79:1859–1872
Flury B (1988) Common principal components and related multivariate models. Wiley, New York
Franch M, Llorente GA, Montori A, Richter-Boix A, Carranza S (2007) Discovery of an introduced population of Discoglossus pictus beyond its known distributional range. Herpetol Rev 38:356–359
Geniez P, Cheylan M (1987) Atlas de distribution des reptiles et des amphibiens du Languedoc-Roussillon. Laboratoire de Biogéographie et Ecologie des Vertébrés, Montpellier
Gervasi SS, Foufopoulos J (2008) Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct Ecol 22:100–108
Goater CP (1994) Growth and survival of postmetamorphic toads: interactions among larval history, density and parasitism. Ecology 75:2264–2274
Gomez-Mestre I, Saccoccio VL, Iijima T, Collins EM, Rosenthal GG, Warkentin KM (2010) The shape of things to come: linking developmental plasticity to post-metamorphic morphology in anurans. J Evol Biol 23:1364–1373
Gosner KL (1960) A simplified table for staging anuran embryos larvae with notes on identification. Herpetologica 16:183–190
Gotthard K, Sören N (1995) Adaptive plasticity and plasticity as an adaptation: a selective review of plasticity in animal morphology and life history. Oikos 74:3–17
Handrigan GR, Wassersug RJ (2007) The anuran Bauplan: a review on the adaptive, developmental, and genetic underpinnings of frog and tadpole morphology. Biol Rev 82:1–25
Harris RN (1999) The anuran tadpole: evolution and maintenance. In: McDiarmid RW, Altig R (eds) Tadpoles. The biology of anuran larvae. University of Chicago Press, Chicago, pp 279–294
Hensley FR (1993) Ontogenetic loss of phenotypic plasticity of age at metamorphosis in tadpoles. Ecology 74:2405–2412
Hourdry J, Beaumont A (1985) Les métamorphoses des amphibiens. Masson, Paris
Huxley JS (1932) Problems of relative growth. Lincoln MacVeagh Dial, New York
James RS, Wilson RS (2008) Explosive jumping: extreme morphological and physiological specializations of Australian rocket frogs. Physiol Biochem Zool 81(2):176–185
Kulkarni SS, Gomez-Mestre I, Moskalik CL, Storz BL, Buchholz DR (2011) Evolutionary reduction of developmental plasticity in desert spadefoot toads. J Evol Biol 24:2445–2455
Laurila A, Pakkasmaa S, Crochet PA, Merilä J (2002) Predator-induced plasticity in early life history and morphology in two anuran amphibians. Oecologia 132:524–530
Leips J, Travis J (1994) Metamorphic responses to changing food levels in two species of hylid frogs. Ecology 75(5):1345–1356
Leips J, Mcmanus MG, Travis J (2000) Response of treefrog larvae to drying ponds: comparing temporary and permanent pond breeders. Ecology 81:2997–3008
Lind MI, Persbo F, Johansson F (2008) Pool desiccation and developmental thresholds in the common frog, Rana temporaria. Proc R Soc Lond B 275:1073–1080
Llewelyn J, Phillips BL, Alford RA, Schwarzkopf L, Shine R (2010) Locomotor performance in an invasive species: cane toads from the invasion front have greater endurance, but not speed, compared to conspecifics from a long-colonised area. Oecologia 162:343–348
Llorente GA, Montori A, Santos X, Carretero MA (1995) Atlas dels amfibis i reptils de Catalunya i Andorra. El Brau, Barcelona
Llorente GA, Montori A, Santos X, Carretero MA (1997) Discoglossus pictus. In: Pleguezuelos JM (ed) Distribución y biogeografía de los anfibios y reptiles en España y Portugal. Asociación Herpetológica Española—Universidad de Granada, Granada, pp 137–139
Llorente GA, Montori A, Santos X, Carretero MA (2001) Discoglossus pictus (Otth 1837). Sapillo pintojo mediterráneo. In: Pleguezuelos JM, Márquez R, Lizana M (eds) Atlas y libro rojo de los anfibios y reptiles de España. Ministerio de Medio Ambiente, Madrid, pp 91–93
Loman J, Claesson D (2003) Plastic response to pond drying in tadpoles Rana temporaria: tests of cost models. Evol Ecol Res 5:179–194
Márquez-García M, Correa-Solis M, Sallaberry M, Méndez MA (2009) Effects of pond drying on morphological and life-history traits in the anuran Rhinella spinulosa (Anura: Bufonidae). Evol Ecol Res 11:803–815
Martínez-Solano Í (2009) Sapillo pintojo mediterráneo—Discoglossus pictus Otth 1837. In: Salvador A, Martínez-Solano Í (eds) Enciclopedia virtual de los vertebrados españoles. Museo Nacional de Ciencias Naturales, Madrid, pp 1–13. http://www.vertebradosibericos.org/
McCoy MW (2007) Conspecific density determines the magnitude and character of predator-induced phenotype. Oecologia 153:871–878
McCoy MW, Bolker BM, Osenberg CW, Miner BG, Vonesh JR (2006) Size correction: comparing morphological traits among populations and environments. Oecologia 148:547–554
Merilä J, Laurila A, Lindgren B (2004) Variation in the degree and costs of adaptive phenotypic plasticity among Rana temporaria populations. J Evol Biol 17:1132–1140
Morey S, Reznick D (2000) A comparative analysis of plasticity in larval development in three species of spadefoot toads. Ecology 81:1736–1749
Morey SR, Reznick D (2004) The relationship between habitat permanence and larval development in California spadefoot toads: field and laboratory comparisons of developmental plasticity. Oikos 104:172–190
Newman RA (1989) Developmental plasticity of Scaphiopus couchii tadpoles in an unpredictable environment. Ecology 70:1775–1787
Newman RA (1992) Adaptive plasticity in amphibian metamorphosis. Bioscience 42:671–678
Newman RA (1998) Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with responses to changing food level. Oecologia 115:9–16
Newman RA, Dunham AE (1994) Size at metamorphosis and water loss in a desert anuran (Scaphiopus couchii). Copeia 1994:372–381
Plaistow SJ, Lapsley CT, Beckerman AP, Benton TG (2004) Age and size at maturity: sex, environmental variability and developmental thresholds. Proc R Soc Lond B 271:919–924
Pujol-Buxó E, San-Sebastián O, Garriga N, Llorente GA (2012) How does the invasive/native nature of species influence tadpoles’ plastic responses to predators? Oikos. doi:10.1111/j.1600-0706.2012.20617.x
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Richter-Boix A, Llorente GA, Montori A (2004) Responses to competition effects of two anuran tadpoles according to life-history traits. Oikos 106:39–50
Richter-Boix A, Llorente GA, Montori A (2006) Effects of phenotypic plasticity on post-metamorphic traits during pre-metamorphic stages in the anuran Pelodytes punctatus. Evol Ecol Res 8:309–320
Richter-Boix A, Garriga N, Montori A, Franch M, San Sebastian O, Villero D, Llorente GA (2012) Effects of the non-native amphibian species Discoglossus pictus on the recipient amphibian community: niche overlap, competition and community organization. Biol Invasions. doi:10.1007/s10530-012-0328-4
Rose CS (2005) Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends Ecol Evol 20:129–135
Rowe L, Ludwig D (1991) Size and timing of metamorphosis in complex life cycles: time constraints and variation. Ecology 72:413–427
Rudolf VHW, Rödel M-O (2007) Phenotypic plasticity and optimal timing of metamorphosis under uncertain time constraints. Evol Ecol 21:121–142
Salvador A, García París M (2001) Anfibios españoles. Identificación, historia natural y Distribución. Esfagnos, Talavera de la Reina
Savage RM (1952) Ecological, physiological and anatomical observations on some species of anuran tadpoles. Proc Zool Soc Lond 122:467–514
Savage RM (1962) The ecology and life history of the common frog: Rana temporaria temporaria. Hafner, New York
Semlitsch RD (1987) Paedomorphosis in Ambystoma talpoideum: effects of density, food, and pond drying. Ecology 68:994–1002
Smith DC (1987) Adult recruitment in chorus frog: effects of size and date at metamorphosis. Ecology 68:344–350
Tejedo M, Reques R (1994) Plasticity in metamorphic traits of natterjack tadpoles: the interactive effects of density and pond duration. Oikos 71:295–304
Touchon JC, Warkentin KM (2011) Thermally contingent plasticity: temperature alters expression of predator-induced colour and morphology in a neotropical treefrog tadpole. J Anim Ecol 80:79–88
Travis J (1984) Anuran size at metamorphosis: experimental test of a model based on intraspecific competition. Ecology 65:1155–1160
Van Buskirk J, Saxer G (2001) Delayed costs of an induced defense in tadpoles? Morphology, hopping, and development rate at metamorphosis. Evol Int J Org Evol 55:821–829
Walton M (1988) Relationships among metabolic, locomotory, and field measures of organismal performance in the fowler’s toad (Bufo woodhousei fowleri). Physiol Zool 61:107–118
Wassersug RJ, Sperry DG (1977) The relationship of locomotion to differential predation on Pseudacris triseriata (Anura: Hylidae). Ecology 58:830–839
Watkins TB (2001) A quantitative genetic test of adaptive decoupling across metamorphosis for locomotor and life-history traits in the pacific tree frog, Hyla regilla. Evol Int J Org Evol 55:1668–1677
Wells KD (2007) Complex life cycles and the ecology of amphibian metamorphosis. In: Wells KD (ed) The ecology and behavior of amphibians. University of Chicago Press, Chicago, pp 599–644
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341
Wilbur HM (1977) Propagule size, number, and dispersion patterns in Ambystoma and Rana sylvatica. Am Nat 111:43–68
Wilbur HM (1980) Complex life cycles. Ann Rev Ecol Syst 11:67–93
Wilbur HM (1987) Regulation of structure in complex systems: experimental temporary pond communities. Ecology 68:1437–1452
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis: nonnormal distributions of competitive ability reflect selection for facultative metamorphosis. Science 182:1305–1314
Zug GR (1972) Anuran locomotion: structure and function. I. Preliminary observations on relation between jumping and osteometrics of appendicular and postaxial skeleton. Copeia 4:613–624
Acknowledgments
We thank the Departament de Medi Ambient i Habitatge of the Generalitat de Catalunya for their support and permission to collect clutches. We also thank Marc Franch for providing the clutches for this study. B. Bolker provided helpful statistical consultation. Francesc Oliva (Departament d’Estadística), Ross Alford (handling editor) and two anonymous reviewers gave helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ross Alford.
Rights and permissions
About this article
Cite this article
Enriquez-Urzelai, U., San Sebastián, O., Garriga, N. et al. Food availability determines the response to pond desiccation in anuran tadpoles. Oecologia 173, 117–127 (2013). https://doi.org/10.1007/s00442-013-2596-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2596-9