Abstract
After over 30 years of research, it was recently shown that nectar amino acids increase female butterfly fecundity. However, little attention has been paid to the effect of nectar amino acids on male butterfly reproduction. Here, we show that larval food conditions (nitrogen-rich vs. nitrogen-poor host plants) and adult diet quality (nectar with or without amino acids) affected the amount of consumed nectar in Coenonympha pamphilus males. Furthermore, amino acids in the nectar diet of males increased progeny’s larval hatching mass, irrespective of paternal larval reserves. Our study takes the whole reproductive cycle of male butterflies into account, and also considers the role of females in passing male nutrients to offspring, as males’ realized reproduction was examined indirectly via nuptial gifts, by female performance. With this comprehensive approach, we demonstrate for the first time that nectar amino acids can improve male butterfly reproduction, supporting the old postulate that nectar amino acids generally enhance butterfly fitness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Floral nectar is the most common and widespread adult butterfly food source (Gilbert and Singer 1975), and provides sugars, water, minerals, and amino acids for pollinators (Ziegler 1956; Lüttge 1961; Baker and Baker 1986). Furthermore, female Heliconius butterflies can utilize amino acid-rich pollen (Gilbert 1972; Dunlap-Pianka et al. 1977; O’Brien et al. 2003), and fruit-feeding Bicyclus anynana females can use amino acids from their adult diet to increase their reproduction (Bauerfeind and Fischer 2009; but see Bauerfeind and Fischer 2005). However, female butterflies of nectar-feeding species have for long been thought to be unaffected by nectar amino acids (Murphy 1983; Moore and Singer 1987; Hill 1989; Hill and Pierce 1989; O’Brien et al. 2000; Romeis and Wäckers 2002; Mevi-Schütz and Erhardt 2003), although butterfly-pollinated flowers contain higher levels of amino acids than flowers that are pollinated, for instance, by bees (Baker and Baker 1986). Only recently, it has also been shown that nectar amino acids can increase female butterfly reproduction (Mevi-Schütz and Erhardt 2005; Cahenzli and Erhardt 2012a). In contrast, effects of nectar amino acids on male butterfly reproduction are unknown.
The amount of acquired nitrogen is a key factor for fitness and reproduction in insects (Schoonhoven et al. 2006), and nitrogen is primarily acquired during the larval phase (Boggs 1997; O’Brien et al. 2002). However, larval host plants often do not provide optimal amounts of nitrogen (Schoonhoven et al. 2006). To maximize fitness, nutrients are required in optimal levels according to an animal’s stage of development and current environmental circumstances (Simpson and Raubenheimer 1993). Furthermore, allocation patterns of nutrients at different developmental stages are not independent of each other, and the importance of larval reserves declines with increasing quality of adult nutrition (Boggs 2009).
In Lepidoptera, spermatophores of males are an additional nutritional resource for females (Boggs and Gilbert 1979). However, spermatophores of Lepidoptera are a costly physiological product built from limited resources (Oberhauser 1988), and the common assumption that males have an almost unlimited reproductive capacity seems to be obsolete in Lepidoptera (Lewis and Wedell 2007). This suggests that male butterflies have a demand for amino acid-rich food sources in their adult diet (e.g., floral nectar) in order to supplement larval resources for the production of spermatophores. Furthermore, radiotracer studies of several butterfly species demonstrated that amino acids acquired during male larval and adult feeding built into spermatophores can enhance female fitness and reproduction (Boggs and Gilbert 1979; Boggs and Watt 1981; Boggs 1981; Wedell 1996; Wiklund et al. 1993; Karlsson 1998). Thus, male and female resource budgets can be linked via nuptial gifts transferred to females at mating (Boggs 2009). Hence, the role of female butterflies in passing male nutrients to offspring should be included in analyzing male butterfly reproduction. We therefore examined males’ realized reproduction indirectly via nuptial gifts, by female performance, in an analogous way as in a previous study (Cahenzli and Erhardt 2012b). This approach allowed for a more realistic assessment of male contributions to offspring than purely measuring male reproductive traits such as spermatophore mass or nitrogen content, as no relationship was found between ejaculate mass and protein content of ejaculates produced by Pieris rapae and P. brassicae males mating for the first time (Bissoondath and Wiklund 1996), and as spermatophore mass did not influence female reproductive output in several other butterfly species (Jones et al. 1986; Oberhauser 1989; Svärd and Wiklund 1991; Ward and Landolt 1995) and was therefore not a clear indicator for realized fecundity. In addition, amino acids from spermatophores are not necessarily invested in egg production, but can also be incorporated into female somatic tissue (Boggs and Gilbert 1979; Boggs and Watt 1981; Boggs 1981; Wedell 1996; Wiklund et al. 1993). Nevertheless, spermatophore quality can affect the amount of females’ nitrogen put into reproduction (Wedell 1996).
Given the many indications that male butterflies may benefit from additional amino acids in their adult diet due to significant investments in reproduction, the objective of the present study was to investigate effects of nectar amino acids on the reproduction of male butterflies, taking into account male larval reserves and influences of females on passing male nutrients to offspring. We expected that adding amino acids in the adult diet of males enhances male and female fitness, and that this effect is more pronounced in males emerging with little larval reserves.
Materials and methods
Study species
The small heath butterfly, Coenonympha pamphilus L. (Lepidoptera: Satyrinae), occurs in unfertilized to lightly fertilized grasslands (Lepidopterologen-Arbeitsgruppe 1987). Larvae feed on a variety of grasses differing in nutritional quality (Goverde and Erhardt 2003), and varying resources from the larval phase may therefore be common in this species. We considered C. pamphilus as an appropriate study species to measure effects of nitrogen on reproduction for several reasons. First, nitrogen acquired during the whole life cycle can affect reproduction. Second, C. pamphilus females emerge with only 5–13 % mature eggs of their total potential egg number (Goverde et al. 2002; Goverde and Erhardt 2003). Thus, females of this species seem to have a particularly high potential to utilize male-derived nutrients from nuptial gifts for egg production. Third, C. pamphilus females can use nectar amino acids to increase their reproductive success (Cahenzli and Erhardt 2012a). However, C. pamphilus is monandrous (Wickman 1985) and males of polyandrous species invest more in spermatophores (Svärd and Wiklund 1989) and may therefore seem more appropriate to study effects of male-derived nutrients for female fitness. On the other hand, C. pamphilus males with their own territories can copulate as many as four times more than non-resident males (Wickman 1985), therefore incurring non-trivial costs in producing ejaculates (Svärd 1985), and females of most butterfly species mate only once or just one to two times during their lifetime (Ehrlich and Ehrlich 1978; Svärd and Wiklund 1989).
The butterflies used in this experiment originated from 16 C. pamphilus females caught on an unfertilized meadow in the northern Jura mountains (Liesberg BL, Switzerland).
Plant material
Larval food plants Festuca rubra were grown in 750-ml plastic pots filled with untreated calcareous soil from the butterfly’s origin place. Plants were grown in a greenhouse at the University of Basel with supplement sunlight (1,000 W broad spectrum, light period from 0600 to 2000 hours) during cloudy weather conditions and a day/night cycle of 25 °C/19 °C. All pots were watered when necessary. High-quality larval food plants were obtained by fertilizing half the pots once a week with 50 ml Algoflash (N:P:K = 1:1:1; Laboratoire Algochemie Z.I. Nord, Chateau-Renault, France). The low-quality larval food plants received only water. Prior to introducing the larvae, dry leaves (drying by 80 °C for 48 h) were ground for leaf nitrogen (N) analysis using a CHN analyser (LECO instruments, model 1932; St. Joseph, MI, USA).
Larval rearing
To account for possible nitrogen allocation patterns from larval to the adult stage, and to test whether larval reserves influence potential effects of nectar amino acids on male reproduction, the nitrogen level in larval food was controlled by rearing larvae on high-quality or low-quality host plants. Male larvae from the 16 ovipositing females were randomly assigned to either the high- or low-quality larval host plants, and were reared separately in order to later trace back each butterfly to the ovipositing female (lineage). After 2 weeks, larvae were separated and kept individually in Petri dishes. We reared unsexed larvae on high- and low-quality host plants and used only females raised on low-quality larval food to minimize effects of female nutritional conditions. Females raised on high-quality plants were released. The larvae continued to receive their assigned larval food quality diet. The high-quality larval food group received an abundant supply of fertilized F. rubra ad libitum, whereas the low-quality larval food group was reared on unfertilized host plants. To prevent compensatory feeding of the low-quality larval group, last instar larvae received a limited quantity of unfertilized host plants (ca. 50 % of the amount of the high-quality larvae). Measuring the amount and quality of available food in the low-quality feeding treatment is necessary to avoid overestimating effects of a low nitrogen level in host plants (Carvalho et al. 2005).
Butterfly diet
Males were kept in plastic boxes (0.6 l), whereas females were placed in individual nylon mesh cages (20 cm × 20 cm × 40 cm). Male butterflies from the high- and low-quality larval food groups were randomly assigned to a nectar diet treatment consisting either of a nectar mimic with amino acids (AA), or without amino acids (NAA). Females were fed with nectar without amino acids to minimize effects of female nutritional conditions. Male butterflies were fed three times before they were allowed to mate once with an unrelated female. All butterflies were fed by hand (Cahenzli and Erhardt 2012a, b). Four treatment groups resulted: high-quality larval food and adult diet with amino acids (high/AA, n = 14), high-quality larval food and adult diet without amino acids (high/NAA, n = 19), low-quality larval food and adult diet with amino acids (low/AA, n = 17) and low-quality larval food and adult diet without amino acids (low/NAA, n = 22).
A nectar mimic of the plant Lantana camara was used in this experiment. Although this plant does not naturally occur in the habitat of C. pamphilus, it was used in several former studies (Alm et al. 1990; Mevi-Schütz et al. 2003; Mevi-Schütz and Erhardt 2003, 2005; Cahenzli and Erhardt 2012a). The nectar mimic of the group fed without amino acids contained only sucrose, glucose, and fructose, whereas the diet of the amino acid-fed group corresponded to the complete nutrient spectrum of L. camara nectar, additionally containing nonessential and essential amino acids (for exact composition, see Alm et al. 1990).
Preliminary experiments showed that C. pamphilus butterflies rejected a daily feeding (Cahenzli and Erhardt 2012a, b). We therefore fed the butterflies their respective nectar diet every second day and allowed them to consume nectar until they voluntarily left the feeding station. The butterflies did not recognize the artificial feeding station as a natural nectar source. We therefore placed the butterflies beside the nectar-filled tube and dipped the rolled out proboscis with the help of a needle into the nectar mimic to initiate feeding. To measure the amount of nectar consumed we used a 100-μl Hamilton syringe.
Reproductive parameters
Butterflies were weighed within 24 h after emerging, and the longevity of each butterfly was recorded (number of days from hatching out of the pupa to death). We counted and collected all eggs of every single female every day. Eggs were placed in Petri dishes covered with nylon mesh until the larvae hatched.
Progeny’s egg duration (number of days from when the egg was laid to eclosion), hatching success of eggs (number of eggs hatched per butterfly), and larval hatching mass of offspring (mg) were recorded for all eggs collected from each butterfly. Freshly hatched larvae were weighed within 24 h.
Statistical analysis
Males’ larval development and reproductive traits were analyzed with mixed-effects models (Table 1) (Crawley 2007). Males’ larval development was tested against the categorical variable larval food quality (low, high) and the factor lineage. Larval duration was log n transformed.
The reproductive traits were tested against the categorical variables larval food quality (low, high) and nectar amino acid diet (AA/NAA), the continuous covariates male and female emergence mass and the average amount of nectar consumed per feeding by male butterflies, and the factor lineage (Table 1). Male longevity, egg hatching success and egg duration were analyzed with generalized linear mixed-effects models due to non-normal data structure (Crawley 2007). The average amount of nectar mimic consumed was tested against the categorical variables larval food quality and nectar diet and the continuous covariate male emergence mass. To test if males fed with amino acid-rich nectar consumed more nectar than males fed nectar lacking amino acids, irrespective of male emergence mass, the continuous variable ‘amount of consumed nectar’ was divided by male emergence mass and analyzed with a two-sided t test. Furthermore, a mixed-effects model with temporal pseudoreplication (repeated measures on the same females) was used to test if larval hatching mass changed over female oviposition period (Crawley 2007). The model used day of oviposition and individual and males’ larval and adult diet as factors.
There was no significant difference in average amount of nectar mimics consumed among females paired with males from different treatment groups (F 1,52 = 1.03, P = 0.4). Therefore, the continuous variable females’ amount of consumed nectar was not incorporated in the analyses of reproductive parameters. A stepwise model reduction was employed, with the least significant interaction always removed first (Crawley 2007). Two-sided t tests were performed between treatment groups.
Correlation analysis was used to examine if female emergence mass affected total number of eggs laid and longevity positively or negatively, and to characterize the relationship between longevity and the average amount of consumed nectar. We also used correlation analysis to detect a possible trade-off between the total number of eggs laid and progeny’s larval hatching mass. All statistical analyses were calculated with R Statistical Software (v.2.9.1; R Development Core Team 2009).
Results
Male larval development
Fertilized larval host plants (6.80 ± 0.07 g N/100 g dry weight) had a significantly higher nitrogen content than unfertilized plants (5.35 ± 0.18 g N/100 g dry weight, t 19.94 = 7.59, P < 0.001). Larval host plant quality significantly affected larval duration of males (F 1,55 = 96.66, P < 0.001), whereas lineage had no significant effect (F 1,13 = 0.03, P = 0.9). Males reared on high-quality host plants had a significantly shorter larval duration (27.12 ± 3.31 days) than males reared on low-quality host plants (37.51 ± 5.74 days, t = 9.62, n = 72, P < 0.001).
Male emergence mass was significantly affected by larval diet quality (F 1,55 = 27.72, P < 0.001), but lineage had no significant effect (F 1,13 = 0.001, P > 0.99). Males reared on high-quality host plants had a significantly higher emergence mass (25.66 ± 5.16 mg) than males reared on low-quality host plants (20.10 ± 4.28 mg, t = 5.51, n = 72, P < 0.001).
Male reproduction
Total number of eggs laid was significantly affected by female emergence mass, whereas males’ nectar quality, males’ larval food quality, male emergence mass, the average amount of males’ consumed nectar and lineage had no significant effect (Tables 1, 2). Heavier females laid more eggs than lighter females (r = 0.30, n = 72, P = 0.02).
Progeny’s egg duration was not significantly affected by males’ nectar diet quality, male emergence mass, males’ average amount of nectar mimic consumed, female emergence mass or lineage, whereas males’ larval food quality had a significant effect (Tables 1, 2). Egg duration of progeny descending from males that had been reared on high-quality host plants (6.25 ± 0.07 days) was significantly longer than from males that had been reared on low-quality host plants (6.02 ± 0.05 days, t = 2.89, n = 72, P = 0.005).
Hatching success of eggs was not significantly influenced by males’ larval food quality, male emergence mass, males’ average amount of nectar mimic consumed, or female emergence mass or lineage, whereas males’ nectar diet quality had a marginal effect (Tables 1, 2). However, there was no significant difference between hatching success of eggs of females mated to males fed with nectar containing (0.88 ± 0.03) or lacking amino acids (0.91 ± 0.02, t = 0.94, n = 72, P = 0.35).
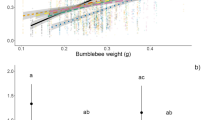
Nectar diet quality of males had a significant effect on progeny’s larval hatching mass, whereas males’ larval food quality, male emergence mass, the males’ average amount of consumed nectar, female emergence mass and lineage had no significant effect (Tables 1, 2). Females mated to males fed with amino acid-rich nectar produced marginally heavier larvae (0.190 ± 0.004 mg) than females mated to males fed with nectar lacking amino acids (0.181 ± 0.003 mg, t = 1.84, n = 72, P = 0.07). Mixed-model analysis with temporal pseudoreplication showed a significant effect of day of oviposition on the time pattern of progeny’s larval hatching mass (t = 15.73, n = 72, P < 0.001), whereas adult diet had a marginal effect (t = 1.93, n = 72, P = 0.058). Individual (t = 0.40, n = 72, P = 0.69) and larval diet (t = 0.68, n = 72, P = 0.50) had no significant effect. During the first 6 days of the female oviposition period, larvae descending from males fed with amino acid-rich nectar (AA: 0.195 ± 0.004 mg) were significantly heavier than larvae descending from males fed with nectar lacking amino acids (NAA: 0.186 ± 0.003 mg, t = 2.01, n = 72, P = 0.049), whereas there was no difference after day 6 (AA: 0.175 ± 0.004 mg, NAA: 0.174 ± 0.004 mg, t = 0.12, n = 72, P = 0.91, Fig. 1). There was no trade-off between number of eggs and progeny’s larval hatching mass in the offspring of males fed high-quality larval food (r = 0.11, n = 33, P = 0.55), whereas there was a marginal trade-off in the offspring of males that had been reared on low-quality host plants, since females that produced lighter larvae tended to lay more eggs (r = 0.57, n = 39, P = 0.06).
Mixed-model analysis with temporal pseudoreplication showed a significant effect of day of oviposition on time patterns of progeny’s larval hatching mass in Coenonympha pamphilus (t = 15.73, n = 72, P < 0.001). During the first 6 days of female oviposition period, larvae descending from males fed with amino acid-rich nectar were significantly heavier than larvae descending from males fed with nectar lacking amino acids (t = 2.01, n = 72, P = 0.049), whereas there was no difference after day 6 (t = 0.12, n = 72, P = 0.91)
Males’ nectar consumption was not significantly affected by male emergence mass and lineage. Males’ larval food quality and nectar diet quality had a significant effect on nectar consumption (Tables 1, 2). Males that had been reared on high-quality larval host plants (6.55 ± 0.23 μl) consumed significantly more nectar per feeding than males that had been reared on low-quality host plants (4.73 ± 0.11 μl, t = 7.74, n = 72, P < 0.001), and males consumed significantly more amino acid-rich nectar (0.28 ± 0.01 μl nectar/mg body mass) than nectar lacking amino acids (0.24 ± 0.01 μl nectar/mg body mass, t = 2.54, n = 72, P = 0.014).
Longevity of male butterflies was not significantly affected by nectar diet quality, males’ larval food quality, emergence mass, or lineage, whereas the average amount of consumed nectar mimic had a marginal effect (Tables 1, 2). However, there was no correlation between average amount of consumed nectar mimic and longevity (r = −0.16, n = 72, P = 0.30).
Longevity of female butterflies was influenced by female emergence mass (F 1,52 = 6.22, P = 0.017); emergence mass and longevity were positively correlated (r = 0.37, n = 72, P = 0.003), since heavier females lived longer than lighter females. Males’ adult nectar diet quality (F 1,52 = 2.07, P = 0.16), male emergence mass (F 1,52 = 2.40, P = 0.13), the males’ average amount of nectar mimic consumed (F 1,52 < 0.01, P = 0.95), and lineage (F 1,52 = 1.32, P = 0.27) had no significant effect. Males’ larval food quality had a marginal effect on female longevity (F 1,52 = 2.99, P = 0.09); females mated with males that had been reared on high-quality larval host plants tended to live longer than females mated with males that had been reared on low-quality larval host plants.
Discussion
Effects of larval food conditions on male butterfly reproduction
In this experiment, larval food quality significantly affected male emergence mass, but had no significant effects on the total number of eggs laid, progeny’s larval hatching mass, and hatching success of eggs (Table 1), suggesting that resources acquired during the larval phase play only a limited role for male reproduction in C. pamphilus. In contrast, initial spermatophore mass was related to male emergence mass in other butterfly species (Oberhauser 1988; Bissoondath and Wiklund 1996; but see Svärd 1985). However, larval food conditions significantly affected males’ larval duration and emergence mass, and by hatching earlier and with a larger body size, the probability of occupying a territory increases, resulting in higher mating success, since larger males in territories mate significantly more often than males without a territory (Wickman 1985). Thus, larval food conditions may still have fitness-relevant effects.
Males’ larval food quality significantly affected nectar consumption (Table 1), and heavier males consumed more nectar than lighter males. Bigger males can ingest more food per feeding, and a bigger body size requires more energy for somatic maintenance. But larval food quality of males also affected their nectar consumption independently from emergence mass, indicating that larval and adult feeding in holometabolous insects are interconnected (Boggs 1997).
We did not find a trade-off between the number of eggs and progeny’s larval hatching mass in females mated with males fed high-quality larval food, whereas a marginal trade-off appeared in females mated with males that had been reared on low-quality larval host plants, since females that laid more eggs tended to produce lighter larvae, indicating a lower spermatophore quality or a reduced nutrient quantity in spermatophores secreted by males that had been reared on low-quality larval host plants. Resource allocation differs under unconstrained, benign conditions and stressful, resource-poor environments (Boggs 2009).
Effects of nectar amino acids on male butterfly reproduction
Male reproductive success is influenced not only by larval resources but also by adult food conditions (Cahenzli and Erhardt 2012b). In our study, hatching mass of larvae descending from males fed with amino acid-rich nectar was increased during the first 6 days of female oviposition period. It is likely that males used nectar amino acids to enhance spermatophore quality, and that females reacted to better spermatophore quality by increasing larval hatching mass. However, larval hatching mass did not differ anymore between treatment groups (AA vs. NAA) after day 6 of the female oviposition period, suggesting that females had depleted potential male resources (Fig. 1). Previous studies showed that female butterflies can incorporate male-derived nutrients almost immediately into eggs (Boggs and Gilbert 1979; Boggs and Watt 1981; Boggs 1997). However, C. pamphilus males transfer relatively small ejaculates, corresponding to a mere 1.5 % of male body mass (Svärd and Wiklund 1989). The present findings are all the more relevant because they show that nectar-derived nutrients can improve male reproductive success even in a species where males deliver small nuptial gifts, documenting a nutritional pathway likely also present in other butterfly species producing bigger spermatophores than C. pamphilus and potentially relying more on nuptial gifts.
Several previous studies with other butterfly species showed benefits of increased egg and larval size (Murphy et al. 1983; Braby 1994; Fischer et al. 2003, 2006; Seko and Nakasuji 2004; but see Wiklund and Persson 1983; Wiklund and Karlsson 1984). C. pamphilus females like other Satyrid butterflies often lay their eggs on unfavorable plant material, and freshly hatched larvae must find better host plants (Wiklund 1984). In general, larger hatchling larvae can travel longer distances, thereby increasing the likelihood to find appropriate larval food plants (Murphy et al. 1983). Thus, floral nectar amino acids in butterfly diet may increase the reproductive success of males by inducing heavier larval hatching mass of offspring.
In a previous study, Lederhouse et al. (1990) found that a combination of electrolytes and amino acids enabled males of Papilio glaucus to produce more offspring than control males. However, actual effects of amino acids on offspring were not tested in that study. Furthermore, Beck (2007) found that males of some tropical butterfly species fed an amino acid-rich sucrose solution lived significantly longer. However, the amino acid concentration used in that study was at an unnatural high level, and the amount of consumed nectar was not measured.
In nectar preference tests carried out so far, C. pamphilus males did not discriminate between nectar containing or lacking amino acids (Mevi-Schütz et al. 2003). In the present study, nectar quality significantly affected nectar consumption (Table 1), and males fed amino acid-rich nectar consumed significantly more nectar than males fed with nectar without amino acids, showing a latent preference in males for amino acids in their adult diet. It is likely that the increased nectar consumption was caused by a tendency to achieve the optimal quantity of nitrogen for maximizing fitness (Simpson and Raubenheimer 1993). However, males increasing their amount of consumed amino acid-rich nectar also ingested more carbohydrates, and female butterflies can also use nitrogen from other sources to synthesize non-essential amino acids with nectar carbohydrates (O’Brien et al. 2002).
As in a previous study (Cahenzli and Erhardt 2012b), the number of eggs produced was not significantly affected by any of the measured male reproductive parameters (Table 1). C. pamphilus females obviously used male-derived nutrients primarily to increase progeny’s larval hatching mass rather than for increasing offspring number (Table 2; Cahenzli and Erhardt 2012b). In contrast, female larval reserves clearly affected egg number (Table 1; Cahenzli and Erhardt 2012a, b). Thus, nutrients acquired in the larval stage are also used as reproductive endowment for oocytes, as also, e.g., in Heliconius charitonius (Dunlap-Pianka 1979).
Our study shows for the first time that nectar amino acid uptake during the adult phase has fitness relevant effects for male reproduction, and that adult feeding does not only cover energy requirements for general maintenance, including flight expenditure (Willers et al. 1987). Thus, the results of the present study support previous findings suggesting a co-evolutionary pollination process between butterflies and flowers.
References
Alm J, Ohnmeiss TE, Lanza J, Vriesenga L (1990) Preference of cabbage white butterflies and honey bees for nectar that contains amino acids. Oecologia 84:53–57
Baker HG, Baker I (1986) The occurrence and significance of amino acids in floral nectar. Plant Syst Evol 151:175–186
Bauerfeind SS, Fischer K (2005) Effects of food stress and density in different life stages on reproduction in a butterfly. Oikos 111:514–524
Bauerfeind SS, Fischer K (2009) Effects of larval starvation and adult diet derived amino acids on reproduction in a fruit-feeding butterfly. Entomol Exp Appl 130:229–237. doi:10.1111/j.1570-7458.2008.00814.x
Beck J (2007) The importance of amino acids in the adult diet of male tropical rainforest butterflies. Oecologia 151:741–747. doi:10.1007/s00442-006-0613-y
Bissoondath CJ, Wiklund C (1996) Male butterfly investment in successive ejaculates in relation to mating system. Behav Ecol Sociobiol 39:285–292. doi:10.1007/s002650050291
Boggs CL (1981) Nutritional and life-history determinants of resource allocation in holometabolous insects. Am Nat 117:692–709
Boggs CL (1997) Dynamics of reproductive allocation from juvenile and adult feeding: radiotracer studies. Ecology 78:192–202
Boggs CL (2009) Understanding insect life histories and senescence through a resource allocation lens. Funct Ecol 23:27–37. doi:10.1111/j.1365-2435.2008.01527.x
Boggs CL, Gilbert LE (1979) Male contribution to egg production in butterflies: evidence for transfer of nutrients at mating. Science 206:83–84
Boggs CL, Watt WB (1981) Population structure of Pierid butterflies. IV. Genetic and physiological investment in offspring by male Colias. Oecologia 50:320–324. doi:10.1007/BF00344970
Braby MF (1994) The significance of egg size variation in butterflies in relation to hostplant quality. Oikos 71:119–129
Cahenzli F, Erhardt A (2012a) Enhancing offspring quality or quantity? Different ways for using nectar amino acids in female butterflies. Oecologia. doi:10.1007/s00442-012-2254-7
Cahenzli F, Erhardt A (2012b) Nectar sugars enhance fitness in male Coenonympha pamphilus butterflies by increasing longevity or realized reproduction. Oikos. doi:10.1111/j.1600-0706.2012.20190.x
Carvalho GB, Kapahi P, Benzer S (2005) Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods 2:813–815. doi:10.1038/nmeth798
Crawley MJ (2007) The R book. Wiley, West Sussex
Dunlap-Pianka HL (1979) Ovarian dynamics in Heliconius butterflies: correlations among daily oviposition rates, egg weights, and quantitative aspects of oogenesis. J Insect Physiol 25:741–749. doi:10.1016/0022-1910(79)90126-4
Dunlap-Pianka H, Boggs CL, Gilbert LE (1977) Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science 197:487–490. doi:10.1126/science.197.4302.487
Ehrlich AH, Ehrlich PR (1978) Reproductive strategies in the butterflies: I. Mating frequency, plugging and egg number. J Kansas Entomol Soc 51:666–697
Fischer K, Bot ANM, Brakefield PM, Zwaan BJ (2003) Fitness consequences of temperature-mediated egg size plasticity in a butterfly. Funct Ecol 17:803–810
Fischer K, Bot ANM, Brakefield PM, Zwaan BJ (2006) Do mothers producing large offspring have to sacrifice fecundity? J Evol Biol 19:380–391. doi:10.1111/j.14209101.2005.01046.x
Gilbert LE (1972) Pollen feeding and reproductive biology of Heliconius butterflies. Proc Natl Acad Sci USA 69:1403–1407. doi:10.1073/pnas.69.6.1403
Gilbert LE, Singer MC (1975) Butterfly ecology. Annu Rev Ecol Evol S 6:365–397. doi:10.1146/annurev.es.06.110175.002053
Goverde M, Erhardt A, Niklaus PA (2002) In situ development of a satyrid butterfly on calcareous grassland exposed to elevated carbon dioxide. Ecology 83:1399–1411. doi:10.2307/3071952
Goverde M, Erhardt A (2003) Effects of elevated CO2 on development and larval food-plant preference in the butterfly Coenonympha pamphilus (Lepidoptera, Satyridae). Glob Change Biol 9:74–83. doi:10.1007/s00265-003-0601-8
Hill CJ (1989) The effect of adult diet on the biology of butterflies. II. The common crow butterfly, Euploea core corinna. Oecologia 81:258–266
Hill CJ, Pierce NE (1989) The effect of adult diet on the biology of butterflies: the common imperial blue, Jalmenus evagoros. Oecologia 81:249–257
Jones KN, Odendall FJ, Ehrlich PR (1986) Evidence against the spermatophore as paternal investment in Checkerspot Butterflies (Euphydryas: Nymphalidae). Am Midl Nat 116:1–6. doi:10.2307/2425932
Karlsson B (1998) Nuptial gifts, resource budgets, and reproductive output in a polyandrous butterfly. Ecology 79:2931–2940
Lederhouse RC, Ayres MP, Scriber JM (1990) Adult nutrition affects male virility in Papilio glaucus L. Funct Ecol 4:743–751. doi:10.2307/2389441
Lepidopterologen-Arbeitsgruppe (1987) Tagfalter und ihre lebensräume, Band 1. Schweizerischer Bund für Naturschutz, Switzerland
Lewis Z, Wedell N (2007) Effect of adult feeding on male mating behaviour in the butterfly Bicyclus anynana (Lepidoptera: Nymphalidae). J Insect Behav 20:201–213. doi:10.1007/s10905-007-9075-2
Lüttge U (1961) Über die zusammensetzung des nektars und den mechanismus seiner sekretion. I. Planta 56:189–212
Mevi-Schütz J, Erhardt A (2003) EVects of nectar amino acids on fecundity of the wall brown butterfly (Lasiomata megera L.). Basic Appl Ecol 4:413–421
Mevi-Schütz J, Erhardt A (2005) Amino acids in nectar enhance butterfly fecundity: a long awaited link. Am Nat 165:411–419
Mevi-Schütz J, Goverde M, Erhardt A (2003) Effects of fertilization and elevated CO2 on larval food and butterfly nectar amino acid preference in Coenonympha pamphilus L. Behav Ecol Sociobiol 54:36–43. doi:10.1007/s00265-003-0601-8
Moore RA, Singer MC (1987) Effects of maternal age and adult diet on egg weight in the butterfly Euphydryas editha. Ecol Entomol 12:401–408
Murphy DD (1983) Nectar sources as constraints on the distribution of egg masses by the checkerspot butterfly, Euphydryas chalcedona (Lepidoptera: Nymphalidae). Environ Entomol 12:463–466
Murphy DD, Launer AE, Ehrlich PR (1983) The role of adult feeding in egg production and population dynamics of the checkerspot butterfly Euphydryas editha. Oecologia 56:257–263
Oberhauser KS (1988) Male monarch butterfly spermatophore mass and mating strategies. Anim Behav 36:1384–1388. doi:10.1016/S0003-3472(88)80208-2
Oberhauser KS (1989) Effects of spermatophores on male and female monarch butterfly reproductive success. Behav Ecol Sociobiol 25:237–246. doi:10.1007/BF00300049
O’Brien DM, Boggs CL, Fogel ML (2002) Renewable and non-renewable resources: amino acid turnover and allocation to reproduction in Lepidoptera. Proc Natl Acad Sci USA 99:4413–4418
O’Brien DM, Boggs CL, Fogel ML (2003) Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proc R Soc Lond B 270:2631–2636. doi:10.1098/rspb.2003.2552
O’Brien DM, Schrag DP, Martínez del Rio C (2000) Allocation to reproduction in a hawkmoth: a quantitative analysis using stable carbon isotopes. Ecology 81:2822–2831. doi:10.1890/0012-9658(2000)081[2822:ATRIAH]2.0.CO;2
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Romeis J, Wäckers FL (2002) Nutritional suitability of individual carbohydrates and amino acids for adult Pieris brassicae. Physiol Entomol 27:148–156. doi:10.1046/j.1365-3032.2002.00281.x
Schoonhoven LM, Van Loon JJA, Dicke M (2006) Insect-plant biology, 2nd edn. Oxford University Press, Oxford
Seko T, Nakasuji F (2004) Effect of egg size variation on survival rate, development and fecundity of offspring in a migrant skipper, Parnara guttata guttata (Lepidoptera: Hesperiidae). Appl Entomol Zool 39:171–176
Simpson SJ, Raubenheimer ED (1993) A multi-level analysis of feeding behaviour: the geometry of nutritional decisions. Philos Trans R Soc Lond B 342:381–402. doi:10.1098/rstb.1993.0166
Svärd L (1985) Paternal investment in a monandrous butterfly Pararge aegeria. Oikos 45:66–70. doi:10.2307/3565223
Svärd L, Wiklund C (1989) Mass and production-rate of ejaculates in relation to monandry polyandry in butterflies. Behav Ecol Sociobiol 24:395–402. doi:10.1007/BF00293267
Svärd L, Wiklund C (1991) The effects of ejaculate mass on female reproductive output in the European swallowtail butterfly, Papilio machaon (L.) (Lepidoptera: Papilionidae). J Insect Behav 4:33–42. doi:10.1007/BF01092549
Ward KE, Landolt PJ (1995) Influence of multiple matings on fecundity and longevity of female cabbage looper moths (Lepidoptera: Noctuidae). Ann Entomol Soc Am 88:768–772
Wedell N (1996) Mate quality affects reproductive effort in a paternally investing species. Am Nat 148:1075–1088. doi:10.1086/285972
Wickman PO (1985) Territorial defense and mating success in males of the small heath butterfly Coenonympha pamphilus L. Anim Behav 33:1162–1168. doi:10.1016/S0003-3472(85)80176-7
Wiklund C (1984) Egg-laying patterns in butterflies in relation to their phenology and the visual apparency and abundance of their host plants. Oecologia 63:23–29
Wiklund C, Karlsson B (1984) Egg size variation in satyrid butterflies: adaptive vs. historical, “Bauplan”, and mechanistic explanations. Oikos 43:391–400
Wiklund C, Persson A (1983) Fecundity, and the relation of egg weight to offspring fitness in the speckled wood butterfly Pararge aegeria, or why don’t butterfly females lay more eggs? Oikos 40:53–63
Wiklund C, Kaitala A, Lindfors V, Abenius J (1993) Polyandry and its effect on female reproduction in the green-veined white butterfly (Pieris napi L.). Behav Ecol Sociobiol 33:25–33
Willers JL, Schneider JC, Ramaswamy SB (1987) Fecundity, longevity and caloric patterns in female Heliothis virescens: changes with age due to flight and supplemental carbohydrate. J Insect Physiol 33:803–808. doi:10.1016/0022-1910(87)90027-8
Ziegler H (1956) Untersuchungen über die leitung und sekretion der assimilate. Planta 47:447–500
Acknowledgments
We thank J. Mevi-Schütz for reviewing the manuscript, D. Aydin for his valuable comments, R. Brandl, K. Fischer and two anonymous referees for their helpful reviews of the manuscript, V. and R. Cahenzli, Freiwillige Akademische Gesellschaft Basel, Basler Stiftung für biologische Forschung and Emilia Guggenheim-Schnurr Stiftung for their financial support, and C. Körner for use of the greenhouse. This work was supported by the Fonds zur Förderung des akademischen Nachwuchses der Universität Basel (Project 65051 to A. Erhardt).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Klaus Fischer.
Rights and permissions
About this article
Cite this article
Cahenzli, F., Erhardt, A. Nectar amino acids enhance reproduction in male butterflies. Oecologia 171, 197–205 (2013). https://doi.org/10.1007/s00442-012-2395-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2395-8