Abstract
While seasonal redistribution of fine root biomass in response to fluctuations in groundwater level is often inferred in phreatophytic plants, few studies have observed the in situ growth dynamics of deep roots relative to those near the surface. We investigated the root growth dynamics of two Banksia species accessing a seasonally dynamic water table and hypothesized that root growth phenology varied with depth, i.e. root growth closest to the water table would be influenced by water table dynamics rather than surface micro-climate. Root in-growth bags were used to observe the dynamics of root growth at different soil depths and above-ground growth was also assessed to identify whole-plant growth phenology. Root growth at shallow depths was found to be in synchrony with above-ground growth phenophases, following increases in ambient temperature and soil water content. In contrast, root growth at depth was either constant or suppressed by saturation. Root growth above the water table and within the capillary fringe occurred in all seasons, corresponding with consistent water availability and aerobic conditions. However, at the water table, a seasonal cycle of root elongation with drawdown in summer followed by trimming in response to water table rise and saturation in winter, was observed. The ability to grow roots year-round at the capillary fringe and redistribute fine root biomass in response to groundwater drawdown is considered critical in allowing phreatophytes, in seasonally water-limited environments, to maintain access to groundwater throughout the year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Knowing when roots grow and die is critical if we are to understand whole plant performance in water limited environments. Root growth is controlled by endogenous constraints on carbon availability (Joslin et al. 2001; Palacio and Montserrat-Martí 2007) and environmental factors such as changes in soil temperature and water availability (Teskey and Hinckley 1981). Environmental factors that affect root growth show considerable variability at the soil surface as opposed to the very consistent conditions found at depth (Voroney 2007). A root system that extends to deeper soil horizons can be beneficial to plants in seasonally water-limited environments, such as that experienced in Mediterranean-type climates with hot, dry summers (Dodd et al. 1984; Richards et al. 1995; Groom 2004). In addition, a deep rooting habit increases the likelihood of plants being phreatophytic (using groundwater; Meinzer 1923). Phreatophytic plants in water-limited environments can be identified by the presence of evergreen foliage, maintenance of high predawn leaf water potentials and high transpiration and leaf growth rates despite water deficits in the vadose zone (unsaturated soil horizons) during the dry season or prolonged drought (Poole and Miller 1975; Murray et al. 2003).
By definition, phreatophytes maintain a connection to the water table and capillary fringe (zone immediately above the water table where capillary rise of groundwater occurs) to avoid severe water deficit and eventual plant death (Scott et al. 1999; Sperry and Hacke 2002; Cooper et al. 2003). Water tables of unconfined aquifers are, however, often dynamic, fluctuating in response to the seasonal changes in the balance between recharge and discharge (Freeze and Cherry 1979; Castelli et al. 2000). As water tables fall over summer they leave behind an aerated zone high in plant available soil moisture, which can be conducive to root growth (Imada et al. 2008). In addition, summer groundwater uptake by phreatophytic plants (Zencich et al. 2002) implies that plant roots follow the receding water table, maintaining ‘connection’ throughout the year. A rising water table saturates part of the rooting zone that was previously occupying the unsaturated capillary fringe, therefore, restricting root growth (Kramer and Boyer 1995) due to anaerobic, water saturated conditions (Kozlowski 1997). Consequently, roots of phreatophytic plants are likely to undergo a seasonal cycle of root elongation and trimming in response to fluctuating water tables. However, the dynamics of root growth in the zone closest to the water table have rarely been observed, particularly for deep-rooted phreatophytes (Naumburg et al. 2005).
The spatial distribution of root growth and function is often implied through indirect means. For example, water source partitioning studies on phreatophytes using the natural abundance of water isotopes have implied root activity in the vicinity of the water table (Busch et al. 1992; Zencich et al. 2002). Chemical tracers have also been used to the same effect and have inferred water uptake, thus root activity, from as deep as 76 m (Lubczynski 2009). While these are useful indicators of root growth at depth, there remains a paucity of data on root growth phenology at the water table interface, and the seasonal cues, if any, controlling root growth at depth are also poorly studied. Mini-rhizotrons are increasingly being used to observe root turnover and seasonality (Eamus et al. 2006); however, such techniques are limited to the top 1–2 m of soil (Hendrick and Pregitzer 1997; Steinaker et al. 2010) and therefore have limited applicability to deep-rooted phreatophytes. Alternative approaches, such as root in-growth bags have been shown to be effective in repeated observations of root activity (Böhm 1979).
Measurement of in situ root growth at different soil depths is required to advance our current limited understanding of mature phreatophyte responses to seasonal change in water table depth. To observe the seasonality of root growth at different soil depths, particularly at the root-water table interface, we used root in-growth bags to investigate the dynamics of phreatophytic Banksia root growth on the Swan Coastal Plain in southwest Australia. We hypothesized that deep root growth (closest to the water table) is less responsive to seasonal changes in soil micro-climatic conditions than shallow root growth. Secondly, we hypothesised that the greatest influence on deep root growth would be water table dynamics, with more root activity (mass of in-growth) seen as the water table recedes in summer and less activity when the water table rises and saturates the roots in winter. To identify whole-plant synchronicity in growth phenology, we also assessed above-ground growth phenophases over the same period.
Methods
Study site and species selection
Observations of above- and below-ground phenology were made at a site located in Whiteman Park, a 2,600 ha conservation reserve approximately 20 km northeast of Perth, Western Australia (31°48′S, 115°56′E). The climate is warm Mediterranean, with mild, wet winters and hot, dry summers (Gentilli 1972). The average rainfall for Perth is 778 mm (average for years 1944–2010; Bureau of Meteorology 2010) and 85 % of rainfall occurs between May and October. The period between November and April experiences little precipitation, in combination with high evaporation and warm temperatures. Because of the warm Mediterranean-type climate and low water holding capacity of the sandy soils of the region, there is a strong seasonal pattern in soil water availability. Water in the vadose zone is generally unavailable to plants during the summer months, with many species reliant on access to deep soil moisture stores or groundwater (Zencich et al. 2002). The Gnangara groundwater mound underlies the park, supporting a number of groundwater dependent ecosystems including large areas of phreatophytic Banksia woodland. To make root sampling logistically possible, an area with a depth to water table ranging from 3 m in spring to 3.75 m in autumn was selected. The soil profile was predominately medium to coarse sand to depths well below the water table. Dominant tree species at the site were Banksia attenuata and B. ilicifolia, although B. menziesii, Adenanthos cygnorum, Allocasuarina fraseriana and Nuytsia floribunda were also present. The two study species, B. attenuata and B. ilicifolia were selected on the basis they were the dominant overstorey species at the site and were known to be phreatophytic (Zencich et al. 2002; Canham et al. 2009).
Hydrological variables
Meteorological data were recorded at a weather station (EnviroStation™, ICT International, Armidale, NSW, Australia) situated 2 km from the study site. Rainfall and ambient temperature were logged at half hourly intervals for the duration of the study period. Soil volumetric water content was determined at the study site every 6 weeks using a neutron moisture meter (Didcot Instrument Co., Abingdon, Oxon, UK). Neutron counts (measured at 0.25 m depth intervals to water table, at 3 replicate measurements per interval) were converted to volumetric water content according to methods outlined by Greacen (1981) using calibration curves for the soil type present at the site. Groundwater depth was recorded daily at the study site using a monitoring bore and groundwater logger (miniTROLL, In Situ Inc., Fort Collins, Colorado, USA) for 12 months prior to and during the study.
Root growth
Ten individuals of mature (reproductive age and minimum basal diameter of 10 cm) B. attenuata and B. ilicifolia were selected at the study site for investigation of below ground root growth phenology. The average distance between the study trees was 8 m. Root in-growth bags were 90 mm in diameter and 30 cm long, and constructed from aluminum flywire with a mesh size of 1 mm. Bags were filled with a 1:5 mixture of peat and clean, white sand and wetted to field capacity (<0.07 cm3 cm−3 volumetric water content) prior to installation to attract root in-growth. Each root bag, therefore, contained a maximum of 133 ml of water at the time of installation which is equivalent to 0.38–0.74 % (per bag) of mean daily water uptake, depending on season and species (Dodd and Bell 1993; R. Froend, unpublished data). A nylon cord was attached to each bag to allow retrieval. Bags were placed at four different depths around each plant with the depth of root bag placement being based on observations of depth to groundwater during the previous year. Groundwater depth was greatest in April (3.7 m, Autumn) and shallowest in October (3 m, Spring). To determine root growth at the water table and capillary fringe, root bags were placed at 3.5–3.7 and 2.7–2.9 m. This meant that root bags placed at 3.5–3.7 m would be at the capillary fringe in April and then as the water table rose to its highest level in October, the bags would be submerged. The 2.7–2.9 m bags were above the capillary fringe in April and within the capillary fringe in October. Bags were also placed at 1.3–1.5 and 0–0.2 m to observe root in-growth in the vadose zone which becomes dry during summer and autumn.
Root in-growth bags were installed via hand-augured access holes. Casings of 90 mm diameter PVC tubes were installed at each access hole to prevent collapse and allow root bag installation and retrieval. Casing length was measured prior to root in-growth bag installation to ensure that 25 cm was left uncovered at the bottom of the access hole to allow contact between the bag and the surrounding soil. The top of the PVC casing of each access tube was sealed with a water-tight cap.
Root bag sampling and measurement took place over 12 months, divided into four periods; April to July 2008 (autumn to early winter), July to October 2008 (late winter to spring), October 2008 to January 2009 (late spring to summer) and January to April 2009 (late summer to autumn). Each root bag was left for 12 weeks during one of the measurement periods to allow in-growth to occur. Once retrieved, a replacement bag was installed via the access tube for the next measurement period. This procedure resulted in 10 replicate in-growth bags per species per measurement period per depth interval. Once retrieved, bags were wrapped in plastic and transported to the laboratory for sorting. Root bags were then cut open and the material inside initially sorted by picking out larger, more obvious Banksia roots by hand. The remaining material was then sieved (2 mm) to separate sand from fine roots, and then all material remaining in the sieve was sorted once again by hand to ensure no root material was missed. Roots were washed in water to ensure all sand particles were removed. This was more problematic for the cluster roots, generally found in the 0.2 m samples, which were soaked and agitated by hand until sand could be removed. Root material was then placed in paper bags and dried at 60 °C for 36 h. Prior to weighing, any sand that remained was removed at this stage by agitating the roots whilst still in the bags. Almost all root material resulting from ingrowth during each 3 month sampling period was live at the time of harvesting. Occasional dead root material was found although it was considered not sufficient to report separately. Emphasis was on quantifying total root ingrowth during each 3 month period (season). Roots were identified on the basis of their morphology and colour using reference samples taken from excavated root material known to be B. attenuata and B. ilicifolia root material.
Above-ground growth phenology and xylem pressure potentials
Above-ground phenological parameters were recorded over a 2 year period, including the same 12 months of the root bag trial, using the same 10 individual trees per species. The timing of vegetative growth and flowering was recorded fortnightly using the nomenclature described by Bell and Stephens (1984). Leaf area index was measured using an LAI meter (LAI 2000, Li-Cor Biosciences, Lincoln, Nebraska, USA) every 3 months between week 9 of 2008 and week 20 of 2009. Leaf area index measurements were made 1 m above the ground surface (above understorey) within a 50 × 50 m plot (that covered all study trees) at 10 m intervals across four gridlines within the plot. Pre-dawn xylem pressure potentials were measured every 6 weeks in the 2 h prior to sunrise, using small twigs cut from three individuals of each species and a Scholander-type pressure chamber (Mk3005 Soil Moisture Equipment Co., Santa Barbara, California, USA). Midday xylem pressure potentials were measured in a similar way between noon and 1 pm.
Data analysis
Total weight of root in-growth at each depth and measurement period was coupled with the corresponding average ambient temperature (°C), total rainfall (mm) and average soil volumetric water content (cm3 cm−3) using multiple linear regressions. Since root in-growth was measured every 12 weeks, environmental parameters were averaged for this period and data were entered into the model using a forward stepwise procedure (p < 0.05 in and p > 0.1 out). All statistical analyses were performed using SPSS v. 17.
Results
Weather during the 12 month study period was typical of the Mediterranean-type climate of the southwest of Western Australia. Summer (December to January) was hot, with weekly average temperatures as high as 27 °C in week 1 2009 (Fig. 1). High average temperatures continued into the autumn months of March and April. Lowest temperatures were observed during the winter months of June to August with average temperatures ranging from 17 to 20 °C. The majority of rain fell between May and October, although there was also rainfall in November 2008. A strong seasonal cycle of drying and rewetting of the vadose zone was evident in the measurements of volumetric water content (Fig. 2), with almost complete depletion of soil water, other than the capillary fringe, during the dry months of January to March. At its deepest, the water table was 3.76 m from the soil surface in April 2008 and responded rapidly to precipitation during winter (Fig. 3), reaching a minimum depth of 2.81 m in August 2008.
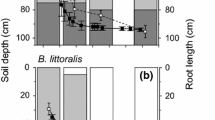
Root in-growth mass for Banksia attenuata (a) and Banksia ilicifolia (b) at four soil depths during each measurement period and flowering and leaf growth phenology during the study. Phenophase percentages refer to the year week in which 20 or 50 % of study individuals displayed the given phenophase (n = 10). DTGW depth to groundwater
The root zone at 2.7 m was not inundated by the water table at any time during the study but was within or just above the capillary zone from July to January (mid-winter to mid-summer). This is reflected in root in-growth throughout the year in this zone (Fig. 3), with the lowest mass being recorded during the July–October period when water tables were at their highest. In contrast, root in-growth bags at 3.7 m were saturated from late autumn until early in the following summer and no root in-growth occurred during this period. However, root in-growth was observed at this depth in early summer and early autumn, when the water table receded and no longer saturated the bags. The mass of root in-growth was comparatively low in the zone closest to the water table and capillary fringe (Fig. 3b).
Patterns in root in-growth were similar in both study species (Fig. 3a, b) and the lowest root mass at all soil depths was observed in the winter and early spring (July–October) sampling period. This coincides with high soil moisture content (Fig. 2), shallow water tables and lowest air temperatures during the study (Fig. 1). Leaf growth did not occur during this period although B. ilicifolia flowering had begun. As air temperatures increased and soil water content declined in spring to summer (October to January), root in-growth mass increased at all depths that were not saturated, which coincided with periods of active leaf growth and flowering (Fig. 3). Root in-growth continued in the mid-summer to early autumn (January–April) period for both species with root mass being greatest at 0.2 m. Shallow (0.2 m) root mass (cluster roots) was low in mid-autumn, winter and early spring sampling periods, and highest during the warmer mid-spring to summer months. The substantial increase in root in-growth at the shallow depths during the spring and summer months was not seen at the 2.7 m and 3.7 m depths. At 3.7 m, no root in-growth was found for either species when root bags were submerged by groundwater. Significant environmental predictors of root growth phenology as determined by multiple linear regression, were only found for shallow (0.2 m) and intermediate depths (1.5 m). Average air temperature (r 2 = 0.61, p < 0.01) and soil volumetric water content (r 2 = 0.49, p < 0.01) were predictors of root growth phenology at 0.2 m for B. attenuata and B. ilicifolia respectively. At intermediate depths in the soil profile (1.5 m), volumetric water content was the only significant predictor of root in-growth in bags placed under B. attenuata (r 2 = 0.32, p < 0.05). Root growth in this part of the soil profile was highest in the January–April sampling period for both species, coinciding with the period of leaf growth and least soil moisture availability. In contrast with shallower soil depths, root activity at the two deepest parts of the profile was less variable and therefore did not show a statistically significant relationship with seasonal changes in ambient temperature or unsaturated zone soil moisture.
Predawn (ΨPD) and midday (ΨMD) shoot water potential measurements over the course of the study did not show any evidence of seasonal plant water deficit, with all predawn measurements above −0.5 MPa for both species, irrespective of season (Fig. 4). This suggests the study plants had consistent access to water despite a large proportion of the vadose zone being essentially dry from December 2008 to April 2009. Midday shoot water potentials (ΨMD) varied throughout the seasons but were generally lower during the observed period of leaf growth, flowering and shallow root growth. Average leaf area index of the overstorey (which includes the study species) at the site was consistent at approximately 1 (Fig. 4).
Discussion
Our assessment of root in-growth in this study suggests a dynamic pattern in the growth of two phreatophytic species of Banksia. Provided soil conditions are conducive and that there are no endogenous limitations to root growth, B. attenuata and B. ilicifolia displayed the ability to grow roots at any time in response to fluctuating soil water conditions. This was demonstrated most clearly at the capillary fringe, where root growth was constant between seasons. The capacity for constant root growth can be considered an essential prerequisite for phreatophytes if they are to respond to fluctuating water tables in water-limited environments.
This study also highlights the differences in root function with depth in a dimorphic root system that interfaces with a water table. Root growth of B. attenuata near the surface was responsive to the seasonal influences of temperature and vadose zone soil water content. These shallow roots were primarily cluster roots (Purnell 1960) which are typical of the O and A soil horizons in Banksia woodlands (Lamont 2003). There was little root growth at this soil depth during the cool, wet winter season (i.e. between April and October) which is contrary to observations made by Lamont and Bergl (1991), who found that cluster root growth was initiated by the first rain events of winter. However, studies of chaparral in southern California and Chilean matorral suggest that fine root growth in the top 0.2 m begins after winter rain, reaching maximum production in spring and early summer (Kummerow et al. 1978; Montenegro et al. 1982). Root growth in the shallow parts of the soil profile in Mediterranean-type ecosystems is likely to be opportunistic and influenced by the large seasonal variations in temperature, as well as changes in water and nutrient availability. Root in-growth bags were wetted prior to installation which may have allowed some root growth to be independent of soil conditions at the time of placement, e.g. by allowing some unseasonal cluster root production. However, root growth as an artefact of the method used appears to be limited since temperature was found to be the best predictor of root growth for one of the species at shallow depth. In addition, the relatively small amount of water added prior to installation would not have had a prolonged effect over the 12 weeks the bag was buried in the field.
While seasonal variability in root growth at the capillary fringe is often inferred in phreatophytic trees, few studies have observed the dynamics of their root growth at the depths measured in this study. In contrast to the pronounced seasonality of environmental variables and root growth near the soil surface, one may expect conditions and root growth to be more stable at deeper soil depths. The magnitude of diurnal and seasonal changes in soil temperature decreases with depth (Voroney 2007), and generally does not have an influence below 1 m (Popiel et al. 2001). Instead, water availability and soil aeration appear to be drivers of root growth at the water table and capillary fringe interface. Root growth within the capillary fringe occurred throughout the seasons, corresponding with consistent water availability and aerobic conditions. Deep root systems enable access to these consistent, available water sources, which permit phreatophytic plants in seasonally water-limited environments to survive and meet their water requirements throughout the year.
Seasonal fluctuations of the water table routinely saturate part of the root zone, which can impact on root activity (Kozlowski 1997; Mahoney and Rood 1998; Castelli et al. 2000). Root growth at or near the water table, was initially observed in autumn when this zone was high in moisture. As the water table rose in winter and early spring the zone became saturated and no root growth was observed until the water table fell again during the following summer and autumn. The roots of the majority of plants are unable to tolerate saturated conditions, primarily due to low oxygen availability (Kozlowski 1997) and it is assumed the anoxic conditions associated with the rising water table prevented root ingrowth from the surrounding soil. By summer and autumn the falling water table leaves an aerated, high moisture environment suitable for root growth, implying a seasonal cycle of root growth in response to water table rise, and subsequent re-growth as the water table falls.
Root elongation in response to a declining water table has been observed for a wide range of species, including phreatophytic plants under glasshouse conditions (Mahoney and Rood 1991; Kranjcec et al. 1998; Horton and Clark 2001; Stave et al. 2005). However, in situ observations of mature tree root growth dynamics at depth following water table fluctuations are rare. Root redistribution is thought to occur as phreatophytic plants adjust root growth in response to a rising and declining water table (Naumburg et al. 2005). As the rising water table inhibits root growth in saturated soil, growth may continue and be encouraged elsewhere in the unsaturated soil just above the water table (Castelli et al. 2000; Martin and Chambers 2002). Imada et al. (2010) described the proliferation of roots of Populus alba cuttings in the zone above a dynamic water table. Cuttings with a fluctuating water table had a higher dead, fine root biomass than those with a static water table. However, the live fine root biomass was similar between the control and the treatments, suggesting that plants maintain total fine root biomass, redistributing growth in response to inundation of part of the root zone. Our measurements of root in-growth by mature phreatophytic Banksia trees support these findings and suggest that close to the water table, there is a seasonal redistribution of fine root biomass in response to fluctuations in groundwater level and distribution of the capillary fringe.
Both B. attenuata and B. ilicifolia demonstrated restricted above-ground growth periods, with predominant leaf growth and flowering occurring from late spring to autumn; a pattern observed both in this study and in Bell and Stephens (1984). As new leaves develop, older leaves are shed, and the balance between the loss of old leaves and new growth results in little overall change in canopy cover, with LAI remaining consistent throughout the year in Banksia woodland (this study and Farrington et al. 1989; Veneklass and Poot 2003). New shallow root growth (cluster roots) to meet nutrient requirements peaked during the predominant leaf growth and flowering period, coinciding with warmer temperatures and moist shallow soils. Growth of cluster roots continued however into summer and autumn despite the very dry vadose zone, suggesting hydraulic redistribution from deep to shallow lateral roots to maintain live cluster root mass (Dawson and Pate 1996).
In contrast with the synchrony seen between shallow root and above-ground growth phenophases, root growth at depth was either constant near or within the capillary zone or responsive to saturation by a rising water table. For phreatophytic Banksia, the ability to grow roots year-round at the water table or capillary fringe would be highly beneficial to maintaining above-ground phenological processes. Although the mass of roots at depth may be relatively small, water uptake by deep roots accessing more consistent water sources can account for up to 60 % of the total plant water balance (Canadell et al. 1996). Root activity and water uptake at depth near the water table is influential in allowing phreatophytes to maintain leaf growth, flowering and shallow root growth when water availability in the vadose zone is low.
References
Bell D, Stephens L (1984) Seasonality and phenology of Kwongan species. In: Pate J, Beard J (eds) Kwongan plant life of the sand plain. University of Western Australia Press, Perth, pp 205–226
Böhm W (1979) Methods of studying root systems. Springer-Verlag, Berlin
Bureau of Meteorology (2010) Climate statistics for Australian locations. Retrieved from http://www.bom.gov.au/climate/averages/tables/cw_009021.shtml in September, 2010
Busch DE, Ingraham NL, Smith SD (1992) Water uptake in woody riparian phreatophytes of the southeastern United States: a stable isotope study. Ecol Appl 2:450–459
Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108:583–595
Canham CA, Froend RH, Stock WD (2009) Water stress vulnerability of four Banksia species in contrasting ecohydrological habitats on the Gnangara Mound, Western Australia. Plant Cell Environ 32:64–72
Castelli RM, Chambers JC, Tausch RJ (2000) Soil-plant relations along a water-table gradient in Great Basin riparian meadows. Wetlands 20:251–266
Cooper DJ, D’Amico DR, Scott ML (2003) Physiological and morphological response patterns of Populus deltoides to alluvial groundwater pumping. Environ Manage 31:215–226
Dawson TE, Pate JS (1996) Seasonal water uptake and movement in root systems of Australian phreatophytic plants of dimorphic root morphology: a stable isotope investigation. Oecologia 107:13–20
Dodd J, Bell DT (1993) Water relations of the canopy species in a Banksia woodland, Swan Coastal Plain, Western Australia. Aust J Ecol 18:281–293
Dodd J, Heddle EM, Pate JS, Dixon KW (1984) Rooting patterns of sandplain plants and their functional significance. In: Pate J, Beard J (eds) Kwongan plant life of the sand plain. University of Western Australia Press, Nedlands, pp 146–177
Eamus D, Hatton T, Cook P, Colvin C (2006) Ecohydrology: vegetation function, water and resource management. CSIRO Publishing, Collingwood
Farrington P, Greenwood EA, Bartle GA, Beresford JD, Watson GD (1989) Evaporation from Banksia woodland on a groundwater mound. J Hydrol 105:173–186
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall Inc., New Jersey
Gentilli J (1972) Australian Climate Patterns. Thomas Nelson, Melbourne
Greacen EL (1981) Soil water assessment by the neutron method. CSIRO (Division of Soils), Adelaide
Groom PK (2004) Rooting depth and plant water relations explain species distribution patterns within a sandplain landscape. Funct Plant Biol 31:423–428
Hendrick RL, Pregitzer KS (1997) The relationship between fine root demography and the soil environment in northern hardwood forests. Ecoscience 4:99–105
Horton JL, Clark J (2001) Water table decline alters growth and survival of Salix goodingii and Tamarix chinensis seedlings. Forest Ecol and Manag 140:239–247
Imada S, Yamanaka N, Tamai S (2008) Water table depth affects Populus alba fine root growth and whole plant biomass. Funct Ecol 22:1018–1026
Imada S, Yamanaka N, Tamai S (2010) Fine-root growth, fine-root mortality, and leaf morphological change of Populus alba in response to fluctuating water tables. Trees 24:499–506
Joslin JD, Wolfe MH, Hanson PJ (2001) Factors controlling the timing of root elongation intensity in a mature upland oak stand. Plant Soil 228:201–212
Kozlowski TT (1997) Responses of woody plants to flooding and salinity. Tree Physiology Monograph 1:1–29
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press Inc., San Diego
Kranjcec J, Mahoney JM, Rood SB (1998) The responses of three riparian cottonwood species to water table decline. Forest Ecol Manag 110:77–87
Kummerow J, Krause D, Jow W (1978) Seasonal changes of fine root density in the southern Californian chaparral. Oecologia 37:201–212
Lamont BB (2003) Structure, ecology and physiology of root clusters—a review. Plant Soil 248:1–19
Lamont BB, Bergl SM (1991) Water relations, shoot and root architecture, and phenology of three co-occurring Banksia species: no evidence for niche differentiation in the pattern of water use. Oikos 60:291–298
Lubczynski MW (2009) The hydrogeological role of trees in water-limited environments. Hydrogeol J 17:247–259
Mahoney JM, Rood SB (1991) A device for studying the influence of declining water table on poplar growth and survival. Tree Physiol 8:305–314
Mahoney JM, Rood SB (1998) Streamflow requirements for cottonwood seedling recruitment—an integrative model. Wetlands 18:634–645
Martin D, Chambers J (2002) Restoration of riparian meadows degraded by livestock grazing: above and belowground responses. Plant Ecol 163:77–91
Meinzer OE (1923) Outline of groundwater hydrology with definitions. Water-supply paper 494. US Geological Survey
Montenegro G, Araya S, Aljaro ME, Avila G (1982) Seasonal fluctuations of vegetative growth in roots and shoots of Central Chilean shrubs. Oecologia 53:235–237
Murray BR, Zeppel MJ, Hose GC, Eamus D (2003) Groundwater dependent ecosystems in Australia: it’s more than just water for rivers. Ecol Manage Restor 4:110–113
Naumburg E, Mata-Gonzalez R, Hunter R, McLendon T, Martin D (2005) Phreatophytic vegetation and groundwater fluctuations: a review of current research and application of ecosystem response modelling with an emphasis on Great Basin vegetation. Environ Manage 35:726–740
Palacio S, Montserrat-Martí G (2007) Above and belowground phenology of four Mediterranean sub-shrubs: preliminary results on root-shoot competition. J Arid Environ 68:522–533
Poole DK, Miller PC (1975) Water relations of selected chapparal and coastal sage communities. Ecology 56:1118–1128
Popiel LO, Wojtkowiak J, Biernacka B (2001) Measurements of temperature distribution in ground. Exp Therm Fluid Sci 25:301–309
Purnell HM (1960) Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Aust J Bot 8:38–50
Richards MB, Stock WD, Cowling RM (1995) Water relations of seedlings and adults of two fynbos Proteaceae species in relation to their distribution patterns. Funct Ecol 9:575–583
Scott M, Shafroth P, Auble G (1999) Responses of riparian cottonwoods to alluvial water table declines. Environ Manage 23:347–358
Sperry JS, Hacke UG (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Funct Ecol 16:367–378
Stave J, Oba G, Eriksen AB, Nordal I, Stenseth BC (2005) Seedling growth of Acacia tortilis and Faidherbia albida in response to simulated groundwater tables. Forest Ecol Manag 212:367–375
Steinaker DF, Wilson SD, Peltzer DA (2010) Asynchronicity in root and shoot phenology in grasses and woody plants. Global Change Biol 16:2241–2251
Teskey RO, Hinckley TM (1981) Influence of temperature and water potential on root growth of white oak. Physiol Plantarum 52:363–369
Veneklass EJ, Poot P (2003) Seasonal patterns in water use and leaf turnover of different plant functional types in a species rich woodland, south-western Australia. Plant Soil 257:295–304
Voroney RP (2007) The Soil Habitat. In: Eldor AP (ed) Soil microbiology and biochemistry, 3rd edn. Elsevier Inc., USA, pp 25–53
Zencich SJ, Froend RH, Turner JV, Gailitis V (2002) Influence of groundwater depth on the seasonal sources of water accessed by Banksia tree species on a shallow, sandy coastal aquifer. Oecologia 131:8–19
Acknowledgments
This research was conducted under an Australian Postgraduate Award associated with Australian Research Council Linkage Project LP0669240. The authors also wish to acknowledge the support of the Water Corporation of Western Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hermann Heilmeier.
Rights and permissions
About this article
Cite this article
Canham, C.A., Froend, R.H., Stock, W.D. et al. Dynamics of phreatophyte root growth relative to a seasonally fluctuating water table in a Mediterranean-type environment. Oecologia 170, 909–916 (2012). https://doi.org/10.1007/s00442-012-2381-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2381-1