Abstract
Predicting the consequences of predator biodiversity loss on prey requires an understanding of multiple predator interactions. Predators are often assumed to have independent and additive effects on shared prey survival; however, multiple predator effects can be non-additive if predators foraging together reduce prey survival (risk enhancement) or increase prey survival through interference (risk reduction). In marine communities, juvenile reef fish experience very high mortality from two predator guilds with very different hunting modes and foraging domains—benthic and pelagic predator guilds. The few previous predator manipulation studies have found or assumed that mortality is independent and additive. We tested whether interacting predator guilds result in non-additive prey mortality and whether the detection of such effects change over time as prey are depleted. To do so, we examined the roles of benthic and pelagic predators on the survival of a juvenile shoaling zooplanktivorous temperate reef fish, Trachinops caudimaculatus, on artificial patch reefs over 2 months in Port Phillip Bay, Australia. We observed risk enhancement in the first 7 days, as shoaling behaviour placed prey between predator foraging domains with no effective refuge. At day 14 we observed additive mortality, and risk enhancement was no longer detectable. By days 28 and 62, pelagic predators were no longer significant sources of mortality and additivity was trivial. We hypothesize that declines in prey density led to reduced shoaling behaviour that brought prey more often into the domain of benthic predators, resulting in limited mortality from pelagic predators. Furthermore, pelagic predators may have spent less time patrolling reefs in response to declines in prey numbers. Our observation of the changing interaction between predators and prey has important implications for assessing the role of predation in regulating populations in complex communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation is a ubiquitous force in nature (Sih et al. 1985; Ricklefs 1987), fundamental to both the regulation of prey populations (Chase et al. 2002) and in shaping community structure (Menge and Sutherland 1987; Gurevitch et al. 2000; Schmitz and Suttle 2001; Stachowicz et al. 2007). Predatory effects may be direct and lethal, or indirect through altering prey behaviour (Lima 1998), morphology (Van Buskirk 2001) or resource allocation (Schmitz et al. 1997). Most prey face multiple predators in natural systems (Schmitz 2007), and single species predator–prey responses overlook the potential for predators to interact and cause non-independent responses in prey (Vance-Chalcraft et al. 2004). Empirical studies assessing the interactive effects of multiple predators on prey are uncommon (Sih et al. 1998), limiting our understanding of the effects of specific predator declines in systems where predator diversity is high (Schmitz 2007).
Recent experimental manipulations have observed that the effects of multiple predators on prey mortality cannot be predicted by summing the effects of predators in isolation (Vance-Chalcraft and Soluk 2005a, b; Griffen and Byers 2006b; Griffen 2006; Carey and Wahl 2010). Termed a multiple predator effect (MPE; Sih et al. 1998), predators do not act independently when foraging on shared prey and may interact with each other directly, or influence the behaviour of prey (Griffen and Williamson 2008). When predators foraging together are more effective than the sum of their separate effects, this results in risk enhancement for prey (Fig. 1a), which occurs when a prey’s response to one predator increases its risk to another predator (DeWitt and Langerhans 2003). Where predators foraging together are less effective than the expected addition of their separate effects, their interaction results in risk reduction for prey (Fig. 1b). Risk reduction may occur either by interference competition amongst predators (Griffen and Byers 2006a) or by direct predation amongst predators (Vance-Chalcraft et al. 2007). Additive mortality (Fig. 1c), or the lack of an MPE, can occur in two ways: where predators’ interactions with prey are independent and foraging is spatially or temporally discrete (Sokol-Hessner and Schmitz 2002), or where one predator does not significantly affect prey, and hence there is no difference in prey survival between both predators together and the more effective predator in isolation (Sih et al. 1998), known as a trivial case of additivity.
Predicted interaction plots of multiple predator effects on prey survival. In all cases the prey survival is on the y-axis on a logarithmic scale, the presence or absence of predator A is on the x-axis and the two lines represent the presence (B+, solid line) or absence (B−, dashed line) of predator B. a Prediction of risk enhancement, where prey mortality is greater in the presence of both predators than that predicted by adding their effects in isolation. Here the slope of the relationship where B is present is significantly less than the slope of the relationship when predator B is absent. b Prediction of risk reduction under a mutual interference model, where the prey survival in the presence of both predators is significantly greater than in the presence of either predator in isolation. c Prediction of an additive relationship between predators A and B, where the slopes of the lines are statistically similar. We assume significant prey survival effects in the presence of either predator in isolation. If only predator A was significant in isolation, slopes would be similar and significantly different to 0, and y-intercepts similar. If only predator B was significant in isolation, slopes would not be different from 0 and y-intercepts would be significantly different. Both of these scenarios are examples of trivial additivity (sensu Sih et al. 1998)
The nature of interactive effects of multiple predators on prey depends on three fundamental traits of the system under study. Firstly, predators may interact in either an antagonistic or facilitative manner, or may not interact at all (Vance-Chalcraft and Soluk 2005a). Secondly, prey can have discrete behavioural responses to different predators, which cannot all be expressed simultaneously when they are confronted with multiple predators and hence an optimal choice of behaviours must be made (Krupa and Sih 1998; Griffen and Byers 2006a). Thirdly, predator and prey behaviour may respond to changes in density of either group. Increased predator density may increase predator interference and hence prey survival (Griffen and Williamson 2008), whereas increases in prey density may alter prey behaviour (i.e. intraspecific aggression) in ways that could increase the strength of MPEs (Losey and Denno 1998; Vance-Chalcraft and Soluk 2005a). Consequently, the dynamic changes in density of both predators and prey will likely affect the nature and strength of MPEs through time, although this prediction has yet to be explicitly tested.
In the marine reef environment, shoaling fish face two predator guilds, benthic and pelagic predators, which are fundamentally different in hunting mode and the spatial and temporal scales over which they forage (White et al. 2010). Benthic predators are resident on reefs and other benthic habitats, have foraging areas similar to that of their prey and are primarily ambush predators (Holbrook and Schmitt 2002). Pelagic predators are transient in their interactions with prey, forage over larger spatial scales than their prey and are primarily chase predators (White et al. 2010). Whilst benthic predators are confined to the same habitat as prey and are limited to targeting local prey resources, pelagic predators will make decisions about where they forage according to energetic costs and benefits, which are often influenced by prey density (Connell 2000; Sandin and Pacala 2005b). Reefs are therefore a useful model system for investigating the effects of two diverse predator guilds with very different foraging relationships with their prey.
Predation of shoaling reef fish by benthic and pelagic predator guilds also presents mechanisms for MPEs. Shoaling provides anti-predator benefits through increased vigilance and reducing per capita predation risk from pelagic predators (Pitcher and Parrish 1993), and by allowing fish to forage beyond the reach of benthic predators (White et al. 2010). However, when both guilds are present, shoaling fish are ‘sandwiched’ between two spatially discrete predator guilds and hence have limited predator-free space (Hixon and Carr 1997). This creates the possibility of synergistic predation, a risk-enhancement mechanism (Sih et al. 1998). Despite this potential risk-enhancement mechanism, the only other study to manipulate predator guilds targeting shoaling prey detected additive mortality (Hixon and Carr 1997).
Declines in prey density can reduce the effectiveness of shoaling (Godin et al. 1988; Morgan 1988; Stier et al. 2012), and reduce targeting by pelagic predators (Anderson 2001). Therefore the nature of predator–prey interactions and potential MPEs could change in experimental situations where prey are depleted over time. Mortality from predation on reef fishes immediately post-settlement [e.g. 55.7 % of fish lost in the first 2 days post settlement across 24 studies of coral reef fishes (Almany and Webster 2006)] is a strong regulator of populations (Schmitt and Holbrook 1999a, b; Shima 1999; Doherty et al. 2004; Sandin and Pacala 2005a; Anderson et al. 2007) and thus assessing how changes in prey densities influence MPEs is relevant for understanding how predation might influence prey mortality during this life history transition.
In this study we examined the interactive effects of benthic and pelagic predator guilds in causing mortality in a shoaling reef fish. Specifically we tested for the presence of MPEs and aimed to identify whether the presence and nature of MPEs change over time in response to declines in prey densities. We conducted our experiments in the field on artificial patch reefs by manipulating initial predator and prey abundance. We did not confine predators to reefs, in order to accommodate the dynamic foraging decisions made by predators in response to changes in abundance of both predators and prey. Predator abundances continue to decline as a result of human harvest (Parsons 1992; Madin et al. 2010) and habitat loss (Bruno and O’Connor 2005; Dobson et al. 2006). By manipulating entire predator guilds in situ, we aimed to better understand the potential impacts of disturbance to marine predators on wider communities.
Materials and methods
We investigated the effects of benthic and pelagic predator guilds on the mortality of a reef fish (the southern hulafish, Trachinops caudimaculatus) on artificial patch reefs in Port Phillip Bay, Victoria, Australia. We used an additive design for the detection of MPEs, by manipulating the initial presence or absence of benthic predators by physical removals, and excluding pelagic predators by netting reefs, to produce an initial crossed design of four present-absent predator treatments. Prey survival was monitored over time, along with the abundance of benthic predators, and the nature of predator effects assessed using multiplicative statistical models.
Study prey species and artificial reefs
T. caudimaculatus is an abundant and conspicuous rocky reef fish in south-east Australia, forming very large (>1,000 fish) and dense (>100 fish m2) shoals in coastal embayments (Hunt et al. 2011). Pelagic larvae settle to rocky reefs between November and January (late spring/early summer) and use the rocky reef structure as a refuge from pelagic predators, diving for cover when threatened. At dusk, fish retreat to refuge in small cracks and holes where they remain until dawn (J. Ford, personal observation). Fish are highly site-attached after settlement; in over 400 dives observing the species, no movement has been recorded across expanses of open water greater than 20 m. Previous experiments with calcein tagging also indicated no movement of fish to neighbouring reefs (M. Le Feuvre and J. Ford, unpublished data).
The study was conducted near Altona in the north of Port Phillip Bay, Victoria, Australia (37°54′S, 144°49′E). Port Phillip Bay is a sheltered embayment with a water area of 1,930 km2 and is connected to the ocean through a restricted entrance 3 km wide. In November–December 2008 we placed 12 artificial reefs in a linear array at 9–10 m depth, each 150 m apart, on open sand approximately 2 km from natural reef. Each artificial reef unit was approximately 0.75 m3 with a base area of 1.1 m2 and constructed of ten modular units stacked two high in an X design (Electronic supplementary material, Fig. S1). Each module consisted of a plastic milk crate (Viscount Plastics IH120, 355 × 355 × 322 mm) lined with eight bricks (230 × 110 × 75 mm), filled with cushion foam in the centre and covered by a concrete paver. Twenty-four of the 80 bricks in a reef had eight 20-mm-diameter holes running the width of the brick to provide a total of 192 small refuge spaces. Each reef unit was covered with a 6-mm PVC sheet measuring 1,600 × 1,200 mm to provide overhangs of 550 and 150 mm on adjacent sides. Larval hulafish settled naturally to artificial reefs during this period and these fish were removed before the experiments commenced. All experiments were conducted after the end of the 2009–2010 summer settlement period and reefs were naturally fouled with encrusting invertebrates and algae.
Predator identification
Predators were first identified and split into benthic and pelagic predator guilds. Taxa identified as benthic predators were fish of the genera Cristiceps and Heteroclinus (Clinidae), Lotella rhacina (Moridae), Genypterus tigerinus (Ophididae), and invertebrates Nectocarcinus spp. (Portunidae), Hapalochlaena maculosa (Octopodidae) and Sepia apama (Sepiidae). Species identified as pelagic predators were the fish Arripis truttacea (Arripidae), Trachurus novaezelandiae (Carangidae), Chrysophrys auratus (Sparidae), and the squid Sepioteuthis australis (Loliginidae), all of which are commercially and recreationally fished (Department of Primary Industries 2008). In all cases, potential predators were selected either from visual observations of feeding on hulafish (Clinidae, Nectocarcinus spp., Sepia apama, C. auratus and Sepioteuthis australis), or published literature listing the species as those which consume fish [Clinidae, L. rhancina, A. trutacea and T. novaezelandiae (Edgar and Shaw 1995; Hindell 2006)]. Whilst we could find no published gut content studies on H. maculosa, the shared use of shelter holes with T. caudimaculatus, observation of strikes on fish in lab aquaria, and diet studies of other octopuses (Grubert 1999; Smith 2003), suggest that small fish in nocturnal refuges are likely prey.
Benthic predator manipulation
We manipulated the initial presence or absence of benthic predators by clearing all artificial reefs of mobile macrofauna. Divers on SCUBA used a clove oil and ethanol mixture to anaesthetise fish and dip nets to capture them. The modular units and lid were then lifted to the surface by winch onto a boat where they and all remaining cryptic benthic predators were removed. Reefs were redeployed and rebuilt at a location 50 m away from the original location but still 150 m from the nearest artificial reef. Predators were returned to six of the reefs in their original abundances (B+) and six were left without benthic predators (B−). Due to the disturbance involved in deconstructing reefs to remove benthic predators, removal occurred only at the beginning of the experiment and benthic predators subsequently colonised all reefs. B+ and B− therefore refer to the initial manipulation, while benthic predator abundance is treated as a continuous variable in all analyses.
Pelagic predator manipulation
Six reefs were covered in 6-mm nylon diamond mesh nets to exclude pelagic predators (P−), and the remaining were non-netted and access open to pelagic predators (P+). The net stretched over the lid of the reef and approximately 45° downward from the edge of the lid to the substrate, providing approximately 3.8 m3 of enclosed shoaling space. Because we could not prevent the possible colonisation of benthic predators to non-netted reefs that had been cleared of all macrofauna (i.e. the B– P+ treatment) during the course of the experiment, nets were loosely pegged at the base to also allow potential colonisation of benthic predators to netted reefs (i.e. the B– P− treatment).
Cage control experiment
Exclusion devices such as nets have the potential to introduce confounding factors (Doherty and Sale 1985; Steele 1996) by altering prey or predator behaviour. With respect to the current experiment, the most likely confounding factors were the restriction to the natural shoaling behaviour of prey inside the net, and benthic predators using the nets as a structure from which to strike prey. A partial net control, which allowed access by pelagic predators whilst providing net structure, would not provide an accurate estimation of the potential effects of the nets on hulafish survival. While a partial net would allow hulafish to shoal outside the nets, it would still provide a structure for benthic predators and could attract more pelagic predators by providing greater coarse structure (see Connell 1997; Hixon and Carr 1997). A partially caged reef control within a larger cage to exclude pelagic predators (see Steele 1996) was not logistically feasible, as tidal currents and wave action in Port Phillip Bay are too strong to permit construction at depth of multiple large net structures able to withstand inclement weather. Therefore, prior to the main experiment, we used a net control to test and correct for the effects of nets on shoaling behaviour and predation. The net controls consisted of larger width mesh net (19 mm) made from thin nylon string that allowed prey to shoal away from the net, excluded pelagic predators, and provided little structure for benthic predators.
Net controls ran for 7 days on three B+ and B− reefs, with fish translocated and surveyed as described below. Comparison of survival between the large (M+) and small (M−) mesh B+ reefs provided an indication of whether possible changes in prey shoaling behaviour created greater opportunity for predation by benthic predators in the M− nets. Comparison between the M+ and M− B− reefs provided a correction for predation by pelagic predators on prey shoaling outside the nets on the M+ treatment. We note that ideally, such a correction experiment would have run for the full 62 days to incorporate possible changes to net effects through time as prey density declines.
Prey translocation
Juvenile hulafish (<30 days post settlement, 20–30 mm total length) were caught from nearby natural reefs by SCUBA divers by herding fish into a large mouthed (1,200 mm square) set net with a 2-m tapered cod end. During summer hulafish are distinct size-assortative shoalers, with juveniles shoaling with conspecifics of similar size in large groups (>100 fish). We were careful to target only shoals of the smallest newly settled fish, with otolith analysis of 12 sub-sampled fish identifying them as having settled within 12–30 days prior to capture. Fish were all caught on the same day and all fish were mixed into the same holding container. Fifty randomly selected fish were released on reefs from a bag by a SCUBA diver and observed for 2 min, noting any sick or injured fish. Fish swam into the reef structure or shoaled under the overhang and in no cases were fish observed swimming away from the reef.
Surveys
Fish survival was visually surveyed by a diver on SCUBA on days 7, 14, 28 and 62. Nets were cleaned of macroalgae each sampling period. Benthic predators were surveyed on days 0, 28 and 62, as it became clear by day 28 that colonisation by benthic predators was occurring. We interpolated day 7 and 14 benthic predator abundances assuming a linear colonisation rate of benthic predators throughout the experiment, which was the best fit to day 0, 28 and 62 data.
Detecting multiple predator effects
Studies of MPEs have a number of underlying design concerns and assumptions that strongly affect the interpretation of statistical results and resulting ecological application. In this study we use an additive experiment design in manipulating predator abundance, the multiplicative statistical test for predator interactions, and we assumed a multiplicative or log decline of prey through time.
Experimental design of MPE studies fall into two specific categories: additive and substitutive designs, which differ in the way the predator treatments are combined (Griffen 2006). Additive experiments add together set densities for the two predators, resulting in an overall higher predator density in the combined treatment. A substitutive design holds predator density constant across all treatments, while manipulating species richness. The two approaches answer different questions—the additive design examines whether non-additive effects occur due to interspecific competition, whilst the substitutive design compares the effects of interspecific competition to those of intraspecific competition (Griffen 2006). We used an additive design, as our primary question concerned the interaction of the two predator guilds in natural abundances and the consequences for prey survival. Also, a substitutive design would require the maintenance of predator densities which was not feasible in our field environment, and impossible for transient pelagic predators.
Tests for MPEs compare the survival of prey in the both-predator treatment to expected values where risk reduction or risk enhancement does not occur. These expected values are derived from the single-predator treatments using either an additive or multiplicative model. The additive model is inappropriate for experiments where prey depletion is not prevented (Sih et al. 1998), and hence we chose to use the multiplicative model, which is more appropriate for proportion survival data used in this study, and accounts for background or predator-free mortality (Sih et al. 1998). Multiplicative models have been widely used, firstly by Billick and Case (1994) and Wooton (1994), and more recently used by Vonesh and Osenberg (2003), Vance-Chalcraft and Soluk (2005a) and Griffen (2006). Non-additive predator effects can be statistically detected through two-way ANOVA of log-transformed survival data, where a significant interaction between predator treatments indicates non-additivity (Billick and Case 1994; Wooton 1994). The log transformation of the data assumes a logarithmic decline in prey density over time (Wooton 1994), which we observed in our data.
Our experimental design differed to the many previous studies of MPEs in that we did not maintain constant densities of either predator group. We manipulated initial benthic predator number, and surveyed as these fluctuated over time. Pelagic predators were either completely excluded or allowed to access the reef, although we could not survey their abundance. Therefore we treated benthic predator abundance as a continuous variable and pelagic presence or absence as a fixed factor. We tested for significant interactions using one-factor analyses of covariance for each sampling period, with the presence/absence of pelagic predators as the main factor and benthic predator abundance as the covariate. Per capita survival from day 0 was used instead of survival between surveys to investigate whether the detection of MPEs is related to prey depletion over time and to remove the effects of variable prey depletion across replicate reefs.
Estimating an expected survival relationship where the effects of predators are independent and additive is complicated in this experiment by the variability in benthic predator abundance amongst reefs. All previous studies of additive effects have controlled predator numbers (Vance-Chalcraft et al. 2004; Griffen 2006, amongst others), or biomass (Carey and Wahl 2010), and compared expected survival under an additive model to observed survival with both predators present. This approach, however, was not possible in our open plots exposed to natural predator densities.
Instead, where a significant interaction between predator guilds was detected, we produced interaction plots and compared these to predictions of non-additive interactions illustrated in Fig. 1. From these comparisons we can determine the nature of the non-additivity, either risk enhancement or risk reduction. We also attempted to identify trivial instances of additivity in the system, where one predator guild had a significant effect on survival and the other did not. Therefore we identified one of four types of predator interaction at each sampling period: additivity, trivial additivity, risk enhancement or risk reduction.
Results
Cage control experiment
Survival was lower when prey could not escape the net in the presence of benthic predators (M+ B+ = 0.64 ± 0.05 SE, M− B+ = 0.55 ± 0.04 SE), although the difference was not significant (t = 2.15 df = 4, P = 0.1). Survival was lower when fish could escape and shoal outside the net in the absence of benthic predators (M− B− = 0.8 ± 0.02 SE, M+ B− = 0.73 ± 0.06 SE), but again the difference was not significant (t = 0.98 df = 4, P = 0.38). Therefore, the small mesh net used in the main experiment likely had a small and non-significant effect of reduced survival by limiting shoaling behaviour, but this was countered by eliminating predation on fish shoaling outside the nets. Subtracting the survival difference between the M+ B− and the M− B− treatment (i.e. predation on fish shoaling outside nets, 0.07), from the difference between the M+ B+ and M− B+ treatments (i.e. effects of restricting shoaling, 0.09), provides an estimate of the cage effect in the presence of benthic predators: a reduction in per capita survival of 0.02, or approximately 3 %. Thus, as a conservative measure, we increased survival estimates in all netted (P−) reef treatments by 3 % to account for this effect.
Additivity of predator guild mortality
The abundance of benthic predators had a significant negative relationship with prey survival for all sampling periods (Table 1). Pelagic predator presence significantly decreased survival only in the first 7 and 14 days of the experiment and was not significant at 28 and 62 days.
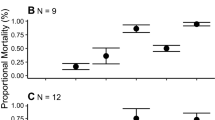
A significant interaction between predator treatments was identified in the first 7 days, indicating a non-additive relationship (Table 1). Comparisons of interaction plots (Fig. 2) showed at 7 days the slope of the observed survival in the P+ treatment was significantly less than (i.e. more negative) than the slope of the P− treatments (Fig. 2a), and follows the prediction for risk enhancement (Fig. 1a). No significant predator treatment interactions were detected for subsequent sampling dates and predator effects were considered additive. Due to the lack of a significant effect of pelagic predators at days 28 and 62, observations at these dates are considered trivial instances of additivity.
Interaction plots of the effects of benthic and pelagic predators on the survival of juvenile hulafish on artificial reefs in Port Phillip Bay, Australia over summer-autumn 2010 for: a days 0–7, b days 0–14, c days 0–28 and d days 0–62. Pelagic predators were present (P+; circles and solid lines) or absent (P−; crosses and dashed lines). P-values correspond to the benthic × pelagic interaction term from each analysis of covariance on log-transformed per capita survival data (Table 1), with NS representing a non-significant interaction. A significant interaction between predator treatments was detected only in the first 7 days and plots reveal a risk-enhancement effect similar to that predicted in Fig. 1a, with the slope of the P+ treatment (−0.14 ± 0.02) significantly less than the slope of the P− treatment (−0.04 ± 0.02). Note the log scale used on the y-axis
Discussion
This is the first study to observe changes in the detection of MPEs through time as prey are depleted, and is the first to test for and detect non-additivity by predator functional groups with discrete foraging domains. We observed risk enhancement to juveniles of the shoaling reef fish Trachinops caudimaculatus by the benthic and pelagic predator guilds in the first 7 of the experiment. Prey mortality was additive at day 14, as both predator guilds significantly affected survival as predicted by the multiplicative model. We observed trivial instances of additivity at days 28 and 62, when only the benthic predator guild significantly affected survival. Changes in the detection of MPEs and the relative effectiveness of predator guilds through time suggests that predator–prey interactions change in response to declining prey abundance and hence mask the original observation of risk enhancement. These results have important implications for how we interpret the results of other studies that manipulated reef fish predators, and provide insight into dynamics of predator–prey relationships in exploited communities.
Risk enhancement and synergistic predation in early post-settlement
Our observation of risk enhancement in the first 7 days of the experiment contrasts with the findings of the only other study to manipulate both predator guilds preying on reef fish (Hixon and Carr 1997), and is different to the majority of predator studies using additive designs that have observed risk reduction (Carey and Wahl 2010; Griffen 2006; Griffen and Williamson 2008; Vance-Chalcraft and Soluk 2005a, b). Hixon and Carr (1997) found that predation on Chromis cyanea from the benthic and pelagic predator guilds were additive at all sampling periods up to 32 days. They hypothesized that the two predator guilds occupy and feed in discrete spaces on the reef and do not interfere with each other; therefore, the authors concluded that the two mortality sources were independent and additive. Risk enhancement, however, is a result of multiple predator exposure increasing the overall risk to prey, and does not require any direct interactions amongst predators (Schmitz 2007). For example, Losey and Denno (1998) observed risk enhancement by two spatially discrete predators when foliage-dwelling predators caused aphids to drop to the ground and were consumed by ground-dwelling predators. Therefore, we hypothesize that where predator foraging domains do not overlap, but provide no predator-free space between domains, risk enhancement can occur.
Although Hixon and Carr (1997) describe the predation of the two guilds as additive, the synergistic predation they describe should result in non-additive risk enhancement. Because the authors numerically summed the mortality from the single predator treatments to produce an expected combined predator mortality, and did not use models of predicted multiplicative mortality used in many studies of non-additivity (e.g. Vonesh and Osenberg 2003; Vance-Chalcraft and Soluk 2005b; Griffen and Williamson 2008; Carey and Wahl 2010), their analysis could have failed to detect a non-additive effect when one was actually present. Applying the multiplicative model from Wooton (1994) to the data presented in Hixon and Carr (1997) results in an observed per capita survival after 32 days in the presence of both guilds (0.35) that is less than that predicted by the additive model (0.41), suggesting prey risk enhancement may be occurring.
Differences in guild specific predation rates may also explain the disparity in detection of MPEs between the two studies. Hixon and Carr (1997) observed a much stronger effect of pelagic predators on prey, likely due to small shoal sizes (20 C. cyanea) reducing the effectiveness of shoaling behaviour, low prey and benthic predator densities (see Carr and Hixon 1995), and increased risk of pelagic predator strikes on C. cyanea at smaller group sizes (see Sandin and Pacala 2005b). Hixon and Carr (1997) may therefore have observed trivial additivity where pelagic predators were much more effective than benthic predators, similar to that observed at days 28 and 62 of the present study. In contrast the present study observed strong effects of both predator guilds on prey in the first 7 days when risk enhancement occurred.
The observed risk enhancement is likely driven by two separate mechanisms: shoaling by prey reducing predation risk in the presence of a single predator, and adjacent predator foraging domains leaving no spatial refuge when both guilds are present. When only benthic predators were present, prey could shoal away from the reef and avoid predation, and when only pelagic predators were present prey could shelter close to the reef. For example, Stier et al. (2012) found no evidence for risk enhancement or risk reduction to shoaling reef fish prey with changes to density of only a single predator type. When both predator guilds were present in our study, such behavioural responses by prey to the presence of one predator guild may increase the risk of predation by the other predator guild (Sih et al. 1998). Prey are in effect ‘sandwiched’ between predators from above and below (Hixon and Carr 1997), with no effective spatial refuge (Krupa and Sih 1998). Large numbers of prey all seeking shelter simultaneously leads to a scramble for limited refuges when a pelagic predator attack occurs (Samhouri et al. 2009). Failure to find refuge and having to ‘queue’ outside of refuges would likely leave prey at greater risk of predation by both benthic predators waiting in ambush and the pelagic chase predators. Such situations could feasibly result in synergistic predation and prey risk enhancement.
Additive predator effects and the decline in pelagic predator effectiveness
Predator interactions shifted from synergistic predation in the first 7 days to an additive relationship for the remainder of the experiment. At day 14 the effect was genuine additivity under the multiplicative model, where both predator guilds significantly affected survival. By days 28 and 62, additivity was ‘trivial’ (Sih et al. 1998) because only the benthic guild significantly affected survival of prey. Benthic predators were in effect the only important predator and the addition of the pelagic predator guild did not influence prey survival beyond its small (non-significant) effect in isolation. Such shifts in the detection of MPEs over the course of the experiment confirm the dynamic nature of predator prey interactions, and highlight important limitations to discrete experiments attempting to observe snapshots in time.
We observed two major shifts that require mechanistic investigation: the change from risk enhancement to additive mortality after the first week, and the decline in mortality from pelagic predators over time. Decreasing prey density can result in reduced risk-enhancement behaviours by prey (Losey and Denno 1998). Reduced detection of prey is also likely to be an important driver of risk reduction if predators are more likely to detect (Krause et al. 1998) or target (Connell 2000) larger groups of prey. Thus there are two possible explanations for these shifts, changes in prey and predator behaviours as prey density declined.
Prey behaviour can change with conspecific density in response to the effectiveness of social or group strategies (Alexander 1974; Maher and Lott 2000). Shoaling can be more successful at higher densities through attack abatement (Pitcher and Parrish 1993; Ioannou and Krause 2008), predator confusion (Milinski 1979), corporate vigilance (Magurran et al. 1985; Krause and Ruxton 2002) and more efficient food-finding abilities (Pitcher et al. 1982). However, at low densities or under low predation pressure, shoaling behaviour is less effective and fish feed as individuals, as observed in guppies (Liley and Seghers 1975) and minnows (Magurran and Pitcher 1987). Similar responses have been observed in the wrasse Thalassoma amblycephalum, where the proportion of time shoaling increased with shoal size (Stier et al. 2012). At low densities T. caudimaculatus shoal closer to the substrate and are more likely to forage individually (Fumei 2011), restricting their foraging domain to areas close to the reef. Therefore the size of prey foraging domains declines with density, and at the smallest group sizes prey no longer overlap with the foraging domain of pelagic predators (Fumei 2011). The cost of not shoaling is increased exposure to, and hence predation by, the benthic guild. This change in foraging behaviour likely contributed to the observation of trivial additivity after 4 weeks, where only benthic predators significantly affected prey survival.
The reduced exposure of prey to pelagic predation through changes to shoaling may have been compounded by a corresponding reduction in targeting of smaller shoals by pelagic predators. Due to their large foraging domains, which encompass many square kilometres and multiple reef habitats, pelagic predators can actively choose which prey aggregations they target (Overholtzer-McLeod 2004). Pelagic predators tend to congregate on larger prey aggregations (Hixon and Carr 1997; Connell 2000; Stewart and Jones 2001), which could provide a greater return on foraging effort (Ioannou and Krause 2008). Although Sandin and Pacala (2005b) observed a higher per capita incidence of predator visits in smaller shoals, predators had either a positive or independent relationship with shoal size. If pelagic predators reduced their targeting of prey over the course of the experiment in response to declining densities, this also may have contributed to the reduced effect of pelagic predators and the trivial observation of additivity. Consequently, the shift in the type of MPE observed is related to both prey and predator responses to prey depletion over time.
Conclusion
The observation of the changing nature of predator interactions from non-additive to additive effects has important implications for both our understanding of predation and how we interpret the results of predator manipulation studies. Whilst short-term non-additive effects may be strong, changes to prey densities and resulting behaviour may negate the detection of these effects over time. Although the use of an additive experimental design means we cannot answer the question of whether predator guilds are substitutable, we can assess how non-impacted communities are likely to respond to declines in or the loss of either predator guild. In our system, benthic predators likely play an important role in regulating prey populations at low densities, whilst pelagic predators are more effective at higher prey densities. A loss of either key functional group through pelagic fishing or benthic habitat change is likely to significantly alter the dynamics of prey populations. Identifying non-additive sources of mortality is, therefore, an important step in understanding the potential effects of biodiversity loss on ecosystem function in marine environments.
References
Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Almany GR, Webster MS (2006) The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25:19–22. doi:10.1007/s00338-005-0044-y
Anderson TW (2001) Predator responses, prey refuges, and density-dependent mortality of a marine fish. Ecology 82:245–257
Anderson TW, Carr MH, Hixon MA (2007) Patterns and mechanisms of variable settlement and recruitment of a coral reef damselfish, Chromis cyanea. Mar Ecol Prog Ser 350:109–116. doi:10.3354/meps07135
Billick I, Case TJ (1994) Higher order interactions in ecological communities: what are they and how can they be detected? Ecology 75:1529
Bruno JF, O’Connor MI (2005) Cascading effects of predator diversity and omnivory in a marine food web. Ecol Lett 8:1048
Carey MP, Wahl DH (2010) Interactions of multiple predators with different foraging modes in an aquatic food web. Oecologia 162:443
Carr MH, Hixon MA (1995) Predation effects on early post-settlement survivorship of coral reef fishes. Mar Ecol Prog Ser 124:31–42
Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ (2002) The interaction between predation and competition: a review and synthesis. Ecol Lett 5:302–315
Connell SD (1997) The relationship between large predatory fish and recruitment and mortality of juvenile coral reef-fish on artificial reefs. J Exp Mar Biol Ecol 209:261–278
Connell SD (2000) Is there safety-in-numbers for prey? Oikos 88:527–532
Department of Primary Industries (2008) Fishery status report 2008. Fisheries Victoria, Melbourne
DeWitt TJ, Langerhans RB (2003) Multiple prey traits, multiple predators: keys to understanding complex community dynamics. J Sea Res 49:143–155. doi:10.1016/S1385-1101(02)00220-4
Dobson A, Lodge D, Alder J, Cumming GS, Keymer J, McGlade J, Mooney H, Rusak JA, Sala O, Wolters V, Wall D, Winfree R, Xenopoulos MA (2006) Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87:1915
Doherty PJ, Sale PF (1985) Predation on juvenile coral reef fishes: an exclusion experiment. Coral Reefs 4:225–234. doi:10.1007/BF00298081
Doherty PJ, Dufour V, Galzin R, Hixon MA, Meekan MG, Planes S (2004) High mortality during settlement is a population bottleneck for a tropical surgeonfish. Ecology 85:2422–2428
Edgar GJ, Shaw C (1995) The production and trophic ecology of shallow-water fish assemblages in southern Australia. II. Diets of fishes and trophic relationships between fishes and benthos at Western Port, Victoria. J Exp Mar Biol Ecol 194:83–106. doi:10.1016/0022-0981(95)00084-4
Fumei S (2011) Growing together: group living behaviour and the relationship between density and growth in a shoaling fish. Masters of Science thesis, Melbourne University
Godin J, Classon L, Abrahams M (1988) Group vigilance and shoal size in a small characin fish. Behaviour 104:29–40. doi:10.1163/156853988X00584
Griffen BD (2006) Detecting emergent effects of multiple predator species. Oecologia 148:702–709. doi:10.1007/s00442-006-0414-3
Griffen BD, Byers JE (2006a) Intraguild predation reduces redundancy of predator species in multiple predator assemblage. J Anim Ecol 75:959–966. doi:10.1111/j.1365-2656.2006.01115.x
Griffen BD, Byers JE (2006b) Partitioning mechanisms of predator interference in different habitats. Oecologia 146:608–614. doi:10.1007/s00442-005-0211-4
Griffen BD, Williamson T (2008) Influence of predator density on nonindependent effects of multiple predator species. Oecologia 155:151–159. doi:10.1007/s00442-007-0889-6
Grubert MA (1999) Diet and feeding strategy of Octopus maorum in southeast Tasmania. Bull Mar Sci 65:441
Gurevitch J, Morrison JA, Hedges LV (2000) The interaction between competition and predation: a meta-analysis of field experiments. Am Nat 155(4):435–453
Hindell JS (2006) Assessing the trophic link between seagrass habitats and piscivorous fishes. Mar Freshwater Res 57:121. doi:10.1071/MF05082
Hixon MA, Carr MH (1997) Synergistic predation, density dependence, and population regulation in marine fish. Science 277:946–949
Holbrook SJ, Schmitt RJ (2002) Competition for shelter space causes density-dependent predation mortality in damselfishes. Ecology 83:2855–2868
Hunt TL, Ford JR, Swearer SE (2011) Ecological determinants of recruitment to populations of a temperate reef fish, Trachinops caudimaculatus (Plesiopidae). Mar Freshwater Res 62:502–509. doi:10.1071/MF10262
Ioannou CC, Krause J (2008) Searching for prey: the effects of group size and number. Anim Behav 75:1383–1388. doi:10.1016/j.anbehav.2007.09.012
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Krause J, Ruxton GD, Rubenstein D (1998) Is there always an influence of shoal size on predator hunting success? J Fish Biol 52:494–501. doi:10.1111/j.1095-8649.1998.tb02012.x
Krupa JJ, Sih A (1998) Fishing spiders, green sunfish, and a stream-dwelling water strider: male-female conflict and prey responses to single versus multiple predator environments. Oecologia 117:258
Liley N, Seghers B (1975) Factors affecting the morphology and behaviour of guppies in Trinidad. Funct Evol Behav 6:92–118
Lima SL (1998) Nonlethal effects in the ecology of predator-prey interactions. Bioscience 48:25
Losey JE, Denno RF (1998) Positive predator–predator interactions: enhance predation rates and synergistic suppression of aphid populations. Ecology 79:2143–2152. doi:10.1890/0012-9658(1998)079[2143:PPPIEP]2.0.CO;2
Madin EMP, Gaines SD, Warner RR (2010) Field evidence for pervasive indirect effects of fishing on prey foraging behavior. Ecology 91:3563
Magurran AE, Pitcher TJ (1987) Provenance, shoal size and the sociobiology of predator-evasion behaviour in minnow shoals. Proc R Soc Lond B Biol Sci 229:439–465. doi:10.1098/rspb.1987.0004
Magurran AE, Oulton WJ, Pitcher T (1985) Vigilant behaviour and shoal size in minnows. J Comp Ethol 64:167–178
Maher CR, Lott DF (2000) A review of ecological determinants of territoriality within vertebrate species. Midl Nat 143:1–29. doi:10.1674/0003-0031(2000)143[0001:AROEDO]2.0.CO;2
Menge BA, Sutherland JP (1987) Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am Nat 130:730–757
Milinski M (1979) Can an experienced predator overcome the confusion of swarming prey more easily? Anim Behav 27:1122–1126. doi:10.1016/0003-3472(79)90060-5
Morgan MJ (1988) The influence of hunger, shoal size and predator presence on foraging in bluntnose minnows. Anim Behav 36:1317–1322. doi:10.1016/S0003-3472(88)80200-8
Overholtzer-McLeod KL (2004) Variance in reef spatial structure masks density dependence in coral-reef fish populations on natural versus artificial reefs. Mar Ecol Prog Ser 276:269–280
Parsons TR (1992) The removal of marine predators by fisheries and the impact of trophic structure. Mar Pollut Bull 25:51
Pitcher TJ, Parrish JK (1993) Functions of shoaling behaviour in teleosts. In: Pitcher TJ (ed) Behaviour of teleost fishes, 2nd edn. Chapman and Hall, London
Pitcher TJ, Magurran AE, Winfield IJ (1982) Fish in larger shoals find food faster. Behav Ecol Sociobiol 10:149–151. doi:10.1007/BF00300175
Ricklefs RE (1987) Community diversity: relative roles of local and regional processes. Science 235:167
Samhouri JF, Vance RR, Forrester GE, Steele MA (2009) Musical chairs mortality functions: density-dependent deaths caused by competition for unguarded refuges. Oecologia 160:257–265. doi:10.1007/s00442-009-1307-z
Sandin SA, Pacala SW (2005a) Demographic theory of coral reef fish populations with stochastic recruitment: comparing sources of population regulation. Am Nat 165:107–119
Sandin SA, Pacala SW (2005b) Fish aggregation results in inversely density-dependent predation on continuous coral reefs. Ecology 86:1520–1530
Schmitt RJ, Holbrook SJ (1999a) Mortality of juvenile damselfish: implications for assessing processes that determine abundance. Ecology 80:35–50
Schmitt RJ, Holbrook SJ (1999b) Settlement and recruitment of three damselfish species: larval delivery and competition for shelter space. Oecologia 118:76–86
Schmitz OJ (2007) Predator diversity and trophic interactions. Ecology 88:2415
Schmitz OJ, Suttle KB (2001) Effects of top predator species on direct and indirect interactions in a food web. Ecology 82:2072–2081
Schmitz OJ, Beckerman AP, O’Brien KM (1997) Behaviorally mediated trophic cascades: effects of predation risk on food web interactions. Ecology 78:1388
Shima JS (1999) Variability in relative importance of determinants of reef fish recruitment. Ecol Lett 2:304–310
Sih A, Crowley P, McPeek M, Petranka J, Stroheimer K (1985) Predation, competition, and prey communities: a review of field experiments. Annu Rev Ecol Syst 16:269
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355. doi:10.1016/S0169-5347(98)01437-2
Smith CD (2003) Diet of Octopus vulgaris in False Bay, South Africa. Mar Biol 143:1127
Sokol-Hessner L, Schmitz OJ (2002) Aggregate effects of multiple predator species on a shared prey. Ecology 83:2367–2372
Stachowicz JJ, Bruno JF, Duffy JE (2007) Understanding the effects of marine biodiversity on communities and ecosystems. Annu Rev Ecol Evol Syst 38:739
Steele MA (1996) Effects of predators on reef fishes: separating cage artifacts from effects of predation. J Exp Mar Biol Ecol 198:249–267. doi:10.1016/0022-0981(96)00011-1
Stewart BD, Jones GP (2001) Associations between the abundance of piscivorous fishes and their prey on coral reefs: implications for prey-fish mortality. Mar Biol 138:383–397
Stier AC, Geange SW, Bolker BM (2012) Predator density and competition modify the benefits of group formation in a shoaling reef fish. Oikos:no–no. doi:10.1111/j.1600-0706.2012.20726.x
Van Buskirk J (2001) Specific induced responses to different predator species in anuran larvae. J Evol Biol 14:482–489. doi:10.1046/j.1420-9101.2001.00282.x
Vance-Chalcraft HD, Soluk DA (2005a) Multiple predator effects result in risk reduction for prey across multiple prey densities. Oecologia 144:472
Vance-Chalcraft HD, Soluk DA (2005b) Estimating the prevalence and strength of non-independent predator effects. Oecologia 146:452–460. doi:10.1007/s00442-005-0201-6
Vance-Chalcraft HD, Soluk DA, Ozburn N (2004) Is prey predation risk influenced more by increasing predator density or predator species richness in stream enclosures? Oecologia 139:117
Vance-Chalcraft HD, Rosenheim JA, Vonesh JR, Osenberg CW, Sih A (2007) The influence of intraguild predation on prey suppression and prey release: a meta-analysis. Ecology 88:2689
Vonesh JR, Osenberg CW (2003) Multi-predator effects across life-history stages: non-additivity of egg- and larval-stage predation in an African treefrog. Ecol Lett 6:503–508. doi:10.1046/j.1461-0248.2003.00470.x
White JW, Samhouri JF, Stier AC, Wormald CL, Hamilton SL, Sandin SA (2010) Synthesizing mechanisms of density dependence in reef fishes: behavior, habitat configuration, and observational scale. Ecology 91:1949–1961
Wooton JT (1994) Putting the pieces together: testing the independence of interactions among organisms. Ecology 75:1544–1551
Acknowledgments
We would like to thank Christian Jung, Dean Chamberlain, Matt Le Feuvre, Madhavi Colton, Mal Lindsay and Jessica Smith for the back-breaking construction of artificial reefs, these again plus Evan Hallein, Simon Pahor, Dan Corrie and Seann Chia for setups and surveys, and John Ahern for boat and technical support. We thank Craig Osenberg for his helpful guidance on determining non-additivity in the system. Operational costs were covered by grants from the Holsworth Wildlife Research Endowment to J. R. F. and the Australia and Pacific Science Foundation to S. E. S. Animal ethics approval was granted under Melbourne University AEC0810932. The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Craig Osenberg.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ford, J.R., Swearer, S.E. Shoaling behaviour enhances risk of predation from multiple predator guilds in a marine fish. Oecologia 172, 387–397 (2013). https://doi.org/10.1007/s00442-012-2508-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2508-4