Abstract
Timing of reproduction and clutch size are important determinants of breeding success, especially in seasonal environments. Several recent bird population studies have shown changes in breeding time and in natural selection on it. These changes have often been linked with climate change, but few studies have investigated how the traits or natural selection are actually connected with climatic factors. Furthermore, the effect of population density on selection has been rarely considered, despite the potential importance of density in demographic processes. We studied variation in natural selection on laying date and on clutch size in relation to measures of spring phenology and population density in a long-term study of pied flycatchers in SW Finland. The phenological stage of the environment at mean egg-laying did not affect the direction of selection on either laying date or on clutch size. There was, however, stronger selection for earlier laying date when the breeding density of the population was high, suggesting that early breeding is not necessarily beneficial as such, but that its importance is emphasized when high population density increases competition. In addition, early breeding was favoured when the pre-breeding period was cool, which may indicate an increased advantage for the fittest individuals in harsher conditions. In the middle of the twentieth century, there was selection for large clutch size, which subsequently ceased, along with an overall decrease in recruit production. Our results indicate that attention should be paid to demographic factors such as breeding density when studying natural selection and temporal changes in it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural selection obviously favours individuals that produce many high-quality offspring. The timing of breeding and clutch size are two key reproductive traits affecting the productivity and thus the fitness of individual birds (Daan et al. 1990; Lack 1954). Correct timing involves beneficially matching with the availability of resources, whereas an optimal clutch size maximizes both quality and quantity of the offspring produced. Clutch size is often negatively correlated with timing of breeding (Garamszegi et al. 2004; Gladbach et al. 2010; Goodenough et al. 2009; von Haartman 1982), and both timing and clutch size are affected by parental condition (Rowe et al. 1994).
In addition to the correct timing, the availability of food, nest sites, and other resources are affected by the density of competitors (Gustafsson 1987). For example, breeding density is related to clutch size and fledgling production (Ahola et al. 2009; Both 1998, 2000; Dhondt et al. 1992). As different trait values may be favoured in different competitive situations, breeding density can also affect the selection. Being early may, for example, be more beneficial under high density than under low density, as some resources become increasingly limited over time in seasonal environments. Climatic effects on life history may thus interact with density. The idea of density-dependent natural selection was first proposed in the 1960s and has been discussed since then (Boyce 1984; MacArthur 1962; Mueller 1997). However, the roles of density and other demographic factors have not recently been considered in studies concerning climate change effects on natural selection.
The timing of breeding has advanced in several bird species in response to climate warming (Dunn 2004). However, the mechanisms behind these changes have barely been studied. In some cases, phenotypic plasticity has been found to sufficiently cover the rate of the changes observed at the population level (e.g. Charmantier et al. 2008; Przybylo et al. 2000). In some other cases, evolutionary changes in response to climate change have been suggested, but evidence for a direct connection between selection and the observed changes at the phenotypic level has rarely been found (Gienapp et al. 2008). There are only a few avian studies that have examined whether selection on a reproductive trait has changed over time (Both and Visser 2001; Gienapp et al. 2006; Visser et al. 1998), or whether selection actually varies in relation to temperature or environmental phenology (van Noordwijk et al. 1995).
Our study species, the pied flycatcher (Ficedula hypoleuca), is an insectivorous passerine bird. It is a long-distance migrant that breeds in Europe and winters in Africa, south of the Sahara Desert (Lundberg and Alatalo 1992). Our earlier studies showed that within-spring variations in temperature trends have caused different trends for the spring arrival times of early and late pied flycatchers in southwestern Finland (Ahola et al. 2004). Although the early part of the population has advanced its arrival, the timing of breeding in our study population has slightly delayed, and clutch size has decreased (Laaksonen et al. 2006). This has happened despite the accelerated progression of the spring phenology of the breeding environment (Laaksonen et al. 2006). Most of these changes are different from those reported from other pied flycatcher populations (Both et al. 2004; Sanz 2003; Sanz et al. 2003), which calls for the examination of the role of natural selection in explaining the contrasting trends. Because of the earlier hypotheses about the density dependence of natural selection and the marked fluctuation in breeding density in this population (Ahola et al. 2007), we thought it meaningful to examine the effect of breeding density on natural selection as well. Thus, in the work reported here, we studied selection on the timing of breeding and on clutch size, and how they were affected by environmental phenology and breeding density in this population.
Materials and methods
Data
Breeding data was used from two nest-box studies in SW Finland. The first data set was collected by Lars von Haartman in Askainen (60°30′N, 21°45′E) during 1954–1994 (although data for the years 1958, 1986, 1990 and 1992 are missing). The number of nest boxes used for monitoring varied from 79 (1993) to 155 (1983) per year (median = 134, N = 37 years). The second data set was collected by T.E. and E.L. in Harjavalta (61°20′N, 22°10′E, 95 km apart from Askainen) during 1991–1998. Pied flycatchers were studied in these study areas before and after this period too, but the data used in this study were restricted to the years 1954–1998; we did this because before 1954 there was no information on ringed nestlings, and after 1999 the pied flycatcher parents were inadequately captured. Thus recruits born after 1998 had a significantly lower probability of being detected. All nesting attempts that were within three kilometres of a polluting industrial complex that was known to affect the breeding parameters of tits and pied flycatchers (Eeva et al. 1997) were excluded from the Harjavalta data. Further from the industrial complex, between 449 (1991) and 599 (1995) nest boxes were studied per year (median = 528, N = 8 years). In both areas, the nest boxes were checked at least once a week, and usually more often during the egg-laying period. Laying dates were determined from the number of eggs observed during the laying period, assuming a laying frequency of one egg per day. Clutches were considered to be complete when no more eggs had appeared and incubation had begun. The number of fledglings was determined from the maximum number of chicks seen at the age of 10 days or older minus any dead chicks remaining in the nest after fledging. If there was any sign of nest predation, the breeding attempt was interpreted as a failure and left out of the data.
The data were restricted to the first nesting attempt of each female during each year which produced fledglings that were ringed (from which recruits could be detected later on). With these restrictions enforced, the data contained information on 2,207 pied flycatcher nests (1,326 and 881 from Askainen and Harjavalta, respectively). Out of these nests, 11,917 (7,147 in Askainen + 4,770 in Harjavalta) pied flycatcher nestlings were ringed and 1,535 (1,187 + 348) breeding males and 2,101 (1,322 + 779) females were trapped (ringing was done during all 42 years). Among the ringed nestlings, 118 recruited to the area. Recruits were produced in 35 of the 42 years of the study. Only one nest produced two recruits, while the other recruits were from separate clutches.
The nesting attempts from the two data sets were pooled, but year-specific biological variables, phenological earliness of mean laying date, and breeding density (see below) were calculated and used separately for the two locations.
Analyses
Temporal trends were analysed as linear regressions. Proportional variables were first arcsine transformed, except for breeding density, as the transformation was not needed for this variable as it only took defined values between 0 and 100 and otherwise met the assumptions of parametric tests.
Since the recruitment rate in this population was relatively low, with only 0–2 recruits produced in some years, the often used yearly standardized selection differentials were not calculated (Falconer and Mackay 1996). Instead, a generalized linear mixed model was built (proc glimmix in SAS 9.2) to elucidate which of the factors determined whether a nest produced a recruit or not. A binary distribution with a logit link function was used for the response variable (0/1). The data from all years were combined by centring the laying date and clutch size of each nest to be the difference from each year’s mean value. The centred laying date and clutch size (one value per nest) were used in the model as explanatory factors, as well as the following candidate explanatory factors (one value per year). (1) Year as a continuous variable to find out possible temporal trends. Year was also included in the model as a random factor (class variable), because the individual breeding attempts within each year were not fully independent of each other (as the breeders were all facing the same environmental conditions). (2) Pre-breeding temperature is the yearly mean temperature of the period that has the strongest impact on the yearly average laying date in this population (period 5th–28th May; Ahola et al. 2007). (3) Phenological earliness of mean laying date is the differential of the yearly mean laying date from the yearly date when the thermal sum of 150°C was reached. The thermal sum is the cumulative sum of daily mean temperatures exceeding +5°C from the beginning of March. The phenology of the main deciduous tree in the study area, the silver birch (Betula pendula Roth), strictly follows thermal sums (Häkkinen et al. 1998). The thermal sum can be considered a useful indicator of the phenology of the environment, including the availability and developmental stage of herbivorous prey items for the pied flycatchers, because the development and growth of these poikilothermic organisms depend largely on the thermal development of spring. The thermal sum of 150°C was chosen because it is reached on average at the same time as the overall average laying date of the pied flycatcher (Laaksonen et al. 2006). Thus, phenological earliness was used to relate the timing of breeding at the population level to spring phenology. (4) Breeding density of the pied flycatchers was measured through the occupancy rate of available nest boxes; i.e. the proportion of nest boxes occupied by pied flycatchers out of all those nest boxes in the study area(s) that were not inhabited by breeding pairs of tits (Parus major, Cyanistes caeruleus, Periparus ater, Lophophanes cristatus and Poecile montanus). The tits initiate breeding before the arrival of the pied flycatchers. This was considered a relevant measure, as in our study system the poor availability of nest holes (nest boxes were along paths at about 40 m intervals) rather than the free territory space around them is the limiting resource, and variation in the number of nest boxes that were available for the pied flycatchers mainly arose from the variation in the number of breeding pairs of tits.
With this model, we were looking for the factors that affect the probability of producing recruits. However, as we were primarily interested in the variation and determinants of natural selection on laying date and clutch size, we added all pairwise interactions between the two centred traits and the other explanatory factors to our model, and mainly focused on them. Such an interaction would indicate that natural selection on the trait is linked to the explanatory factor in question.
We reduced the model by removing the nonsignificant variables one by one, starting from the least significant interactions. The nonsignificance of the dropped variables was checked by re-adding them to the final model one by one (interactions with their main effects). We also ensured that there were no collinearity issues among the explanatory variables on the basis of variation inflation values of the parameter estimates.
Since producing many fledglings obviously enhances the probability of producing a recruit, we also examined whether selection measured by recruit production was independent of fledgling production; i.e. if selection is actualized before or after fledging. This was tested with a separate model where the number of fledglings produced in each nest was added to the final model.
Throughout the study, we used running day numbers set in such a way that the vernal equinox was always day number 80. In the text, however, days are given as calendar dates based on a vernal equinox on 20 March. SAS 9.2 was used for all analyses.
Results
Temporal trends
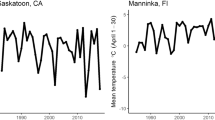
The proportion of nests that produced recruits decreased during 1954–1998 (b = −0.0039 ± 0.0014, t = −2.80, p = 0.0075, N = 45; Fig. 1a) as well as the proportion of recruits of all fledglings (b = −0.0016 ± 0.0006, t = −2.66, p = 0.011, N = 45; Fig. 1b; the analyses on arcsine-transformed values). There was no significant change in average laying date of pied flycatchers during 1954–1998 (b = 0.055 ± 0.041, t = 1.35, p = 0.18, N = 45; Fig. 1c), but the phenological earliness of mean laying date was delayed by more than 1 week (b = 0.22 ± 0.06, t = 3.68, p = 0.0006, N = 45; Fig. 1d), indicating that the timing of breeding was delayed in relation to the advanced phenology. However, there was no directional change in pre-breeding temperature (mean temperature of the period 5–28 May; b = 0.018 ± 0.023, t = 0.75, p = 0.46, N = 42; Fig. 1e). Therefore, the delay in phenological earliness of mean laying date was mainly due to increasing temperatures of earlier spring (e.g. mean temperatures of 1–31 March: b = 0.074 ± 0.028, t = 2.69, p = 0.01, N = 45; and 1–30 April: b = 0.044 ± 0.016, t = 2.72, p = 0.0094, N = 45; Fig. 1f). The average clutch size tended to decrease during this study period, but the change was not statistically significant (b = −0.0045 ± 0.0026, t = −1.76, p = 0.086, N = 45, Fig. 1g; but see Laaksonen et al. 2006 for 1943–2002). The breeding density showed a quadratic trend (year: b = −1.88 ± 0.59, t = −3.17, p = 0.0029, N = 45; year2: b = 0.033 ± 0.011, t = 2.99, p = 0.0046, N = 45). Density was highest during the 1950s and 1960s, and again during the late 1980s and early 1990s (Fig. 1h).
Temporal trends in the yearly a proportion of pied flycatcher nests that produced recruits, b recruitment rate of produced fledglings, c population mean laying date, d phenological earliness of the mean laying date (mean laying date minus the date when the thermal sum of 150°C was reached each year), e mean temperature of the pre-breeding period 5–28 May, f mean temperatures in March (filled dots) and April (open circles), g mean clutch size, and h pied flycatcher breeding density (percentage of nest boxes that were occupied by pied flycatchers among those that were available after breeding pairs of tits) in the pied flycatcher study populations over the study period 1954–1998
Factors affecting natural selection
In the model explaining each nest’s recruit production, the centred laying date had significant interactions with breeding density and pre-breeding temperature, indicating that selection on laying date was connected to these factors. Early laying was favourable, especially when breeding density was high and/or when the pre-breeding temperature was low (Fig. 2a, b; Table 1). The interaction between the centred clutch size and year indicated that there was a temporal change in selection on clutch size. There was selection for a large (centred) clutch size in the 1950s and 1960s, but it gradually disappeared after those times (Fig. 2c; Table 1). During the study, the overall probability of recruit production also decreased, as indicated by the negative main effect of year in the model (Table 1). The significance of centred clutch size in the model showed that, despite the temporal change in overall recruit production, the probability of producing a recruit was higher with a large clutch than with a small one (Table 1).
Probability of producing a recruit as a function of the interactions between a centred laying date and breeding density, b laying date and pre-breeding temperature, and c clutch size and year. In a, the effect of centred laying date is shown separately for rounded values of the 10th (dashed), 50th (dotted) and 90th (solid) percentiles of the breeding density; in b, the effect of centred laying date is shown respectively for the percentiles of pre-breeding temperature, and in c, the different trends in the probability of recruit production are shown for the percentiles of the centred clutch size. The actual values of the percentiles are shown to the right of each line
When added to the model, the number of fledglings produced in each nest had a clear effect on the probability that one of them would return as a recruit (estimate ±SE = 0.365 ± 0.107, t = 3.42, p = 0.0006), as expected. The number of fledglings did not, however, change the main results, indicating that the observed effects on selection take place mainly after fledging.
Discussion
We found that during the last half of the twentieth century, recruitment rate in this pied flycatcher population decreased. Mean timing of breeding did not show any temporal change, but it was delayed in relation to the advanced environmental phenology. We suppose that this was because only early spring has warmed, while the pre-breeding period that most affects the timing of breeding in the pied flycatchers has not (Fig. 1). Despite these phenological patterns, natural selection on laying date was not affected by the difference between the phenology of the environment or by the mean laying date of the population. Instead, we found that high breeding density and cool pre-breeding period strengthened the selection for early laying, indicating that intraspecific competition and supposedly harsh conditions preceding breeding both emphasize the fitness of those individuals that are able to start breeding earlier than others. We found that selection on clutch size changed such that there was selection for large clutch size in the early decades of the study, but that disappeared during the study. None of the other explanatory factors were, however, found to be connected to the selection on clutch size.
Density-dependent selection on laying date
Our results indicate that selection for early laying is stronger when breeding density is high in relation to available nest holes. We suggest that in a high-density situation, there are no high-quality territories for all breeders—some are forced to settle in less productive patches. This emphasizes the superiority of those that are able to occupy the best territories. The decreasing food availability during the breeding season may also add to the density effect, as this may also affect breeders in low-quality territories most strongly.
Whatever the reason, the effect of density on recruitment did not take place before fledging time, since the laying date × density interaction did not change when the number of fledglings was added to the model. This indicates that the density dependence of selection is affected by factors that take effect between fledging and recruitment. For example, a high post-fledging density might decrease post-fledging survival and/or increase the natal dispersal distances of young from late broods (Nilsson 1989; Tinbergen 2005).
The young from early nests may be in a better position compared to those from later nests for many reasons. The offspring raised in the highest quality territories are probably in the best condition at fledging time. If these nests are also the earliest, the fledglings in good condition should be able to start their autumn migration before the fledglings from later nests. Early autumn migration is probably beneficial as the Sahel is then crossed when there are better environmental conditions (before its seasonal dry period; Jenni and Kéry 2003) and because it is then possible to obtain a high-quality wintering territory (Salewski et al. 2002). Furthermore, a good territory at the wintering grounds increases the chances of survival and ensures that the bird is in good condition for the moult, spring migration and breeding.
Since population density has long been considered a factor that affects natural selection, it is surprising how rarely it has been examined recently. Moorcroft et al. (1996) studied coat colour and horn type of Soay sheep and, congruently with our results, found that selection on these traits was stronger when there was a high rather than a low population density. As population density is known to have various effects on many traits of birds (Newton 1998), we recommend that population density should be taken into account in all long-term population studies focusing on variation and temporal changes in natural selection.
Pre-breeding temperature and selection for laying date
Selection for early laying was stronger when the pre-breeding period was cool. We suggest that cool pre-breeding temperatures emphasize the fitness of high-quality individuals, as they are probably better able to time their breeding well, irrespective of the temperature of spring. The early birds may also have an advantage over later ones if their high-quality territories ensure that they are in better condition during cool temperatures. A warm pre-breeding temperature implies the predominance of mild southern and southwestern tail winds for the migrants. Under such favourable conditions, centred laying dates may not be determined as much by individual ability to control their arrival time as by chance if most individuals are able to arrive and start breeding early. This is supported by our earlier findings that both arrival and laying date distributions are more right-skewed in warm springs, meaning that most laying dates are concentrated towards the early end of the range, while they are more normally distributed in cool springs (Laaksonen et al. 2006).
Although the phenology of the environment strongly determines the timing of breeding in many bird species (Crick et al. 1997), only a few studies have found changes in selection on the timing of reproduction to be linked to changes in phenology. Climate warming has been linked to mismatches between timing of breeding and the peak time for food availability (moth caterpillars; Both and Visser 2001; Both et al. 2006; Sanz et al. 2003), as well as to subsequent stronger selection for early laying (Both and Visser 2001) and population declines (Both et al. 2006) in pied flycatcher populations in Central Europe. The idea in these studies was that, along with the warming climate, the earliest birds have been in the best situation to match the hatching of their offspring with the advanced food peak. Thus, because of warmer springs, there would have been selection for early breeding. In our study, selection for early laying appeared with a cool pre-breeding temperature. It might be that different factors affect the selection on laying date in populations breeding in Southern and Central Europe than in Finland. The reason for this may be the less strict food availability peak in Finland. The period when moth caterpillars are available is relatively long (Eeva et al. 1997), and alternative food items such as saw-fly caterpillars occur during a period that overlaps with and extends after that (Eeva et al. 2005). Thus, we suppose that the critical factor in our study population is the food availability in space (territory quality) instead of food availability in time (food peak).
Selection on clutch size
The third result showed that there was selection for large clutch size at the beginning of our study period, but that it disappeared as decades passed. This is in concordance with the tendency of phenotypic mean clutch size to decrease in this population. However, it also appeared that the overall probability of producing recruits decreased during the study. It is unclear what causes these results, considering that the phenological measures, population density and number of produced fledglings from each nest did not. One possibility is that environmental conditions at the breeding grounds have deteriorated independently of the aforementioned explanatory factors, so it has become more difficult to raise large broods to fledge under good conditions. Another possibility is that the result appeared due to a decreasing number of recruits during the study. Due to the decrease in the yearly number of recruits, the favourability of large clutches could have become statistically undetectable.
As clutch size in birds is commonly connected to laying date (“calendar effect”; Crick et al. 1993; Garamszegi et al. 2004; Gladbach et al. 2010; Goodenough et al. 2009; Klomp 1970; von Haartman 1982), the existence of independent selection on clutch size has rarely been reported. In this study, selection on laying date and selection on clutch size were connected with different factors. In addition, as both were analysed in the same model, we can conclude that there is independent selection on laying date and clutch size in this pied flycatcher population. In collared flycatchers (Ficedula albicollis) on the Swedish island of Gotland in the Southern Baltic Sea, selection on laying date and selection on clutch size were found to be closely correlated, and in that population there was no evidence of independent selection on clutch size (Sheldon et al. 2003). Charmantier et al. (2006) found that selection for a larger than average clutch size in mute swans (Cygnus olor) increased after the human-induced increase in food abundance and restriction of predation. With a pedigree, they showed that the trend resulted partly from microevolutionary processes that were independent of selection on laying date (Charmantier et al. 2006).
Selection measures and their usage
Some caution needs to be exercised when inferring selection from measures based on recruitment, since the measured recruitment in a study population may be confounded (e.g. by variation in natal dispersal). Due to the small number of recruits, there is presumably some inaccuracy involved in measuring selection based on them. However, our analysis indicated clear effects of independent explanatory factors on selection. We think that these effects do not appear randomly; their appearance indicates real connections of the selection measures used to the explanatory variables. Another question is whether the surviving young that recruit to breed in their natal area are a sample that represent the whole surviving cohort in regard to their breeding time or clutch size. It is also worth considering that when a significant proportion of the young disperse from their natal population, we do not know which of them will return. Results indicate true natural selection if the natal dispersal distance is not dependent on the studied traits. As natal dispersal distance is known to be connected to population density (Nilsson 1989; Tinbergen 2005), part of the density effect may come from that. However, this is an unavoidable problem in any study in which a significant proportion of the offspring disperse outside of the study area. Our results indicate that the spatial restrictions of population studies should be taken into account when studying subjects connected to recruitment.
References
Ahola M, Laaksonen T, Sippola K, Eeva T, Rainio K, Lehikoinen E (2004) Variation in climate warming along the migration route uncouples arrival and breeding dates. Glob Change Biol 10:1610–1617. doi:10.1111/j.1365-2486.2004.00823.x
Ahola M, Laaksonen T, Eeva T, Lehikoinen E (2007) Climate change can alter competitive relationships between resident and migratory birds. J Anim Ecol 76:1045–1052. doi:10.1111/j.1365-2656.2007.01294.x
Ahola MP, Laaksonen T, Eeva T, Lehikoinen E (2009) Great tits lay increasingly smaller clutches than selected for—a study of climate- and density-related changes in reproductive traits. J Anim Ecol 78:1298–1306. doi:10.1111/j.1365-2656.2009.01596.x
Both C (1998) Density dependence of clutch size: habitat heterogeneity or individual adjustment? J Anim Ecol 67:659–666
Both C (2000) Density dependence of avian clutch size in resident and migrant species: is there a constraint on the predictability of competitor density? J Avian Biol 31:412–417
Both C, Visser ME (2001) Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411:296–298
Both C, Artemyev AV, Blaauw B, Cowie RJ, Dekhuijzen AJ, Eeva T, Enemar A, Gustafsson L, Ivankina EV, Järvinen A, Metcalfe NB, Nyholm NEI, Potti J, Ravussin P-A, Sanz JJ, Silverin B, Slater FM, Sokolov LV, Török J, Winkel W, Wright J, Zang H, Visser ME (2004) Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc R Soc Lond B 271:1657–1662. doi:10.1098/rspb.2004.2770
Both C, Bouwhuis S, Lessells CM, Visser ME (2006) Climate change and population declines in a long-distance migratory bird. Nature 441:81–83. doi:10.1038/nature04539
Boyce MS (1984) Restitution of r- and K-selection as a model of density-dependent natural selection. Annu Rev Ecol Syst 15:427–447
Charmantier A, Perrins CM, McCleery RH, Sheldon BC (2006) Evolutionary response to selection on clutch size in a long-term study of the mute swan. Am Nat 167:453–465
Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC (2008) Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320:800–803. doi:10.1126/science.1157174
Crick HQP, Gibbons DW, Magrath RD (1993) Seasonal changes in clutch size in British birds. J Anim Ecol 62:263–273
Crick HQP, Dudley C, Glue DE, Thomson DL (1997) UK birds are laying eggs earlier. Nature 388:526
Daan S, Dijkstra C, Tinbergen JM (1990) Family planning in the kestrel (Falco tinnunculus): the ultimate control of covariation of laying date and clutch size. Behaviour 114:83–116
Dhondt AA, Kempenaers B, Adriaensen F (1992) Density-dependent clutch size caused by habitat heterogeneity. J Anim Ecol 61:643–648
Dunn P (2004) Breeding dates and reproductive performance. Adv Ecol Res 35:69–87. doi:10.1016/S0065-2504(04)35004-X
Eeva T, Lehikoinen E, Pohjalainen T (1997) Pollution-related variation in food supply and breeding success in two hole-nesting passerines. Ecology 78:1120–1131
Eeva T, Ryömä M, Riihimäki J (2005) Pollution-related changes in diets of two insectivorous passerines. Oecologia 145:629–639. doi:10.1007/s00442-005-0145-x
Falconer DS, Mackay FTC (1996) An introduction to quantitative genetics. Longman, Harlow
Garamszegi LZ, Török J, Tóth L, Michl G (2004) Effect of timing and female quality on clutch size in the Collared Flycatcher Ficedula albicollis. Bird Study 51:270–277
Gienapp P, Postma E, Visser ME (2006) Why breeding time has not responded to selection for earlier breeding in a songbird population. Evolution 60:2381–2388
Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J (2008) Climate change and evolution: disentangling environmental and genetic responses. Mol Ecol 17:167–178. doi:10.1111/j.1365-294X.2007.03413.x
Gladbach A, Gladbach DJ, Quillfeldt P (2010) Seasonal clutch size decline and individual variation in the timing of breeding are related to female body condition in a non-migratory species, the Upland Goose Chloephaga picta leucoptera. J Ornithol 151:817–825. doi:10.1007/s10336-010-0518-8
Goodenough AE, Elliot SL, Maitland DP, Hart AG (2009) Variation in the relationship between lay date and clutch size in three cavity-nesting woodland passerines. Acta Ornithol 44:27–36. doi:10.3161/000164509X464858
Gustafsson L (1987) Interspecific competition lowers fitness in collared flycatchers Ficedula albicollis: an experimental demonstration. Ecology 68:291–296
Häkkinen R, Linkosalo T, Hari P (1998) Effects of dormancy and environmental factors on timing of bud burst in Betula pendula. Tree Physiol 18:707–712
Jenni L, Kéry M (2003) Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants. Proc R Soc Lond B 270:1467–1471. doi:10.1098/rspb.2003.2394
Klomp H (1970) The determinants of clutch size in birds: a review. Ardea 58:1–125
Laaksonen T, Ahola M, Eeva T, Väisänen RA, Lehikoinen E (2006) Climate change, migratory connectivity and changes in laying date and clutch size of the pied flycatcher. Oikos 114:277–290
Lack D (1954) The natural regulation of animal numbers. Clarendon, Oxford
Lundberg A, Alatalo RV (1992) The pied flycatcher. T & AD Poyser, London
MacArthur RH (1962) Some generalized theorems of natural selection. Proc Natl Acad Sci USA 48:1893–1897
Moorcroft P, Albon SD, Pemberton JM, Stevenson IR, Clutton-Brock TH (1996) Density-dependent selection in a fluctuating ungulate population. Proc R Soc B 263:31–38
Mueller LD (1997) Theoretical and empirical examination of density-dependent selection. Annu Rev Ecol Syst 28:269–288
Newton I (1998) Population limitation in birds. Academic, London
Nilsson J-Å (1989) Causes and consequences of natal dispersal in the marsh tit, Parus palustris. J Anim Ecol 58:619–636
Przybylo R, Sheldon BC, Merilä J (2000) Climatic effects on breeding and morphology: evidence for phenotypic plasticity. J Anim Ecol 69:395–403
Rowe L, Ludwig D, Schluter D (1994) Time, condition, and seasonal decline of avian clutch size. Am Nat 143:698–722
Salewski V, Bairlein F, Leisler B (2002) Different wintering strategies of two Palearctic migrants in West Africa—a consequence of foraging strategies? Ibis 144:85–93
Sanz JJ (2003) Large-scale effect of climate change on breeding parameters of pied flycatchers in Western Europe. Ecography 26:45–50
Sanz JJ, Potti J, Moreno J, Merino S, Frías O (2003) Climate change and fitness components of a migratory bird breeding in the Mediterranean region. Glob Change Biol 9:1–12
Sheldon BC, Kruuk LEB, Merilä J (2003) Natural selection and inheritance of breeding time and clutch size in the collared flycatcher. Evolution 57:406–420
Tinbergen JM (2005) Biased estimates of fitness consequences of brood size manipulation through correlated effects on natal dispersal. J Anim Ecol 74:1112–1120. doi:10.1111/j.1365-2656.2005.01010.x
van Noordwijk AJ, McCleery RH, Perrins CM (1995) Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J Anim Ecol 64:451–458
Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM (1998) Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc R Soc Lond B 265:1867–1870
von Haartman L (1982) Two modes of clutch size determination in passerine birds. J Yamashina Inst Ornith 14:214–219
Acknowledgments
We greatly appreciate the contribution of Professor Lars von Haartman, whose lifelong interest in hole-breeding Passerines and dedication to data collection made our study possible. We thank Risto A. Väisänen and the Finnish Museum of Natural History for trusting the data to us, and a number of people who participated in field work in the Harjavalta area. Constructive comments and suggestions by Christiaan Both, Tore Slagsvold, Elina Koivisto and two anonymous reviewers improved the manuscript. A suggestion of new analysis by the other reviewer was especially helpful. Eric Le Tortorec kindly checked the language. The Finnish Meteorological Institute provided the temperature data. This study was funded by the Academy of Finland (project 8119367 to TE), the Kone Foundation (grants to MA and EL) and the Emil Aaltonen Foundation (a grant to TL). The data were collected in compliance with Finnish legislation and with appropriate permissions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ola Olsson.
Rights and permissions
About this article
Cite this article
Ahola, M.P., Laaksonen, T., Eeva, T. et al. Selection on laying date is connected to breeding density in the pied flycatcher. Oecologia 168, 703–710 (2012). https://doi.org/10.1007/s00442-011-2135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2135-5