Abstract
Many hypotheses suggest that pollinators act to maintain or change floral color morph frequencies in nature, although pollinator preferences do not always match color morph frequencies in the field. Therefore, non-pollinating agents may also be responsible for color morph frequencies. To test this hypothesis, we examined whether Raphanus sativus plants with white flowers received different amounts of florivory than plants with pink flowers, and whether florivores preferred one floral color over the other. We found that white-flowered plants received significantly more floral damage than pink-flowered plants in eight populations over 4 years in northern California. Both generalists and specialists on Brassicaceae preferred white petals in choice and short-term no choice tests. In performance tests, generalists gained more weight on white versus pink petals whereas specialists gained similar amounts of weight on pink and white morphs. Because our results suggest that florivores prefer and perform better on white versus pink flowers, these insects may have the opportunity to affect the frequency of color morphs in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flower color is a highly conspicuous plant trait that varies widely within and between species. Many hypotheses about the maintenance of these differences in floral color revolve around the selective pressures of discriminating pollinators (Mueller 1883; Clements and Long 1923; Waser and Price 1981). Such hypotheses are well supported because pollinators are known to prefer certain color morphs over others in several systems (e.g., Gigord et al. 2001; Jones and Reithel 2001, reviewed in Frey 2004). However, these pollinator preferences do not always coincide with realized changes in the frequencies of different color morphs (Irwin and Strauss 2005), which has led researchers to consider the actions of non-pollinator agents of natural selection.
Herbivores are agents that could act as selective forces shaping the relative frequencies of different color morphs in nature. Herbivory on flowers, or florivory, can reduce fitness in plants (e.g., Lehtila and Strauss 1997), and researchers have investigated possible pleiotropic actions of genes involved in pigment production and anti-herbivore traits (Fineblum and Rausher 1997; Armbruster 2002). In particular, anthocyanins, which usually produce red and blue hues in flowers, share parts of the same biosynthetic pathway as flavonoid compounds that deter herbivory (Fineblum and Rausher 1997). In addition, anthocyanin production may also be pleiotropically linked to glucosinolate production (Hemm et al. 2003). Thus, mutations that affect color may simultaneously affect resistance to herbivores and vice versa.
One example of how non-pollinating agents may affect the frequency of color morphs can be found in wild radish, Raphanus sativus (Brassicaceae). Irwin et al. (2003) found that herbivores of R. sativus generally preferred leaves from anthocyanin-free morphs (white and yellow flowers) over those genotypes that produce anthocyanins (pink and bronze flowers). Likewise, Claytonia virginica plants with red flowers sustained greater leaf damage by slugs and lower pathogen infection than white flowers (Frey 2004). Fruit set in this pollen-limited system tended to be higher in plants with more red pigment in their flowers, which suggests that both mutualists and antagonists could simultaneously act on color morph frequencies in this system.
These studies showed that floral colors are linked to resistance in leaves, but resistance based on color may also be present in the flowers themselves. If florivores prefer some floral colors over others and floral color is heritable, there may be selection for color that is not directly related to pollinators. Furthermore, selection on floral color by a mutualist pollinator may be countered by selection from an antagonist florivore if both agents prefer the same colors (Strauss and Irwin 2004). Thus, although there may be indirect selection for floral color via selection on resistance in leaves, there could also be direct selection on floral color by selection of antagonists on flowers.
Despite the intriguing possibilities of these interactions among pollinators and florivores, very little is known about how florivores respond to floral traits like color and whether this response is consistent over time. A few studies suggest that florivores or seed predators can discriminate among either flowers or fruit structures with different colors. Early in the study of flowers, Darwin (1876) related a finding that blue-flowered Aconitum napellus morphs suffered less floral damage by nectar robbers than white-flowered morphs and suggested this might be due to the distasteful nature of blue morphs in the Ranunculaceae. More recently, Johnson et al. (2008) found that herbivores ate less petal area that was colored with anthocyanins than white areas on the same flower and that feeding on blue areas both decreased weight gain and increased mortality rates depending on the herbivore under study. In Acacia ligulata, Whitney and Stanton (2004) found that seed predators damaged fewer red-ariled fruit than ones with yellow arils in 1 year of their study. Despite these advances, we are still in need of long-term surveys of florivores to determine if a pattern of damage on pigmented versus non-pigmented flowers is persistent in the field. We also have little data showing enhanced preference or performance of florivores on floral or fruit morphs with differing levels of anthocyanin production. This information is important if we want to understand if non-pollinating agents can exert selection pressure on floral morph color.
In order to provide information on the long- and short-term preferences of florivores on flowers differing in anthocyanin production, we aim to answer three questions using California wild radish, R. sativus. First, does florivory differ among white and pink color morphs in natural populations? Second, do generalist and specialist florivores prefer one color morph over another in short-term laboratory assays? Third, does flower color affect florivore performance in the laboratory? This work complements previous studies investigating selection on R. sativus flower color from pollinators (Stanton 1987) and folivores (Irwin et al. 2003).
Materials and methods
Study system
Raphanus sativus in California is an annual plant produced from crosses between R. raphinistrum, jointed charlock, and agronomic R. sativus cultivars (Hegde et al. 2006). Its seeds germinate during the first part of the wet season in California’s Mediterranean climate (October–December), with plants flowering between March and July. Floral color phenotype (bronze, pink, white, and yellow) is controlled by two independently segregating loci. (Panestsos 1964; Irwin et al. 2003). There is no evidence that floral display and silique size differ among color morphs (Stanton 1987; Irwin et al. 2003). Although the plant makes four color morphs, we concentrated on only white and pink flowering plants because they differ in the presence or absence of anthocyanins. Because there is variation in the amount visible pigment in radish flowers, we limited our surveys of pink morphs to those flowers that were uniformly pink in color and had no visible white sectors and used only white flowers without any visible pink pigment.
Florivores on R. sativus include larvae of diamondback moths (Plutella xylostella), tiger moth larvae (Platyprepia virginalis), western flower thrips (Frankliniella occidentalis), an unidentified weevil, and an unidentified aphid species (K. Shiojiri, personal communication; A.M., personal observation.). Foliar herbivores include larvae of Pieris rapae, Plutella xylostella, Spodoptera exigua, Tricoplusia ni, cabbage aphids (Brevicoryne brassicae), gray garden slugs (Agriolimax reticulates), flea beetles (Phyllotreta spp.), and earwigs (Forficula auricularia) (Irwin et al. 2003; Karban and Nagasaka 2004).
Defensive chemicals found in leaves or other parts of a plant may be effective at deterring these florivores. For example, glucosinolates are the most prevalent defensive chemicals found in Raphanus species, and are effective in facilitating resistance against many herbivores (Glen et al. 1990; Li et al. 2000; Renwick 2002). Raphanus sativus has both constitutive glucosinolate resistance and indicible glucosinolate resistance in both petals and leaves (Strauss et al. 2004).
Survey of damage
In 2006, we began a florivory survey of eight populations of R. sativus (Table 1). We chose populations on a linear transect across northern California, between Bodega Bay in the west and the Cosumnes River in the east. Sites were at least 2 km apart. Populations were usually in roadside ditches and other disturbed areas where R. sativus thrives. The main florivores in these populations were western flower thrips, diamondback moths, tiger moth larvae, and an unidentified weevil. We placed two 40-m linear transects through each population and surveyed plants every 2 m until we assayed 100 plants. In some populations, there were not enough plants to complete 100 replicates, so we assayed as many as possible. For each plant, we chose the most distal open and receptive flowers on the longest raceme to examine for damage. We only considered a flower damaged if the insect produced a hole through the entire petal, not just the top epidermal layer. The dependent variable for each plant was the proportion of open flowers with damage, and the unit of replication was at the individual plant level.
We surveyed these populations in mid-May, before the peak of flowering, for 4 years. In total, we surveyed 2,270 individual plants. We analyzed the data using a three-way, fully crossed ANOVA with year and flower color as fixed factors and population as a random effect. If we found significant interactions, we ran appropriate main-effect ANOVAs.
Choice tests
In 2008, we performed an experiment to determine florivore preference, using approximately seven plants grown from each of the eight populations surveyed in 2006–2009 as sources for the petals. We used both a generalist herbivore, the beet armyworm, S. exigua, and a specialist on Brassicaceae, P. xylostella. Both florivores were obtained from a commercial source (Benzon Research, Carlisle, PA, USA). In the S. exigua trials, we placed entire petals of either white or pink flowers in a 150-mm-wide Petri dish used as the experimental arena. First instar larvae of S. exigua were allowed to feed for 24 h, after which we coded the damage as ‘1’ for any damage and ‘0’ for no damage. For the P. xylostella tests, we used the same assay. A total of 49 S. exigua and 38 P. xylostella larvae were used in these assays. We then tested for flower color preferences within each species with a Wilcoxon signed-rank matched pairs t test. We also performed post hoc power analyses on these data because the sample sizes were relatively small.
No-choice tests
During the summer of 2008, we performed a series of no-choice experiments with S. exigua and P. xylostella. We used a graded response to quantify insect damage where 0 = no damage, 1 = 1–25 % damage, 2 = 26–50 % damage, 3 = 51–75 % damage, and 4 = 76–100 % damage. We used 103 S. exigua and 136 P. xylostella neonates for this experiment. We placed individual larvae into Petri dishes with damp cotton balls and gave each of them a single petal disk of one color to feed on for 24 h. We used a two-way ANOVA with petal color and insect species as fixed, fully crossed factors and the damage score as the response variable.
Performance tests
Although damage to petals may be indicative of initial palatability, we also wanted to know if performance, as measured by weight gain, was dependent on flower color. We conducted an experiment where we fed larvae either only white petals or only pink petals for an extended period of time and measured their weight gain. We used a single population (LAND) for all of the experiments in order to control for population-level genetic effects. The methods for experiments using S. exigua and P. xylostella were identical.
After growing approximately 45 plants from 10 maternal lines of R. sativus within LAND, we placed each larva in a Petri dish arena with a moist cotton ball and initially fed it two petals, either pink or white, from a randomly chosen plant. Every 2 days until the 10th day, we removed the old petals and replaced them with two new petals from the initial plant. After 10 days, we weighed each larva to estimate performance during this period. A total of 48 S. exigua and 44 P. xylostella larvae were used in the experiment. Data were ln-transformed to improve the normality of residuals and were analyzed using a two-way ANOVA with herbivore type and petal color as fully crossed effects.
Results
Survey
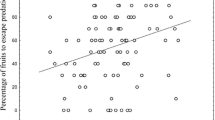
We found that floral damage depended on the year of the survey (F 3, 2,255 = 6.62, P = 0.0024), and that plants with white flowers suffered a higher proportion of floral damage than plants with pink flowers [Pink = 24.30 ± 1.18 % (1 SE), White = 32.75 ± 1.18 %, F 1,2255 = 23.58, P < 0.0001; Fig. 1]. There were no significant year by color (F 3, 2,255 = 1.25, P = 0.321) or population by color (F 7, 2,255 = 0.63, P = 0.722) interactions. For the random effects, there were no effects of population (Variance component = 0.02, 95 % CI = −0.014–0.053), or the three-way-interaction among year, population, and color (Variance component <0.001, 95 % CI = −0.005 to 0.007).
Choice test
In the choice test, S. exigua larvae preferred white petals versus pink petals [Pink mean score = 0.521 ± 0.083 (1 SE), White mean score = 1.00, Wilcoxon signed-rank matched pairs test, S = 138, P < 0.0001; Fig. 2]. The post hoc power for this analysis at α = 0.05 was 0.99. Plutella xylostella larvae also consumed more white petal tissue than pink petal tissue (Pink mean score = 0.421 ± 0.081, White mean score = 0.816 ± 0.091, Wilcoxon signed-rank matched pairs test, S = 114, P = 0.016; Fig. 2). The post hoc power for this analysis at α = 0.05 was 0.68.
Mean damage petal scores from a 24-h choice tests between pink and white petals for the specialist Plutella xylostella (n = 38) and the generalist Spodoptera exigua (n = 49). All white petals damaged by S. exigua had the highest possible damage score (1). Bars indicate mean ± SE (*P < 0.05, ***P < 0.001)
No-choice test
In the no-choice tests, both S. exigua and P. xylostella inflicted more damage on white petals than on pink petals [white damage score mean = 0.889 ± 0.066, pink damage score mean = 1.19 ± 0.066 (1 SE), F 1,235 = 10.17, P = 0.0016, data not shown]. For both petal morphs, S. exigua larvae caused significantly more damage than P. xylostella (S. exigua damage score mean = 1.144 ± 0.072, P. xylostella damage score mean = 0.936 ± 0.062, F 1, 235 = 4.83, P = 0.029, data not shown). There was no significant effect of the color by insect interaction (F 1, 235 = 0.069, P = 0.793).
Long-term performance tests
In the long-term no-choice test, all larvae, regardless of species, weighed more when fed white petals versus pink petals (F 1, 88 = 8.61, P = 0.004) and P. xylostella larvae weighed more than S. exigua larvae, regardless of petal color (F 1, 88 = 82.75, P < 0.0001). There was a significant interaction between petal color and herbivore species (F 1, 88 = 19.27, P < 0.0001). Within P. xylostella, there was no evidence of an effect of petal color on larvae weight (F 1, 42 = 2.12, P = 0.152; Fig. 3) but within S. exigua larvae fed on white petals weighed 84 % more than larvae fed on pink petals (F 1, 46 = 19.1, P < 0.0001; Fig. 3).
Discussion
Our survey results showed that florivores damage a higher proportion of petals on white flowers versus pink flowers and that this is consistent across populations and survey years. We also found that florivory rates were variable across years within populations, regardless of the petal color. This variation is not surprising, given the large variation in percent leaf herbivory observed across R. sativus populations in northern California (Karban and Nagasaka 2004). To our knowledge, our work is one of the longest records of florivore damage on multiple populations in the literature.
In the behavioral tests, S. exigua and P. xylostella both preferred white petals over pink petals in the laboratory (Fig. 2) and also ate more white petal tissue versus pink petal tissue when given no choice. For the long-term performance tests, S. exigua larvae gained more weight on white petals versus pink petals, but the specialist P. xylostella gained roughly equal amounts of weight on both colors (Fig. 3).
Although our results indicate a pattern of preference and performance, our work does not tell us what mechanism may be affecting the consumption of white versus pink petals. One clue is that petal color had little effect on the long-term performance of the specialist P. xylostella but did have an effect on the generalist S. exigua. Given that specialist herbivores on the Brassicaceae are often either unaffected or stimulated by glucosinolates whereas generalists are deterred (e.g., Lankau 2007), pink petals may simply have higher constitutive levels of glucosinolates than white petals. For example, Hemm et al. (2003) found that mutations in Arabidopsis thaliana that disrupt the biosynthesis of glucosinolates and pleiotropically affect anthocyanin production. Despite this possibility, workers found that constitutive glucosinolate levels do not differ among pink and white petals in plants from the BODE population used in our study (Strauss et al. 2004). Instead, Strauss et al. (2004) found that leaf damage induced higher concentrations of glucosinolates in pink flowers than white flowers, which may explain the pattern of increased damage on white petals we saw in the natural populations because leaf damage can be extremely widespread both within and across populations (Karban and Nagasaka 2004; A. McCall, personal observation). Induction of glucosinolates through leaf damage does not explain why white petals were preferred in the laboratory, where undamaged plants were used.
An alternative reason for the preference and performance patterns we observed is that the constitutive levels of glucosinolates could have actually differed between the color morphs in the particular populations we used. We tested florivores on different populations than Strauss et al. (2004), and since there are often population-level differences in herbivore resistance in plants (e.g., Newton et al. 2010), constitutive or induced levels of resistance between the color morphs could have been different in our study.
Defenses other than glucosinolates could be linked to anthocyanin production. For example, phenolic defensive compounds could be produced at higher concentrations in pink flowers because these compounds share a biosynthetic pathway with anthocyanins (Koes et al. 1994; Fineblum and Rausher 1997). In this way, plants with red flowers might deter herbivores through the action of the defensive phenolics rather than of the conspicuous and co-occurring anthocyanins. For example, tobacco budworm larvae, Heliothis virescens, gained less weight and had a greater mortality rate when fed on cotton flowers colored by cyanidin-3-β-glucoside than when fed on white flowers (Hedin et al. 1983). It is also possible that the anthocyanins themselves either deter feeding or interfere with digestion. When anthocyanin mixtures from Petunia hybrida flowers were added to the diets of H. zea and Trichoplusia ni larvae, both insects gained less weight after 3 days than those insects reared on control diets (Johnson et al. 2008). Despite these results, anthocyanin content may not be the sole factor affecting preference in our study because specialists did not experience a differential weight gain on the two different color morphs, while generalists did. This suggests that defenses more specialized than anthocyanins, like glucosinolates, are influencing preference for white over pink in this system.
One final insight about the mechanisms behind the patterns we found comes from studies on leaf herbivores in R. sativus. Our patterns of performance for S. exigua are the opposite of what Irwin et al. (2003) found in larvae fed anthocyanin-free or anthocyanin-containing leaves. In that study, S. exigua larvae performed better on leaves from pink- and bronze-flowered plants whereas we found that S. exigua larvae performed better on anthocyanin-free flowers, suggesting that resistance compounds in leaves are somehow different than those employed in flowers. This scenario is entirely possible, given that some plants deploy different types or different levels of defenses in different tissue types (Smallegange et al. 2007).
Florivores and other herbivores may impact plants simultaneously in nature. We know that insect damage to leaves can have large effects on maternal and paternal fitness (Strauss et al. 1996), and that foliar herbivores other than Lepidopterans prefer and perform better on anthocyanin-free color morphs (Irwin et al. 2003). Our work adds to this story by suggesting that florivores may select on floral color through flower consumption. Thus, at least two types of herbivores, florivores and folivores (Irwin et al. 2003) have now been shown to prefer anthocyanin-free plants versus anthocyanin-containing radish plants.
Although we showed that larvae prefer and perform better on white petals, we did not test the adult oviposition preferences. An alternative mechanism behind the pattern that we see in the field is that adult moths prefer to oviposit on white-flowering plants. Indeed, Irwin et al. (2003) showed that adults of the P. rapae preferred to oviposit on anthocyanin-free color morphs than anthocyanin-containing morphs, but only when the plants were flowering. While we cannot directly address whether S. exigua or P. xylostella adults would show the same preference, we do think that larvae may be able to choose among color morphs in the field because R. sativus often grows in dense patches where racemes of one plant grow nearly intertwined with adjacent plants (A. McCall, personal observation).
We also do not know whether florivory on R. sativus has any effect on fitness components. Others have found that smaller flowers tend to receive fewer syrphid pollinator visits than larger ones (Strauss et al. 1996), so it is possible that floral damage that reduces petal width or length could similarly affect pollination. Further work should either protect flowers from damage or apply artificial florivory to all color morphs to see if they are differentially affected by florivory.
Conclusions
Our work shows that white flowers receive significantly more florivore damage than pink flowers over many populations and several years. Using both choice and no-choice tests, we show that both specialists and generalists prefer white petals in the short term, but that longer-term performance on white petals is higher only for generalists rather than specialists. These findings, in combination with other studies on wild radish, indicate that florivore preference may coincide with folivore preference, possibly affecting selection on petal morph color.
References
Armbruster WS (2002) Can indirect selection and genetic context contribute to trait diversification? A transition-probability study of blossom-colour evolution in two genera. J Evol Biol 15:468–486
Clements FE, Long FL (1923) Experimental pollination, vol 336. Carnegie Institution Publication, Washington, DC
Darwin C (1876) The effects of cross and self fertilisation in the vegetable kingdom. Murray, London
Fineblum WL, Rausher MD (1997) Do floral pigmentation genes also influence resistance to enemies? The W locus in Ipomoea purpurea. Ecology 78:1646–1654
Frey FM (2004) Opposing natural selection from herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae). Evolution 58:2426–2437
Gigord LDB, Macnair MR, Smithson A (2001) Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.). Proc Natl Acad Sci USA 98:6253–6255
Glen DM, Jones H, Fieldsend JK (1990) Damage to oilseed rape seedlings by the field slug Deroceras reticulatum in relation to glucosinolate concentrations. Ann Appl Biol 117:197–208
Hedin PA, Jenkins JN, Collum DH, White WH, Parrott WL, Macgown MW (1983) Cyanidin-3-beta-glucoside, a newly recognized basis for resistance in cotton to the tobacco budworm heliothis virescens (Fab) (Lepidoptera, Noctuidae). Experientia 39:799–801
Hegde SG, Nason JD, Clegg JM, Ellstrand NC (2006) The evolution of California’s wild radish has resulted in the extinction of its progenitors. Evolution 60:1187–1197
Hemm MR, Ruegger MO, Chapple C (2003) The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell 15:179–194
Irwin RE, Strauss SY (2005) Flower color microevolution in wild radish: evolutionary response to pollinator-mediated selection. Am Nat 165:225–237
Irwin RE, Strauss SY, Storz S, Emerson A, Guibert G (2003) The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 84:1733–1743
Johnson ET, Berhow MA, Dowd PF (2008) Colored and white sectors from star- patterned petunia flowers display differential resistance to corn earworm and cabbage looper larvae. J Chem Ecol 34:757–765
Jones KN, Reithel JS (2001) Pollinator-mediated selection on a flower color polymorphism in experimental populations of Antirrhinum (Scrophulariaceae). Am J Bot 88:447–454
Karban R, Nagasaka K (2004) Are defenses of wild radish populations well matched with variability and predictablity of herbivory? Evol Ecol 18:283–301
Koes RE, Quattrocchio F, Mol JNM (1994) The flavonoid biosynthetic-pathway in plants—function and evolution. BioEssays 16:123–132
Lankau RA (2007) Specialist and generalist herbivores exert opposing selection on a chemical defense. New Phytol 175:176–184
Lehtila K, Strauss SY (1997) Leaf damage by herbivores affects attractiveness to pollinators in wild radish, Raphanus raphanistrum. Oecologia 111:396–403
Li Q, Eigenbrode SD, Stringam GR, Thiagarajah MR (2000) Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J Chem Ecol 26:2401–2419
Mueller H (1883) The fertilisation of flowers. MacMillan, London
Newton E, Bullock JM, Hodgson D (2010) Temporal consistency in herbivore responses to glucosinolate polymorphism in populations of wild cabbage (Brassica oleracea). Oecologia 164:689–699
Panestsos CP (1964) Sources of Variation in Wild Populations of Raphanus (Cruciferae). PhD thesis, University of California, Berkeley
Renwick JAA (2002) The chemical world of crucivores: lures, treats and traps. Ent Exper Appl 104:35–42
Smallegange RC, van Loon JJA, Blatt SE, Harvey JA, Agerbirk N, Dicke M (2007) Flower vs. leaf feeding by Pieris brassicae: glucosinolate-rich flower tissues are preferred and sustain higher growth rate. J Chem Ecol 33:1831–1844
Stanton ML (1987) Reproductive-biology of petal color variants in wild populations of Raphanus sativus I: pollinator response to color morphs. Am J Bot 74:178–187
Strauss SY, Irwin RE (2004) Ecological and evolutionary consequences of multispecies plant–animal interactions. Annu Rev Ecol Evol Syst 35:435–466
Strauss SY, Conner JK, Rush SL (1996) Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. Am Nat 147:1098–1107
Strauss SY, Irwin RE, Lambrix V (2004) Optimal defense theory and flower petal colour predict variation in the secondary chemistry of wild radish. J Ecol 92:132–141
Waser NM, Price MV (1981) Pollinator choice and stabilizing selection for flower color in Delphinium nelsonii. Evolution 35:376–390
Whitney KD, Stanton ML (2004) Insect seed predators as novel agents of selection on fruit color. Ecology 85:2153–2160
Acknowledgments
We would like to thank Peter Connors and Jackie Sones at the Bodega Marine Laboratory and Reserve for the use of their facilities and access to radish populations. We would also like to thank the California Department of Fish and Game for access to the YOLO site. Special thanks to Amy Chang and Amber Wright for help in the field. R. Karban, J. Lau, and T. Schultz, and two anonymous reviewers made helpful comments on an earlier version of this manuscript. These experiments comply with the current laws of the country in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Florian Schiestl.
Rights and permissions
About this article
Cite this article
McCall, A.C., Murphy, S.J., Venner, C. et al. Florivores prefer white versus pink petal color morphs in wild radish, Raphanus sativus . Oecologia 172, 189–195 (2013). https://doi.org/10.1007/s00442-012-2480-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2480-z