Abstract
Life history characteristics and resulting fitness consequences manifest not only in an individual experiencing environmental conditions but also in its offspring via trans-generational effects. We conducted a set of experiments to assess the direct and trans-generational effects of food deprivation in the Glanville fritillary butterfly Melitaea cinxia. Food availability was manipulated during the final stages of larval development and performance was assessed during two generations. Direct responses to food deprivation were relatively minor. Food-deprived individuals compensated, via increased development time, to reach a similar mass as adults from the control group. Delayed costs of compensatory growth were observed, as food-deprived individuals had either reduced fecundity or lifespan depending on the type of feeding treatment they had experienced (intermittent vs. continuous). Female food deprivation did not directly affect her offspring’s developmental trajectory, but the way the offspring coped with food deprivation. Offspring of mothers from control or intermittent starvation treatments reached the size of those in the control group via increased development time when being starved. In contrast, offspring of mothers that had experienced 2 days of continuous food deprivation grew even larger than control animals, when deprived of food themselves. Offspring of food-deprived Glanville fritillary initially showed poor immune response to parasitism, but not later on in development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental conditions in the wild are spatially and temporally heterogeneous. Therefore, organisms are likely to face periods of stressful conditions (those that impose a challenge to the homeostatic state of the organism; Ghalambor et al. 2007) at some stage of their lives, especially in today’s changing environment. Environmental stress is one of the most important sources of natural selection, and as a result various strategies have evolved allowing organisms to cope with environmental variation (Meyers and Bull 2002). Phenotypic plasticity, which is the ability of a genotype to modify its phenotypic expression depending on the environmental conditions (Bradshaw 1965; Pigliucci 2001), is one such strategy. The quality and quantity of food are often heterogeneously distributed, causing environmental stress. Accordingly, studies have demonstrated both adaptive and non-adaptive responses to shortage or suboptimal quality of food (Metcalfe and Monaghan 2001).
Environmental conditions during development can profoundly affect an individual’s adult condition (or state), which in turn will impact life-history and individual fitness (McNamara and Houston 1996). This is because an organism’s adult physiological condition is largely determined during development, especially in holometabolous insects in which resources acquired during larval development are allocated to different adult body parts during metamorphosis. Larval conditions are particularly important for those species that acquire few resources at the adult stage (Boggs 2009). In holometabolous insects, an individual’s growth is often highest during the final instar, so that even a very short period of starvation during this sensitive period can severely impact adult resource allocation patterns, namely fecundity and body maintenance (Bauerfeind and Fischer 2005b; Saastamoinen et al. 2010). In general, stress responses to nutritional limitations seem to be tightly linked with growth responses, and stress often induces a shift in individual’s physiology towards a “survival mode” in which growth is ceased. The duration and magnitude of the stress response is typically proportional to the severity of stress (Lopez-Maury et al. 2008). Once resources become available again, the survival mode is disrupted and growth will continue. In some cases, growth even accelerates afterwards, as individuals compensate for the period of starvation (Hector and Nakagawa 2012). Both the intensity and frequency of food deprivation is likely to have a great impact on the observed life history responses.
Compensation for a period of food deprivation is most likely if the fitness of a phenotype is stable across environments, and hence individuals aim to produce the same target phenotype (for example, large body size) under all environmental conditions (i.e. canalization; Liefting et al. 2009). Compensatory strategies can be costly in the long term (Metcalfe and Monaghan 2001). For example, compensatory growth has been shown to increase metabolic rate and/or elevate disease risk later in life, so that stressed individuals suffer from reduced lifespan even though they appear as fit as those developing under optimal conditions (e.g., Criscuolo et al. 2008; Monaghan et al. 2011). Poor environmental conditions during development may also lead to adaptive changes in resource allocation patterns that prepare individuals for future environmental stress (Metcalfe and Monaghan 2001). Such changes, for example in lipid storage (body maintenance) or thorax ratio (investment in dispersal), are predicted if the quality of the adult environment can be predicted by conditions during development (e.g., Barrett et al. 2009; Harbison et al. 2005; Saastamoinen et al. 2010).
In addition to direct effects, a number of studies on both plants and animals have shown that environmental conditions experienced by a parent can greatly influence the life history trajectory of its offspring (i.e. trans-generational and maternal effects; Mousseau and Fox 1998). Poor environmental conditions experienced by a mother often have a negative impact on the life history of her offspring, for example via low allocation of resources to eggs (Hafer et al. 2011; Marshall and Uller 2007). Poor maternal developmental conditions may hence result in poor quality offspring that suffer from reduced fitness (reviewed in Monaghan 2008). One measure of individual condition is the ability to resist diseases. Parasites and pathogens are ubiquitous threats that most organisms fight through immunity (Beckage et al. 1993). As immunological responses are costly (Schmid-Hempel 2003), poor- compared to well-conditioned individuals may have either poor immune responses or reduced fitness following an immunological challenge (a trade-off). The insect immune system is relatively simple and is characterized by inducible expression of antimicrobial peptides and constitutive melanization–encapsulation responses (e.g., Lavine and Strand 2002; Rantala and Roff 2005; Schmidt et al. 2001; Strand 2008). The latter is a commonly assayed cellular response in insects, in which hemocytes kill a foreign intruder such as a parasitoid egg or larva by enclosing it in a melanized capsule (Gillespie et al. 1997). Hemocyte concentrations or ratios of different types of hemocytes have been shown to vary among species, individuals within species, life stages, and physiological states, and are associated with resistance to disease (Alleyne and Wiedenmann 2001; Bauer et al. 1998; Lavine and Strand 2002; Strand 2008).
Though trans-generational effects of environmental stress often have negative effects on offspring fitness, a number of studies have shown positive effects, e.g., by buffering the offspring against environmental stressors (e.g., Agrawal 2002; Mousseau and Dingle 1991). As an example, mothers exposed to natural enemies can produce more resistant offspring (Agrawal et al. 1999) and mothers living under degraded conditions can produce more dispersive offspring (e.g., Krug and Zimmer 2000).
In the present study, we examined the life history responses of the Glanville fritillary butterfly (Melitaea cinxia) to food deprivation during the final stage of development. First, we imposed 2 days of food deprivation and assessed the developmental responses. Since Glanville fritillary larvae often have to move to new plants in their last instars, we expected that a 2-day starvation period would represent a common occurrence under field conditions. We also assessed whether the responses were dependent on the frequency of the food deprivation. Second, we assessed whether the effects of food deprivation transfer to the offspring via maternal (and/or other non-genetically inherited) effects. We assessed whether the maternal environment affects the offspring’s developmental response to food deprivation, and the immune response of the pre-diapause larvae. The main goal in the trans-generational assessments was to evaluate whether offspring from starved mothers are simply of worse quality or whether they were predisposed to cope with nutritional deprivation via maternal experiences.
Materials and methods
The Glanville fritillary butterfly is an endangered Eurasian butterfly that in Finland only occurs in the Åland Islands. Female butterflies lay clusters of on average 140 eggs (up to 10 clutches; Saastamoinen 2007) in June on the plants Plantago lanceolata and Veronica spicata L. (Plantaginaceae), which are patchily distributed in dry meadow habitats (Kuussaari et al. 2000). There is no systematic difference in larval survival between the two host plant species in the wild. However, in laboratory experiments, the developmental growth trajectories are affected by the host plant species (van Nouhuys et al. 2003). After feeding gregariously for five instars, the larvae spend the winter in diapause in a silken web, after which they go through two to three additional instars and then pupate in the late spring (Saastamoinen, personal observation). In the last instars, larvae often have to leave their natal plants and search for food, making periods of starvation likely (Hellmann 2002; Kuussaari et al. 2004). In the Åland Islands, the Glanville fritillary hosts three species of parasitoids (van Nouhuys and Hanski 2005). For this study, we used the specialist parasitoid Cotesia melitaearum (Kankare et al. 2005), which lay gregarious broods of between 2 and 40 eggs in a Glanville fritillary larva. The number of eggs laid is partially dependent on the size of the host larva when parasitized (Lei et al. 1997; van Nouhuys and Lei 2004). Successful parasitism by this wasp differs among host generations (it has 2–3 generations per host generation; van Nouhuys and Lei 2004), host conditions (van Nouhuys and Laine 2008; van Nouhuys and Punju 2010), and populations. These differences are at least in part due to variation of encapsulation of parasitoid eggs and larvae by host hemocytes (Pakarinen 2011).
Experimental design
Generation 1
A total of 2,040 Glanville fritillary larvae were collected from more than 430 local populations across the Åland Islands in autumn 2009 (up to 3 larvae/family), and kept in diapause (−5 °C) in the laboratory over the winter. In the spring, they were reared ad libitum (28:15 °C, D:N) on either P. lanceolata or V. spicata until the last instar, after which they were randomly assigned to one out of three treatment groups. On the day of the molt into the seventh instar, each larva was weighed and placed in an individual container. The larvae in the “control group” had food available at all times, whereas those in the starvation treatments were food deprived for 2 days. In the “continuous starvation treatment” larvae did not receive food for 2 consecutive days, whereas in the “intermittent starvation treatment” fresh food was given to the larvae for 1 day between the 2 days without food. All individuals were weighed after pupation. After eclosion, butterflies were individually numbered on the hind wing and placed in cylindrical cages with no more than 50 adults per cage (sexes separately). One day after eclosion, ~100 randomly chosen individuals per larval feeding treatment (1:1 sex ratio, all were fed P. lanceolata) were killed to measure thorax ratio (thorax mass/total body mass). The remaining adults were fed honey:water solution ad libitum to assess lifespan. In addition, we measured the fecundity of 139 females (n = 45–49 per treatment). For the fecundity assessments, we randomly chose 3-day-old females from each of the treatment groups and placed them in mating cages with twice the number of randomly selected males from the control group. All mating pairs were removed from the cage and fed after mating. Each female was then placed in a separate cage with a host plant for oviposition and honey:water solution ad libitum. Host plants were checked for eggs every other day until the female died. The number of egg clutches and the number of eggs in each clutch were counted.
Generation 2
Trans-generational effects on post-diapause larval development
To test maternal effects on offspring life history we used offspring of mothers that had experienced one of the three developmental conditions; control, intermittent or continuous starvation (13, 10, and 10 families, respectively). Each family was initially split into two groups (to minimize the effect of common environment), and reared on V. spicata ad libitum under laboratory conditions with a constant temperature regime (28:15 °C, D:N). The day of molting to the final instar each larva was weighed, placed in an individual container and randomly assigned to a feeding treatment. In total, 136 offspring (57, 41, and 31 from control, intermittent, and continuous groups, respectively) were reared to adulthood. Due to the relatively small sample size, we used only the control and intermittent starvation treatment in the F2 generation. We measured mass at last instar, duration of the last instar, pupal weight, and thorax ratio at day 3 of adult life.
Trans-generational effects on encapsulation rate and immunity
To assess maternal effects on immune function of the F2 generation we used the specialist parasitoid species, C. melitaearum. To obtain wasps for this experiment, C. melitaearum cocoons were collected from the natural populations in the Åland Islands in the spring of 2010. The resulting wasps were mated with non-siblings and the females were allowed to parasitize laboratory-reared Glanville fritillary larvae. The resulting progeny were mated with non-siblings and then the females were kept in individual plastic vials under laboratory conditions and fed honey:water (3:1) until needed.
The Glanville fritillary larvae used for the immune response experiment were offspring of females from the control and intermittent feeding treatments (10 families per treatment). All larvae were from the second clutch of each mother (the first clutch was used for the life history assessment as above). Larvae were reared on V. spicata ad libitum in gregarious family groups under laboratory conditions with a constant temperature regime (28:15 °C, D:N). After the larvae molted into the third instar, each family was split into three groups to control for common environment effects. Three replicates of 10 larvae per family (i.e. 30 larvae/family) were parasitized when the larvae were in the third instar. To ensure parasitism, each larva was individually placed in a Petri dish with a single female wasp and the parasitism was observed directly. For each family replicate, three different parasitoids were used in order to remove the effects of individual variation among wasps. Three days after exposure to parasitoids, three randomly chosen larvae (or one-third of the larvae) from each Petri dish (i.e. approximately 9 larvae per family) were dissected under a compound microscope to assess the number of living and encapsulated (dead) parasitoids within each host larva. The remaining host larvae in each family were allowed to develop naturally until diapause. Very few parasitoids egressed from the host before diapause, and hence the larvae were dissected 12 days after entering diapause, and the number of parasitoid larvae was counted. The date when the host entered diapause was noted to assess the development time of the host. Dead host larvae were dissected and recorded.
In addition to the encapsulation response, 84 unparasitized larvae from 18 families including offspring from both control and starved (intermittent only) mothers were assayed for the number and types of hemocytes present in the hemolymph. One microliter of hemolymph was taken during the fourth instar by puncturing each larva with a needle and immediately diluting the hemolymph with 10 μl of insect saline solution. Cells were transferred to a Bürker hemocytometer and counted. For each larva, we calculated the total number of hemocytes and the number of hemocytes of three different types. The hemocytes were identified with phase contrast microscopy on the basis of their characteristic morphology according to (Lackie 1988) as granular cells (GRs), oenocytoids (OEs) and other hemocytes (which were mostly plasmatocytes, but included some cells that were not well enough focused to distinguish from plasmatocytes).
Statistical analyses
Linear mixed model approaches (SAS v.9.2 for Windows; SAS Institute, Cary, NC, USA) were used to examine the effects of larval feeding treatments on phenotypic variation in the first generation as well as to assess maternal effects on parasitism rate and offspring responses to starvation. In the first generation, the phenotypes examined were development time of the final instar, pupal mass, relative allocation to thorax, and adult lifespan. Sex and food plant species (only P. lanceolata for thorax ratio) were included as fixed factors, and population was included as a random factor. As a measure of female fecundity we used clutch size and included clutch number as a factor, pupal mass as a covariate, and female as a random factor. For the second generation, maternal treatment, current treatment, and sex were included as fixed factors and family as a random factor.
To assess maternal effects on parasitism and larval survival, we analyzed the number of living parasitoids (eggs or larvae), the number of dead parasitoids (encapsulated larvae and “cell aggregations” which are disintegrated encapsulated parasitoid eggs or larvae) and whether or not we found any parasitoids within each dissected host individual (0/1). Parasitism (0/1) and larval survival until the diapause were modeled with a binary error distribution. In these analyses, maternal treatment was included as a fixed factor and family as a random factor. The total number of hemocytes, granular cells, and oenocytoids were analyzed with maternal treatment and family nested within maternal treatment as fixed factors. We also conducted family level analyses to assess whether the average number of hemocytes in each family correlated with the average larval survival, development time and ratio of dead to all parasitoids (in both third instar and diapaused larvae). In all the analyses, we used backward model selection by starting with a full model for each trait and sequentially eliminating interaction terms with highest P values.
Results
Direct effects of food deprivation in generation 1
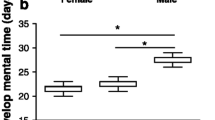
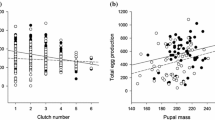
A period of starvation increased development time (Tables 1, 2) with stronger impact under intermittent than continuous food deprivation, especially in females (interaction between sex and feeding treatment; Tables 1, 2; for details, see Appendix A). Despite the influence of starvation on development time, pupal mass and allocation to thorax remained unaffected in both sexes (Tables 1, 2). Starvation further affected lifespan, with individuals experiencing continuous deprivation having the shortest lifespan while those from the intermittent deprivation treatment living the longest (Tables 1, 2; Fig. 1A). Males developed faster, were smaller as pupae, allocated more resources to the thorax and lived longer than females (Tables 1, 2). In general, individuals fed V. spicata developed faster, were lighter as pupae, and had shorter adult lifespan than those fed P. lanceolata (Tables 1, 2). The latter effect was more pronounced in females than in males (significant sex by host–plant interaction; Tables 1, 2; for details, see Appendix A). For the subset of females in which fecundity was assessed (all were fed P. lanceolata), the average clutch size was 140.7 eggs. Larval starvation reduced clutch size such that females from the control group laid significantly more eggs than females from the intermittent feeding treatment (Fig. 1B; for details see Appendix A). The clutch size decreased with clutch number and was influenced by body mass (Table 2). However, the effect of body mass was treatment-specific (a significant interaction between body mass and treatment indicated; Table 2), with a positive correlation between pupal mass and average clutch size only in the continuous starvation treatment (Fig. 2; P = 0.004).
Direct effects (±SE) of food deprivation in the Glanville fritillary butterfly (Melitaea cinxia) during development on A lifespan (least squares means) and B clutch size (least squares means) separately for the control, intermittent and continuous food deprivation treatments. Bars accompanied by the same letter within each panel do not differ significantly in pairwise post hoc comparisons
Effect of body mass on the average clutch size (corrected for clutch number) separately for the control (black circles, solid line), intermittent (grey triangles, long dashed line) and continuous (open circles, short dashed line) food deprivation treatments. The correlation was only significant for the females in continuous treatment (P = 0.004)
Trans-generational effects of food deprivation
Offspring life history
Developmental conditions experienced by the mother did not seem to impact the initial condition of her offspring, as there was no difference in the body mass of the larvae at the beginning of the seventh instar or in the developmental time of the offspring (Table 3). Though the maternal developmental conditions did not impact the pupal mass of her offspring, there was a trend suggesting (interaction between sex and maternal treatment; Table 3) that female offspring of continuously starved mothers were heavier as pupae than those from the intermittent group (for details, see Appendix B). As in the first generation, experiencing starvation during development (intermittent starvation only) increased development time, but had no effect on pupal mass or allocation to thorax in the second generation (Table 3). Similarly, males generally developed faster than did females, were lighter as pupae, and allocated more resources to their thorax (Table 3).
The effect of food deprivation on development time was also influenced by the maternal conditions (Fig. 3A; Table 3; interaction between maternal food treatment and offspring food treatment). A closer examination indicated that offspring of the mothers in the control group developed more slowly in response to food deprivation than offspring of mothers in the intermittent group (Fig. 3A; Appendix B). Maternal treatment also influenced the offspring response to food deprivation in terms of body mass (Fig. 3B; Table 3; interaction between maternal food treatment and offspring food treatment), as individuals whose mothers had experienced continuous starvation attained a high body mass when starved themselves (Fig. 3B; Appendix B). In contrast, the response to starvation in terms of body mass was slightly negative or absent in intermittent starvation and control groups, respectively.
Direct and trans-generational effects (±SE) on A development time and B pupal mass (least squares means). Current developmental treatment is represented by the x-axes and maternal treatment by the different lines. Dots accompanied by the same letter within each panel do not differ significantly in pairwise post hoc comparisons
Offspring immunity
There was a small effect of maternal feeding treatment on successful parasitism by C. melitaearum. No parasitoid offspring were found in 17 % of the parasitized larvae dissected at the very early stage of parasitoid development (3 days after parasitism). The probability of being parasitized did not differ between the two maternal treatment groups (P = 0.39). Similarly, the number of dead parasitoids (0.3 on average) found inside the host upon dissection was not affected by maternal treatment (P = 0.99). Larvae of starved mothers did, however, have more living parasitoids in them at day 3 (1.4 and 1.8 for control and starvation groups, respectively; F 1,156 = 5.8, P = 0.017).
The rest of the parasitized larvae in each family were dissected after the initiation of the winter diapause. At this stage, we found no difference between the treatment groups in any of the response variables (P = 0.48, P = 0.40 and P = 0.53 for whether or not the host was parasitized, the number of living parasitoids and the number of dead parasitoids, respectively). The average number of parasitoids per larva at the end of pre-diapause development was 1.7, which was the same as that of the control group at day 3. There was no difference between the treatment groups in the rate of survival to diapause (P = 0.39), though larvae with more parasitoids in them took longer to reach diapause (F 1,232 = 14.5, P = 0.0002).
Among the unparasitized larvae dissected, we found significant differences among families in the number of granular hemocyte cells (F 16,66 = 2.5, P = 0.005) and the number of oenocytoids (F 15,44 = 3.2, P = 0.002), but not in the number of other hemocytes (mostly plasmatocytes) (P = 0.25) nor in the total number of hemocytes (P = 0.35). The maternal treatment (control or starvation) did not have a significant effect in any of the hemocyte categories (P = 0.69, P = 0.79, P = 0.27, and P = 0.88 for the total number of hemocytes, granular cells, oenocytoids, and other hemocytes, respectively). Larval survival to diapause was positively correlated with the average number of granular cells and the average number of oenocytoids (F 1,14 = 5.4, P = 0.035 and F 1,14 = 9.6, P = 0.008, respectively), but not with the number of other hemocytes (P = 0.60).
Discussion
Organisms in the wild are faced with a wide range of environmental conditions, and are expected to show some degree of adaptation and resilience to challenges such as short term food deprivation. Environmental fluctuations occur in all natural environments and will become more severe with increasing global change (Pachauri and Reisinger 2007). We found that the Glanville fritillary butterfly responds to an episode of larval starvation by increasing development time, and hence attains the same adult size as well-fed individuals. Though food-deprived individuals are able to compensate in terms of body mass, some negative effects are still evident later on in life. These effects differ between types of starvation, as individuals that experienced two intermittent days of food starvation have reduced clutch size, whereas individuals deprived of food for two consecutive days have a reduced lifespan. In addition to these direct effects, we also found trans-generational effects of food deprivation. The offspring of food-deprived mothers did not differ from those of control mothers in survival or initial body mass. However, when the offspring of food-deprived mothers were starved themselves, their compensatory responses were dependent on the type of food deprivation the mother had experienced. Starved offspring of mothers from the intermittent feeding treatment developed slowly and remained smaller than control individuals, whereas offspring of mothers that experienced starvation for two consecutive days increased in body mass via compensatory growth when starved themselves. Offspring of starved mothers are initially more vulnerable to parasitism by C. melitaearum, but later in development that vulnerability is overcome, at least under the conditions of this experiment. While we found strong variation of hemocyte concentration among families, and that families with high average hemocyte concentrations had low mortality, there was no association of maternal conditions with constitutive immunity of the offspring.
Compensatory growth after food deprivation
A general prediction is that developmental plasticity is favored when the environment experienced by the developing organism is a good predictor of future environmental conditions, and can thus serve as a cue for an appropriate phenotype (Liefting et al. 2009; Meyers and Bull 2002). In insects in which metamorphosis results in both a change in morphology and a change in ecological niche (i.e. larvae and adult have different diets), the conditions for the larvae do not necessarily predict the conditions for the adults. Therefore, poor dietary conditions for a larva may result in smaller adult size (e.g., Fox et al. 1999; Saastamoinen et al. 2010), rather than a plastic response of delayed development without reduction in body size (Barrett et al. 2009; Bauerfeind and Fischer 2005b). Resource limitation during development may, however, hinder individuals from attaining an optimal size or critical body size for pupation, resulting in prolonged development or faster growth rate once conditions improve (Esperk and Tammaru 2010).
The Glanville fritillary butterfly seems to canalize body size by increasing development time in response to poor nutritional conditions and, at least in females, development time has the greatest increase under intermittent food deprivation. In general, the canalization of body mass implies either that there is a critical body size that needs to be achieved prior to pupation or that the fitness benefit of large body size is equal across all environmental conditions, and hence should be maximized. As in many other Lepidoptera (e.g., Garcia-Barros 2000; Jimenez-Perez and Wang 2004), we found that for females larger body mass is correlated positively with fecundity but only in the continuous starvation treatment. Previous work on the Glanville fritillary under semi-natural conditions has suggested benefits of large body size under poor environmental conditions when compensation for small body reserves by adult feeding is prevented (Saastamoinen 2007).
The larvae of the Glanville fritillary butterfly feed on P. lanceolata and V. spicata in Åland. In the spring, the gregariously feeding larvae easily consume an entire host plant and, as both plant species often have a patchy distribution in a habitat patch (Kuussaari et al. 2004), experiencing food deprivation while searching for a new host plant may be quite common. Glanville fritillary larvae, both starved and not-starved, grew faster but reached a smaller final size when fed V. spicata than fed P. lanceolata. Development of the Glanville fritillary is known to depend on the host plant species (e.g., van Nouhuys et al. 2003; Saastamoinen et al. 2007), though long-term and large-scale survey data do not show any systematic difference in the survival of larvae using the two host plants in natural populations (van Nouhuys et al. 2003).
Contrasting responses to different types of food deprivation
Poor nutritional conditions followed by compensatory strategies can come at a cost, which may not be apparent until later in life and/or under particular environmental conditions (e.g., Dmitriew and Rowe 2011; Helle et al. 2012). Experiencing 2 days of starvation did entail delayed costs for the Glanville fritillary. Interestingly, the two types of starvation treatments resulted in different responses. First, individuals (effect was stronger in females) from the intermittent starvation group took more time than those from the continuous starvation treatment to attain the equal body size with the control group. Second, the individuals that experienced intermittent starvation were almost 10 % less fecund in terms of clutch size than those in the control treatment. Negative effects on fecundity in response to starvation are expected and commonly found (Bauerfeind and Fischer 2005a, b; Boggs and Ross 1993), as reproduction is generally a nutrition-limited process. In Lepidoptera, resources allocated to reproduction can be derived from reserves stored during development and/or current feeding (O’Brien et al. 2004). The reduced fecundity due to larval food deprivation that we observed highlights the importance of stored resources for fecundity of the Glanville fritillary (see also Saastamoinen et al. 2009). However, the lowered fecundity occurred without any differences in body mass or body allocation patterns. Finally, though individuals in the intermittent feeding treatment had low fecundity they had the longest lifespan. This suggests that, in the intermittent treatment group, the compensation in terms of body size results from investment in body maintenance rather than fecundity. A positive correlation between longevity and food limitation is also commonly observed in experimental studies of insects, and is often explained by individuals entering a “survival mode” in which they use less resources in response to a shortage of food (e.g., Baldal et al. 2006; Bishop and Guarente 2007).
Individuals that experienced continuous rather than intermittent starvation on the other hand had a 5 % shorter lifespan than the control group. Reduced lifespan may be caused by increased metabolic rate or decreased immunity (e.g., Moret and Schmid-Hempel 2000; Parsons 1993). Increased energy metabolism due to food limitation may result from compensatory growth (increased growth rate) after resources become plentiful again. If an increase in metabolic rate becomes fixed it may accelerate senescence in adults (e.g., Criscuolo et al. 2008). Decreased adult survival in response to starvation has also been observed in other insects (e.g., Boggs and Freeman 2005; Dmitriew and Rowe 2007). It is notable that, whatever the mechanism behind the reduced lifespan in the continuous starvation treatment, it had little consequence since it did not decrease individual fecundity. In the wild, where oviposition may be time limited and environmental conditions may be less favorable, the fitness consequences of reduced lifespan may be higher (Dmitriew and Rowe 2011).
Trans-generational effects of developmental condition
Poor conditions are known to have impacts across generations (e.g., Hafer et al. 2011). Such trans-generational effects occur if a parent’s phenotype influences the phenotype of its offspring (Räsänen and Kruuk 2007). These effects could be negative if a mother, for example, sacrifices the quality of her offspring due to resource depletion. Alternatively, if the developmental environment of a mother provides a reliable indicator of the environmental conditions that her progeny will encounter, maternal effects may evolve as a mechanism for trans-generational phenotypic plasticity (Fox and Mousseau 1998; Mousseau and Dingle 1991). Parents may, for example, provide offspring with increased tolerance of environmental contaminants (Marshall 2008), food shortages (Bashey 2006), desiccation (Yoder et al. 2006), and increased temperatures (Salinas and Munch 2011). We found that individuals whose mothers had experienced continuous starvation had high compensatory growth after experiencing food deprivation themselves, becoming larger as pupae than offspring of mothers that had food available at all time. As discussed above, large body size in many insects, including the Glanville fritillary butterfly, often entails fitness benefits (Saastamoinen 2007).
To assess the quality of the offspring in an entirely different way, we compared the susceptibility to parasitism and hemocyte concentration of larvae with starved and non-starved mothers. It is known that Lepidoptera combat parasitism by Cotesia parasitoids by encapsulating parasitoid eggs and larvae (Alleyne and Wiedenmann 2001; Bukovinszky et al. 2009; Laurentz et al. 2012; Okech and Overholt 1996). Initially, the offspring of starved individuals contained more living parasitoid eggs and larvae than the offspring of well-fed mothers, suggesting that they were immunologically compromised. This could result both if C. melitaearum had laid large broods of eggs in the offspring of food-deprived mothers or if we failed to detect parasitoid eggs killed quickly by offspring of well-fed mothers. The first case would suggest that the wasps were assessing host conditions, and favoring those with starved mothers. This would be adaptive for the wasp if it reflected high host quality (Vet et al. 1990; Vinson 1976) or low expected host immune response. In the second case, the progeny of non-starved mothers would have had a stronger initial immune response to parasitism, indicating that maternal starvation reduced the immune response of her offspring. We cannot distinguish between the two outcomes. However, in the end, the number of C. melitaearum larvae present at host diapause, which reflects the brood size in the spring (van Nouhuys and Punju 2010), was equal in the two groups. Thus, there is evidence that maternal starvation leads to increased vulnerability to C. melitaearum which, under the conditions of this experiment, was overcome partway through host development. A similar pattern of response was observed in a study comparing parasitism of the Glanville fritillary fed mildew-infected and uninfected host plants (van Nouhuys and Laine 2008).
Hemocytes are involved in the encapsulation of parasitoids as well as other foreign bodies (Gillespie et al. 1997). In general, higher hemocyte concentrations are expected to be associated with ability to fight infection (Gillespie et al. 1997). As Alleyne and Wiedenmann (2001) found, using related host and parasitoid species, we detected no association of hemocyte concentration with rate of encapsulation of Cotesia parasitoids. However, some Glanville fritillary larvae died during the experiment due to disease rather than parasitism. Mortality due to disease was low in families containing high average concentration of granular cells and oenocytoids. Thus, there was family level variation of susceptibility to disease associated with hemocyte concentration, but hemocyte concentration was not associated with feeding treatment. An adaptive explanation of the lack of plasticity of immune response to food deprivation is that the threat of disease is constant, so the immune response of offspring should not be modified by the experience of the parents.
Conclusions
We found that the Glanville fritillary butterfly is able to cope with environmental variation during the sensitive period of larval development. Both females and males canalized their body size via compensatory growth after food deprivation by increasing their development time. Though the compensatory growth is successful in terms of body mass, it also entails costs, which are related to the type of starvation they experience. Deprivation for 2 consecutive days seems less costly than deprivation for 2 days separated by 1 day of feeding. That is, individuals that experienced the continuous deprivation treatment were able to compensate with a shorter increase in development time, and consequently maintained higher fecundity. Their offspring also withstood food deprivation better than those experiencing intermittent deprivation.
The compensatory response of the Glanville fritillary, though different in the two deprivation treatments, suggests that a mother’s experience of a 2-day starvation period during development could be a reliable indicator of environmental conditions that her offspring are likely to encounter a year later. The local habitat patches in the Åland Island metapopulation vary in host plant density, the density of nectar plants, and the degree of within-patch spatial heterogeneity, as well as environmental conditions, especially precipitation (Hanski and Meyke 2005). It may be that poor nutritional conditions during the final stages of development signify a low density or scattered distribution of food plants that may persist within the habitat patch through the following year.
Studies on other insects have shown that as an adaptive response to nutritional limitation or high density during development, resource allocation patterns may be altered allowing individuals to move away from the crowded and/or poor quality habitats (Boggs and Freeman 2005; Saastamoinen et al. 2010). Characteristics associated with dispersal of the Glanville fritillary butterfly are also known to evolve in response to local landscape structure (e.g., Hanski et al. 2006). In our study, however, characteristics closely associated with dispersal (thorax ratio) were not affected by maternal food deprivation. In combination with the previous work on dispersal of the Glanville fritillary, ours suggests that the butterfly employs a mixed strategy with respect to local environmental conditions. The role of poor maternal environment for offspring immunity is less clear. Some evidence suggests that offspring from the starved mothers are more vulnerable to parasitism than the offspring of non-starved mothers, but this was only apparent in the very young larvae, and maternal condition was unrelated to the hemocyte concentration of their offspring. This suggests a lack of plasticity of immune response which may be adaptive where disease and parasitism is a constant threat to larvae. Overall, our study provides insight into how organisms may respond to environmental challenges they encounter in the wild.
References
Agrawal AA (2002) Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology 83:3408–3415
Agrawal AA, Laforsch C, Tollrian R (1999) Transgenerational induction of defenses in animals and plants. Nature 401:60–63
Alleyne M, Wiedenmann RN (2001) Encapsulation and hemocyte numbers in three lepidopteran stemborers parasitized by Cotesia flavipes-complex endoparasitoids. Entomol Exp Appl 100:279–293
Baldal EA, Brakefield PM, Zwaan BJ (2006) Multitrait evolution in lines of Drosophila melanogaster selected for increased starvation resistance: the role of metabolic rate and implications for the evolution of longevity. Evolution 60:1435–1444
Barrett ELB, Hunt J, Moore AJ, Moore PJ (2009) Separate and combined effects of nutrition during juvenile and sexual development on female life-history trajectories: the thrifty phenotype in a cockroach. Proc R Soc Lond B 276:3257–3264
Bashey F (2006) Cross-generational environmental effects and the evolution of offspring size in the Trinidadian guppy Poecilia reticulata. Evolution 60:348–361
Bauer E, Trenczek T, Dorn S (1998) Instar-dependent hemocyte changes in Pieris brassicae after parasitization by Cotesia glomerata. Entomol Exp Appl 88:49–58
Bauerfeind SS, Fischer K (2005a) Effects of adult-deprived carbohydrates, amino acids and micronutrients on female reproduction in a fruit-feeding butterfly. J Insect Physiol 51:545–554
Bauerfeind SS, Fischer K (2005b) Effects of food stress and density in different life stages on reproduction in a butterfly. Oikos 111:514–524
Beckage NE, Thompson SA, Federici BA (eds) (1993) Parasites and pathogens of insects. Academic, New York
Bishop NA, Guarente L (2007) Genetic links between diet and lifespan:shared mechanisms from yeast to humans. Nat Rev Genet 8:835–844
Boggs CL (2009) Understanding insect life histories and senescence through a resource allocation lens. Funct Ecol 23:27–37
Boggs CL, Freeman KD (2005) Larval food limitation in butterflies: effects of adult resource allocation and fitness. Oecologia 144:353–361
Boggs CL, Ross CL (1993) The effect of adult food limitation on the life-history traits in Speyeria mormonia (Lepidoptera: Nymphalidae). Ecology 74:433–441
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. Adv Genet 13:115–155
Bukovinszky T et al (2009) Consequences of constitutive and induced variation in plant nutritional quality for immune defence of a herbivore against parasitism. Oecologia 160:299–308
Criscuolo F, Monaghan P, Nasir L, Metcalfe NB (2008) Early nutrition and phenotypic development: “catch-up” growth leads to elevated metabolic rate in adulthood. Proc R Soc Lond B 275:1565–1570
Dmitriew C, Rowe L (2007) Effects of early resource limitation and compensatory growth on lifetime fitness in the ladybird beetle (Harmonia axyridis). J Evol Biol 20:1298–1310
Dmitriew C, Rowe L (2011) The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS One 6:e17399
Esperk T, Tammaru T (2010) Size compensation in moth larvae: attention to larval instars. Physiol Entomol 35:222–230
Fox CW, Mousseau TA (1998) Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, New York, pp 159–177
Fox CW, Czesak ME, Savalli UM (1999) Environmentally-based maternal effects on development time in the seed beetle, Stator pruininus (Coleoptera: Bruchidae): consequences of larval density. Environ Entomol 28:217–223
Garcia-Barros E (2000) Body size, egg size, and their interspecific relationships with ecological and life history traits in butterflies (Lepidoptera: Papilionoidea, Hesperioidea). Biol J Linn Soc 70:251–284
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Gillespie JP, Kanost MR, Trenczek T (1997) Biological mediators of insect immunity. Annu Rev Entomol 42:611–643
Hafer N, Ebil S, Uller T, Pike N (2011) Transgenerational effects of food availability on age at maturity and reproductive output in an asexual collembolan species. Biol Lett 7:755–758
Hanski I, Meyke E (2005) Large-scale dynamics if the Glanville fritillary butterfly: landscape structure, population processes, and weather. Ann Zool Fenn 42:379–395
Hanski I, Saastamoinen M, Ovaskainen O (2006) Dispersal-related life history trade-offs in a butterfly metapopulation. J Anim Ecol 75:91–100
Harbison ST, Chang S, Kamdar KP, Mackay TFC (2005) Quantitative genomics of starvation stress resistance in Drosophila. Genome Biol 6:R36
Hector KL, Nakagawa S (2012) Quantitative analysis of compensatory and catch-up growth in diverse taxa. J Anim Ecol. doi:10.1111/j.1365-2656.2011.01942.x
Helle H, Koskela E, Mappes T (2012) Life in varying environments: experimental evidence for delayed effects of juvenile environment on adult life history. J Anim Ecol. doi:10.1111/j.1365-2656.2011.01937.x
Hellmann J (2002) The effect of an environmental change on mobile butterfly larvae and the nutritional quality of their hosts. J Anim Ecol 71:925–936
Jimenez-Perez A, Wang Q (2004) Effect of body weight on reproductive performance in Cnephasia jactatana (Lepidoptera: Tortricidae). J Insect Behav 17:511–522
Kankare M, Stefanescu C, Van Nouhuys S, Shaw MR (2005) Host specialization by Cotesia wasps (Hymenoptera: Braconidae) parasitizing species-rich Melitaeini (Lepidoptera: Nymphalidae) communities in north-eastern Spain. Biol J Linn Soc 86:45–65
Krug P, Zimmer R (2000) Developmental dimorphism: consequences for larval behavior and dispersal potential in a marine gastropod. J Exp Biol 203:1741–1754
Kuussaari M, Singer M, Hanski I (2000) Local specialization and landscape-level influence on host use in a herbivorous insect. Ecology 81:2177–2187
Kuussaari M, van Nouhuys S, Hellmann J, Singer M (2004) Larval biology. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford, pp 138–160
Lackie AM (1988) Haemocyte behavior. Adv Insect Physiol 21:85–178
Laurentz M, Reudler JH, Mappes J, Friman V, Ikonen S, Lindstedt C (2012) Diet quality can play a critical role in defence efficacy against parasitoids and pathogens in the Glanville fritillary (Melitaea cinxia). J Chem Ecol 38(1):116–125
Lavine MD, Strand MR (2002) Insect hemocytes and their role in immunity. Insect Biochem Mol Biol 32:1295–1309
Lei GC, Vikberg V, Nieminen M, Kuussaari M (1997) The parasitoid complex attacking the Finnish populations of Glanville fritillary Melitaea cinxia (Lep: Nymphalidae), an endangered butterfly. J Nat Hist 31:635–648
Liefting M, Hoffmann AA, Ellers J (2009) Plasticity versus environmental canalization: population differences in thermal responses along a latitudinal gradient in Drosophila serrata. Evolution 63:1954–1963
Lopez-Maury L, Marguerat S, Bähler J (2008) Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat Rev Genet 9:583–593
Marshall DJ (2008) Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology 89:418–427
Marshall DJ, Uller T (2007) When is a maternal effect adaptive? Oikos 116:1957–1963
McNamara JM, Houston AI (1996) State-dependent life histories. Nature 380:215–221
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Meyers LA, Bull JJ (2002) Fighting change with change: adaptive variation in an uncertain world. Trends Ecol Evol 17:551–557
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond B 363:1635–1645
Monaghan P, Heidinger BJ, D’Alba L, Evans NP, Spencer KA (2011) For better or worse: reduced adult lifespan following early-life stress is transmitted to breeding partners. Proc R Soc Lond B 279:709–714
Moret Y, Schmid-Hempel P (2000) Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168
Mousseau TA, Dingle H (1991) Maternal effects in insect life histories. Annu Rev Entomol 36:511–534
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
O’Brien DM, Boggs CL, Fogel ML (2004) Making eggs from nectar: the role of life history and dietary carbon turnover in butterfly reproductive resource allocation. Oikos 105:279–291
Okech SHO, Overholt WA (1996) Comparative biology of Cotesia chilonis (Hymenoptera: Braconidae) on selected African gramineous stemborers. Biocontrol Sci Technol 6:595–602
Pachauri RK, Reisinger A (eds) (2007) IPCC, Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, Geneva, Switzerland
Pakarinen S (2011) Host-parasitoid relationship in different Cotesia melitaearum and Melitaea cinxia populations around the Baltic Sea. MSc thesis, University of Helsinki, Helsinki
Parsons PA (1993) Evolutionary adaptations and stress: energy budgets and habitats preferred. Behav Genet 23:231–238
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. Johns Hopkins University Press, Baltimore
Rantala MJ, Roff DA (2005) An analysis of trade-offs in immune function, body size and development time in the Mediterranean Field Cricket, Grylluis bimaculatus. Funct Ecol 19:323–330
Räsänen K, Kruuk LEB (2007) Maternal effects and evolution at ecological time-scales. Funct Ecol 21:408–421
Saastamoinen M (2007) Life-history, genotypic, and environmental correlates of clutch size in the Glanville fritillary butterfly. Ecol Entomol 32:235–242
Saastamoinen M, van Nouhuys S, Nieminen M, O’Hara B, Suomi J (2007) Development and survival of a specialist herbivore, Melitaea cinxia, on host plants producing high and low concentrations of iridoid glycosides. Ann Zool Fenn 44:70–80
Saastamoinen M, Ikonen S, Hanski I (2009) Significant effects of Pgi genotype and body reserves on lifespan in the Glanville fritillary butterfly. Proc R Soc Lond B 276:1313–1322
Saastamoinen M, van der Sterren D, Vastenhout N, Zwaan BJ, Brakefield PM (2010) Predictive adaptive responses: condition-dependent impact of adult nutrition and flight in the tropical butterfly Bicyclus anynana. Am Nat 176:686–698
Salinas S, Munch SB (2011) Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol Lett 15:159–163
Schmid-Hempel P (2003) Variation in immune defence as a question of evolutionary ecology. Proc R Soc Lond B 270:357–366
Schmidt O, Theopold U, Strand M (2001) Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. Bioessays 23:344–351
Strand MR (2008) The insect cellular immune response. Insect Sci 15:1–14
van Nouhuys S, Hanski I (2005) Metacommunities of butterflies, their host plants and their parasitoids. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago, pp 99–121
van Nouhuys S, Laine A-L (2008) Population dynamics and sex ratio of a parasitoid altered by fungal-infected diet of host butterfly. Proc R Soc Lond B 275:787–795
van Nouhuys S, Lei GC (2004) Parasitoid-host metapopulation dynamics: the causes and consequences of phenological asynchrony. J Anim Ecol 73:526–535
van Nouhuys S, Punju E (2010) Coexistence of competing parasitoids: which is the fugitive and where does it hide? Oikos 119:61–70
van Nouhuys S, Singer M, Nieminen M (2003) Spatial and temporal patterns of caterpillar performance and the suitability of two host plant species. Ecol Entomol 28:193–202
Vet LEM, Lewis WJ, Papaj DR, van Lenteren JC (1990) A variable response model for parasitoid foraging behavior. J Insect Behav 31:471–490
Vinson SB (1976) Host selection by insect parasitoids. Annu Rev Entomol 21:109–134
Yoder JA, Tank JL, Rellinger EJ (2006) Evidence of a maternal effect that protects against water stress in larvae of the American dog tick, Dermacentor variabilis (Acari: Ixodidae). J Insect Physiol 52:1034–1042
Acknowledgments
We would like to acknowledge Lea Heikkinen, Terhi Lahtinen, Linda Peltola and Suvi Ikonen for their assistance in the experiments. We thank Ilkka Hanski, Klaus Fischer, and two anonymous referees for their useful comments on the manuscript. This research was funded by the Academic Academy of Finland Grants (Nos. 132697 to M.S., and 213547 and 130958 to S.v.N.) and by the European Research Council Advanced Grant (232826 to I. Hanski).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Klaus Fischer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saastamoinen, M., Hirai, N. & van Nouhuys, S. Direct and trans-generational responses to food deprivation during development in the Glanville fritillary butterfly. Oecologia 171, 93–104 (2013). https://doi.org/10.1007/s00442-012-2412-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2412-y