Abstract
The driving factors of survival, a key demographic process, have been particularly challenging to study, especially for winter migratory species such as the Adélie penguin (Pygoscelis adeliae). While winter environmental conditions clearly influence Antarctic seabird survival, it has been unclear to which environmental features they are most likely to respond. Here, we examine the influence of environmental fluctuations, broad climatic conditions and the success of the breeding season prior to winter on annual survival of an Adélie penguin population using mark–recapture models based on penguin tag and resight data over a 16-year period. This analysis required an extension to the basic Cormack–Jolly–Seber model by incorporating age structure in recapture and survival sub-models. By including model covariates, we show that survival of older penguins is primarily related to the amount and concentration of ice present in their winter foraging grounds. In contrast, fledgling and yearling survival depended on other factors in addition to the physical marine environment and outcomes of the previous breeding season, but we were unable to determine what these were. The relationship between sea-ice and survival differed with penguin age: extensive ice during the return journey to breeding colonies was detrimental to survival for the younger penguins, whereas either too little or too much ice (between 15 and 80% cover) in the winter foraging grounds was detrimental for adults. Our results demonstrate that predictions of Adélie penguin survival can be improved by taking into account penguin age, prior breeding conditions and environmental features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adélie penguin (Pygoscelis adeliae) populations at different locations along the Antarctic coastline and offshore islands are showing contrasting trends (Ainley 2002; Forcada et al. 2006). These differences have been explained as differing responses by the penguin populations to a changing environment, in particular the changing frequency of heavy ice years (Fraser and Trivelpiece 1996; Smith et al. 1999). Unravelling precisely what drives these differential changes is difficult because of the complexity of processes influencing penguin population dynamics (Forcada and Trathan 2009; Jenouvrier et al. 2009; Ainley et al. 2010). Some aspects of these contrasting trends may be explained by different survival rates, because survival is a key demographic parameter that contributes to population change. Therefore, understanding the factors which influence survival and establishing environmental linkages with survival will assist in predicting the impacts of climate change on Adelie penguin populations as changes in the Antarctic ecosystem become more apparent (e.g. Croxall et al. 2002; Parkinson 2002, 2004; Murphy et al. 2007; Barbraud et al. 2008; Le Bohec et al. 2008).
Adélie penguin survival is influenced by factors operating differentially in time and space during the summer months when compared with winter. During summer, breeders can be subject to increased pressure as a consequence of increased energy expenditure during reproduction (Ainley and DeMaster 1980; Lescroel et al. 2009), and all birds can suffer leopard seal predation when predictably concentrated at regularly used entry or exit points around colonies (Ainley et al. 2005). Furthermore, because breeders are restricted to feeding in the immediate marine environment around colonies (Croxall and Prince 1980), they are exposed to changes in food abundance in those waters at that time, abandoning their chicks to ensure their own survival in years of low food availability (Lescroel et al. 2009; Wilson 2009). During winter, Adélie penguins swim many hundreds of kilometres away from their breeding colonies (Kerry et al. 1995; Davis et al. 1996, 2001; Clarke et al. 2003b) where they are thought to forage north of the consolidated pack ice and south of the open water (Wilson et al. 2001). Penguin mortality is highest in winter when temperatures drop and the ice becomes its most extensive (Davis et al. 1996; Ainley 2002). Extreme northward sea-ice (Wilson et al. 2001) and minimal sea-ice (Ballerini et al. 2009) are both thought to negatively affect penguins’ ability to reach optimal winter foraging grounds with starvation being possible, especially for younger birds (Ainley 2002) or those with poor fledging body condition (Williams and Croxall 1990). It is uncertain whether predation on Adélie penguins during the inter-breeding period is prominent, but there is some evidence that they are not a common prey item for leopard seals during winter (Siniff et al. 1980; Costa and Crocker 1996; Hall-Aspland and Rogers 2004) despite leopard seals targeting fledglings as they depart some breeding sites for their winter migration (Ainley et al. 2005).
Attributing the cause of mortality depends on the timing of mortality and whether it can be directly observed; during winter, this is extremely difficult. Consequently, explanations for mortality are sought through linkages with some measurable aspects of the ecosystem. Some mortality factors operate directly, while others have a mediating or indirect influence. For example, sea-ice is proposed to affect penguin survival either through its impact on prey accessibility (Wilson et al. 2001; Ballerini et al. 2009), through its positive effect on primary productivity and the food web (Loeb et al. 1997; Murphy et al. 2007) or through direct crushing of penguins (Penney and Lowry 1967; Kerry et al. 2009). The link between survival and potential mediating or indirect factors such as sea-ice extent, the Southern Oscillation Index (SOI), or the Southern Annular Mode (SAM) (e.g. Wilson et al. 2001; Jenouvrier et al. 2006; Ballerini et al. 2009) may not always be obvious, and explanations for such linkages are often speculative. Given the range of ice environments that Adélie penguins inhabit, it is therefore not surprising that conclusions vary as to which aspects of the environment or broader climatic indices influence survival for different populations (e.g. Jenouvrier et al. 2006; Ballerini et al. 2009).
Penguin populations are expected to grow faster with some intermediate amount of ice or frequency of ice years compared with either extreme of too much or too little ice (Smith et al. 1999). This shape relationship between penguin survival and sea-ice has been demonstrated for one Ross Sea Adélie penguin population (Ballerini et al. 2009) and may be realised in other populations for population size changes and previous environmental conditions (Wilson et al. 2001; Kato et al. 2002). Further exploring the link between the physical environment and survival at a range of sites would enhance our understanding of mechanisms driving Adélie penguin population changes and therefore improve our predictive abilities. Béchervaise Island is one of a small number of Adélie penguin populations where direct measurement of survival is possible because of a long-term tagging and resighting program over a 20-year period. A subset of this data was used to estimate survival for this population (Clarke et al. 2003a), and here we extend that analysis to determine underlying factors contributing to the temporal variability in survival.
In this paper, we investigate how Béchervaise Island Adélie penguin survival is associated with, or driven by, measurable attributes of the naturally fluctuating Antarctic environment and whether this varies according to penguin age. We focus on processes occurring during the inter-breeding period rather than on survival of the newly hatched chicks within a breeding season, which has been described elsewhere (Clarke et al. 2002; Emmerson and Southwell 2008). Survival of young Adélie penguins should be more responsive to environmental fluctuations than that of older birds as they encounter the winter environment in their first year with no previous experience (Wilson et al. 2001; Ainley 2002). We also related penguin survival to reproductive outcomes in the breeding season immediately prior winter to examine whether poorer breeding conditions results in reduced survival the following winter. Finally, we determined the association between survival and a range of environmental variables to examine whether an intermediate value of the environment is associated with an optimal penguin survival rate in line with expectations from the Smith et al. (1999) conceptual model of Adélie penguin population growth.
Materials and methods
Adélie penguin chicks were tagged on Béchervaise Island (67°35′S, 62°49′E) near Mawson Station with electronic identification devices implanted subcutaneously (Clarke and Kerry 1998) and resighted in later years. Annual chick tagging commenced in the 1990/1991 split-year breeding season with up to 300 tagged in each year. Low chick numbers in 1994/1995, 2004/2005 and 2005/2006 meant that very few or no chicks were tagged in those years, nor in 2003/2004 due to logistics. The annual detection of tagged birds commenced in 1991/1992 and this paper uses annual detection histories through until 2006/2007. The tags were glass-coated passive transponders (TIRIS; Texas Instruments) which are supplied in sterilised cartridges and can theoretically last a bird’s life time; implanting details are described in Kerry et al. (1993b). Any known deaths between the time of tagging and departure from the colony were recorded and these birds were removed from the analysis. The term resight describes the detection of a bird carrying an electronic tag allowing individual identification. Resighting was by one of three methods: an automated penguin detection gateway, island wide manual detection of nesting birds, and detailed nest surveys at several sub-colonies. Resighting could occur at any stage during the breeding season from mid-October through until March the following calendar year.
Birds were directed through two ‘gateways’ as they moved around the island by small fences constructed around several larger sub-colonies. Each gateway carried a tag-reader that detected the presence and identity of implanted tags as birds crossed the gateway. Adult Adélie penguins typically pass through the gateway as they return to their nesting sub-colonies on the island while young pre-breeders typically wander extensively around the island.
Tagged birds were manually detected on nests during the incubation period in several larger sub-colonies between 1991/1992 and 1998/1999 and in all sub-colonies between 1999/2000 and 2006/2007. Long-handled tag readers were used to detect tags at a distance of about 10–20 cm from the bird. Each bird sitting on a nest was examined for implanted tags during two occasions each year: (1) 23–29 November, corresponding to the time when predominantly breeding males are present, and (2) 11–18 December, when predominantly breeding females are present (Kerry et al. 1993a). This method detects in excess of 98% of birds sitting on nests (unpublished data).
We monitored nests daily in several sub-colonies from the time of peak attendance (mid-November) until the chick(s) crèched (mid-February). The identity of each breeding bird on a nest was recorded during each nest survey along with the presence of either eggs or chicks.
Physical environmental variables
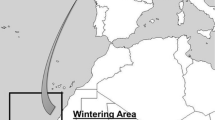
Here, we incorporate physical environmental variables at a temporal and spatial scale that is relevant to the winter migration route of Béchervaise Island Adélie penguins to determine the association between environmental conditions and penguin survival. Based on winter satellite tracks of fledglings and adults, Clarke et al. (2003b) recorded penguins travelling up to 1,500 km away from Béchervaise Island during the inter-breeding period from March until the onset of the following breeding season in October in the years 1995–1998. Because of the large spatial scales involved, we have broadly divided their journey into spatial sectors and months representing different components of their winter migration (Fig. 1).
The expected travel route (indicated by arrows in highlighted area) of Béchervaise Island Adélie penguins (Pygoscelis adeliae) during the inter-breeding period adapted from satellite tracks in Clarke et al. (2003b). Areas with <15, 15–80 and 80% sea-ice concentration (SIC) shown for the beginning of a March, b April, c May and d August 2003 along the coastline between 15 and 80°E. SB-ACC Southern boundary of the Antarctic Circumpolar Current
Initially, the penguins stayed close to the continental shelf break during their rapid westward travel towards their winter foraging grounds to the west of Syowa Station (two sectors defined as OUTBOUND MARCH: between 50 and 65°E during the month of March, and OUTBOUND APRIL: between 30 and 50°E during the month of April; Fig. 1a, b). The birds remained at a similar longitude during the months of May, June and July but travelled northwards as the sea-ice became more extensive (WINTER: between 15 and 30°E; Fig. 1c). They then travelled eastwards in August and September to arrive at the breeding colony in October (RETURN: between 30 and 50°E; Fig. 1d).
We included physical environmental variables for each of these sectors for the relevant months listed above which were considered likely to influence penguin survival during the inter-breeding period. Sea-ice and weather as measured through broad climatic indices were considered the primary measurable aspects of the environment likely to influence survival.
Sea-ice data obtained from satellite images from the National Snow and Ice Data Center (NSIDC) (Cavalieri et al. 1996) were averaged and summed over these longitudinal sectors for each time period. In each case, calculations were based on the ice area between the coastline and 55°S where there is open sea. Sea-ice variables included: (1) total ice area (km2) from the coastline out to 15% sea-ice concentration (SIC), (2) most northerly ice edge latitude at 15% SIC, (3) most northerly ice edge latitude at 80% SIC, (4) foraging ice area (km2), considered as the area of ice between 15 and 80% SIC, (5) distance to ice edge for 15% SIC (km), (6) distance to ice edge for 80% SIC (km), and (7) a measure of ice cover patchiness (ICE SD) calculated as the standard deviation of percentage ice cover for each pixel from the 15% SIC boundary to the coastline.
Because the large number of sea-ice variables leads to the potential for multi-collinearity, we examined pair-wise correlations within each spatial sector for the relevant months in order to reduce, if necessary, the original set to a smaller set of orthogonal parameters. Many of the sea-ice variables were correlated (here we used r > 0.7 as indicative of multi-collinearity between parameters). Our preference when variables were correlated was to include total ice area, and this was highly correlated with ice edge latitudes (both 15 and 80% SIC) and distance to ice edge (both 15 and 80% SIC). This procedure reduced the dataset to three sea-ice variables: ICE AREA, FORAGING ICE AREA and ICE SD. We reduced the parameter set further for the two outbound sectors which were at a time of minimal ice extent during March and April. For these two sectors, we used total ICE AREA which was correlated with the FORAGING ICE AREA. Because ice was minimal in those sectors during the outbound journey in March and April, the ICE SD parameter was based on few values and was not used.
The southern oscillation index (SOI) and the southern annular mode (SAM) were included as broad indicators of climatic conditions around Antarctica. The SOI quantifies the strength of an ENSO event and is calculated from the monthly or seasonal fluctuations in the air pressure difference between Tahiti and Darwin. In the area relevant to this study, positive SOI are associated with decreased temperatures around the Antarctic coastline between 20 and 90ºE (Kwok and Comiso 2002). The SOI was obtained from the Australian Bureau of Meteorology website (www.bom.gov.au) as the average monthly value during the inter-breeding period from March through to the end of September. The SAM is a dominant atmospheric-circulation pattern of the Southern Ocean with positive phases indicating stronger than normal westerly winds around the Antarctic Peninsula (Arrigo et al. 2008) and colder air between 20 and 90ºE during autumn inducing a larger sea-ice cover (Lefebvre and Goosse 2005). Monthly average SAM values were calculated for the inter-breeding period for data obtained from the NOAA Climate Prediction Centre (http://www.cpc.noaa.gov/products/precip/CWlink/daily_ao_index/aao/aao_index.html).
Population parameters indicating penguin or previous breeding season condition
To examine the possibility that penguin survival was related to the conditions either during the breeding season prior to the winter of interest or the condition of the fledglings when they leave the breeding colony, we included Béchervaise Island population parameters for the breeding season immediately prior to the inter-breeding period as covariates in statistical models. Of relevance for penguin survival was annual breeding success (number of chicks reaching crèche per occupied nest) and the foraging trip duration of males and females during the crèching period. In addition, survival of the youngest birds had the potential to be related to chick condition at the end of the breeding season as determined by the mean fledgling weight.
Population parameters were determined according to the CEMP Standard Methods (SC-CAMLR 1997). Breeding success was calculated from island-wide counts of occupied nests and crèched chicks conducted on or around 2 December and 30 January each year, respectively. Foraging trip durations were derived from records obtained from the automated monitoring system of bird crossings and nest censuses of bird presence on nests. Average foraging trip durations were determined for males and females during the crèche period (mid-January through until mid-February). Fledgling weights were obtained for up to 50 chicks during the peak fledging 5-day period each year (generally late February).
Estimating apparent recapture and survival
To estimate recapture and survival probabilities we condensed multiple resightings for each animal to a single detection event for each year. All resighting information was of known aged birds. Resight data was used to determine the most suitable age structure for recapture (p) and survival (ϕ) probabilities and to determine which features of the environment or bird condition most strongly influences these two estimates. To do this, we utilised capture–recapture models in the mark–recapture software MARK v.5.1 (White and Burnham 1999) which estimates “apparent” probabilities where recapture is the probability of being encountered, conditional on being alive and in the sample, and survival is the probability of surviving and returning to the sampling area. Hence, recapture is estimated for each detection effort (i.e. split-year breeding periods) and survival is estimated for the period between detection efforts (i.e. the inter-breeding period).
The first step in the modelling process involves determining the appropriate age structure for the recapture and survival sub-models to define a reference model(s) that satisfactorily describes temporal variation in survival. The process involves goodness-of-fit (GOF) testing, an assessment of the m-array (a matrix of the number of individuals released at each occasion and the number of them next being reencountered at each resighting occasion), and the comparison of models with different age structure specifications. GOF testing assesses the assumptions that: (1) every marked animal present in the population at time (t) has the same probability of recapture (p), and (2) every marked animal in the population immediately after time (t) has the same probability of surviving to time (t + 1). Systematic failure across cohorts indicates a potential need for including age structure. The reference and relevant constant form of that model are then used to determine where variation in survival is related to specific drivers by building ultrastructural CMR models (Grosbois et al. 2008).
Determining the appropriate age structure for modelling survival
The age structure of both the recapture and survival sub-models are of specific interest here because younger penguins may be less likely to survive than older birds and are likely to return to the colony first when they are older than one. Models were defined as a pair of hypotheses to explain the observed variance in the data for each sub-model (one for p and another for ϕ) within MARK. We follow the syntax for model formulation in Lebreton (1992) adapted to our particular interests in age structure. We used RELEASE (Burnham et al. 1987) to examine the GOF of the standard time-dependent CJS model: ϕ(t)p(t). Systematic failure across cohorts of the overall results of Test 2 and Test 3 and the examination of the m-array confirmed the need to incorporate age structure in the recapture and survival sub-models. We used MARK to calculate the overdispersion parameter (\( \hat{c} \)) using the median \( \hat{c} \) approach for our reference model to account for any lack of fit due to overdispersion. We used this approach because of the difficulty in assessing GOF for complex models like this one in mark–recapture analyses (Crawley 2002).
We considered a range of age classes for the recapture sub-model: (1) a single age class, (2a) two age classes: (1, 2+ year), and (3a) three age classes: (1, 2, 3+ years). The data were not able to support further age class differentiation. Because yearlings are unlikely to return to their natal colonies and have subsequent low recapture probabilities (Ballerini et al. 2009), separately identifying survival for birds of ages 0–1 and 1–2 years was not possible. To address this, the first 2 years were set to be equivalent (ϕ 0−1 = ϕ 1−2) when the survival sub-model included age structure. The survival sub-model was hence specified as (1) a single pooled age class, (2b) two age classes: (0–1 = 1–2, 3+ years), (3b) three age classes: (0–1 = 1–2, 3, 4+ years), (4b) four age classes: (0–1 = 1–2, 2, 3, 4+ years), and (5b) five age classes: (0–1, 2, 3, 4, 5+ years). Model specification for “a” models separate the first year from other years while models with a “b” specification have equal first and second year estimates. We also examined fledgling survival with a pooled survival estimate for the older age classes: (2a) two age classes: (0–1, 1+ year) which was possible because robust survival estimates for the older age classes enabled the calculation of the 0–1 age survival parameter separately.
Birds are considered to be of age 0 between when fledglings first leave the breeding colony in March through to the following breeding season when they turn 1 year old. For simplicity, we refer to survival between age 0 and age 1 as “fledgling” survival, survival from age 1 to age 2 as “yearling” survival and survival from any age x to x + 1 as “sub-adult and adult” survival (where x ≥ 1 year in the case of model “a” specification, and x ≥ 2 years in the case of model “b” specification). We acknowledge that for model 2a, sub-adult and adult survival includes yearlings as well as individuals greater than 1 year old.
Both sub-models included time variant (t) and constant (.) options because of the potential for inter-annual variability in the environment to affect survival and recapture. In the case of recapture probabilities, temporal variability also allows for modifications of the gateway or changes in the field protocol. These options were specified in models for each age class separated by a forward slash. Hence the model with two age classes (0, 1+ years) with time varying fledgling survival, constant adult survival and time varying fledgling and pooled sub-adult and adult recapture would be specified as: ϕ(2a t/.) p(2a t/t)).
We compared models with different age structures and time varying or constant options for the survival and recapture sub-models with Akaike’s Information Criterion (Burnham and Anderson 2002). For each model, MARK computed maximum likelihood estimates (MLE) of parameters and the maximised value of the log-likelihood function. Candidate models were ranked on the basis of quasi-likelihood values ΔQAICc and QAICc weights (w i ) corrected for small sample size and model over-dispersion using \( \hat{c} \). ΔQAICc values represent the difference between each model’s QAICc and the minimum value from the candidate set. Models with ΔQAICc ≤ 2 are considered strongly plausible, 3 ≤ ΔQAICc ≤ 7 are considerably less plausible, and models with ΔQAICc ≥ 10 are improbable (Burnham and Anderson 2002). All models used the logit link function. We examined parameter convergence for models before their inclusion in the results tables to avoid over-parameterisation. The a priori candidate model set was a combination of recapture and survival sub-models (see Table 1 for a list of models).
Including model covariates to determine potential driving factors
Once the most appropriate age structure for the survival and recapture sub-models was identified (the reference model), environmental variables, broad climatic indices and population parameters were introduced as covariates to identify which factors were associated with the temporal variation in survival. These analyses were used to determine the statistical support for and magnitude of a covariate on survival (Grosbois et al. 2008), where covariates were based on the distinct geographic locations and times during the over-winter period as described above. While environmental covariates are likely to influence recapture estimates, changes in field protocols may have a greater contribution on capture probabilities than other factors and hence environmental and other covariates were only included for the survival sub-model. To simplify modelling and to minimise the number of parameters in models, covariates were initially included in turn in isolation.
We used an information-theoretic approach to rank competing covariate models in the candidate set and to compare them with the fully time-varying and constant baseline models. In this process, two models which vary by less than two AIC points are considered as having identical support (Burnham and Anderson 2002; Grosbois et al. 2008). Any particular covariate model (co) was considered as having statistical support when the model was as well supported as the general time-varying model (t) and better supported than the baseline constant model (cst). Hence, the effect of a covariate was statistically supported if Δ(cst−co) ≤ −2 and Δ(t-co) ≤ 2 where Δ(cst-co) = QAICc(cst) − QAICc(co) and Δ(t-co) = QAICc(t) – QAICc(co) (Grosbois et al. 2008). Models with Δ(cst-co) < −2 and Δ(t-co) > 2 are an improvement on the constant model, and have some support, but do not express the full temporal variation in survival.
The magnitude of effect of the covariate models was determined by a “coefficient of determination” equivalent. The fraction of the temporal variation in survival accounted for by covariates was calculated as: \( \% {\text{ Deviance explained }} = \frac{{{\text{Dev}}({\text{cst}}) - {\text{Dev}}({\text{co}})}}{{{\text{Dev}}({\text{cst}}) - {\text{Dev}}({\text{t}})}} \), where Dev is the models deviance, cst is the constant model, co is the covariate model, and t is the fully time-varying model. This value quantifies the relative importance of the focal covariate as compared to other drivers in generating variation in the dependent variable (Grosbois et al. 2008). Covariates that exceeded 0.2 in isolation were likely to account for more than 20% of the temporal variation in survival and could be considered as potentially influential.
Because the most parsimonious model included two age classes for penguin survival, covariates were included in models for each age class separately using the reference model with different baseline constant models for each of the two age classes. The baseline constant model for each age class held that particular age class survival parameter constant while the other was time-varying. In either case, covariate models were compared with time-varying and baseline constant models to assess whether covariates affect survival of each age class independently.
Multiple covariate models were examined using the same principles with covariates selected on the basis that they explained the largest percent deviance in isolation. To reduce the chance of over-parameterisation, only two covariates were included at the same time and the degree of correlation between covariates examined prior their inclusion. The most appropriate multiple covariates were determined for each age class separately.
Results
Because the small number of chicks tagged in the breeding seasons of 1990/1991, 1991/1992 and 2003/2004 result in large standard errors for recapture and survival estimates, we only include estimates based on chicks tagged between 1992/1993 and 2002/2003, and the detection histories recorded between 1993/1994 and 2005/2006. A total of 1,187 (43%) birds out of the total 2,734 chicks tagged between 1991/1992 and 2002/2003 were detected at Béchervaise Island during subsequent breeding seasons. Survival estimates are based on a minimum of 4 years of resight data when most birds have returned to the island at least once (Ainley 2002).
Goodness-of-fit assessment of the fully time-dependent CJS model (ϕ(t)p(t)) showed lack of homogeneity of recapture and survival rates between newly tagged and previously tagged birds (Test 2 + Test 3: χ 2 = 3,754.66, df = 64, P ≤ 0.001). An examination of the m-array showed that fledglings are less likely to be observed again in subsequent years and are less likely to return to the colony in the following year compared with previously tagged birds. 87% of previously tagged birds were seen next in the following year compared with only 0.8% of newly tagged birds and 81% of previously tagged birds were seen again compared with 56% of newly tagged birds. On the basis of this, age structure was incorporated into both sub-models and an overdispersion adjustment factor (\( \hat{c} = 1. 5 4 \)) used to address any additional lack of fit due to overdispersion. Model support is similar when varying \( \hat{c} \) between 1 and 3.
The most parsimonious model (model with ΔQAICc = 0; Table 1a) included a survival sub-model with two age classes (0–1 = 1–2, 2+ years) and a recapture sub-model including three age classes (0, 1, 2+ years). Including further subdivision of age classes in the survival sub-model did not improve model support (Table 1a). Including time-varying survival estimates improved model fit irrespective of age class but was only supported in the recapture sub-model for yearlings and sub-adults and adults (Table 1a; Fig. 2a, b). We selected the most parsimonious model (ϕ(2b t/t) p(3a ./t/t)) as our reference model for introducing environmental, climatic and population parameters as covariates. The alternative model with fledgling survival and a pooled older age class of sub-adults and adults (0, 1+ years) had almost as much support as the model with equal survival in the penguins first and second years (0–1, 2+ years) (ΔQAICc = 2.01; Table 1b). Because of this, we present survival estimates for both models. Survival estimates for both models are synchronous through time (Fig. 2a) with reduced survival estimated for the fledglings compared with the pooled estimate obtained for fledglings and yearlings. Estimates of survival for the sub-adult and adult birds and recapture estimates were equivalent between models.
Estimates from the two most parsimonious age class structure models for a apparent survival, two age classes: either equal survival for fledglings and yearlings (model 1: dark triangles), fledgling survival (model 2: light triangles), and pooled sub-adult and adult survival (open squares), and b apparent recapture, three age classes: fledglings, yearlings, and adults. Survival estimated for period between split-year breeding seasons and recapture estimated for each breeding season
In both cases, survival was more variable and lower for the younger birds than survival for the sub-adults and adults (Fig. 2a). Survival for both age classes dropped substantially in the inter-breeding period between 2002/2003 and 2003/2004 irrespective of which modelling framework was used. Mean annual survival between 1992/1993 and 2006/2007 was 53% (SD = 0.149) for fledglings (model 2) or 69% (SD = 0.109) for fledglings and yearlings (model 1), and 88% (SD = 0.072) for sub-adults and adults. Fledglings and yearling survival was relatively more variable than for adults (CV = 16 and 8%, respectively). Apparent recapture probabilities were consistently low and near zero (0.006) for fledglings (Fig. 2b), although fixing this term at zero did not improve model fit (QAICc = 1,8332.5, k = 44, Deviance = 10,985.5) as 1 year olds were occasionally detected on the island. Yearling recapture probabilities varied between 0.1 and 0.75, with consistently higher adult recapture probabilities (0.55–0.85). There was a tendency for adult recapture probabilities to increase through time which was almost certainly due to improvements with automated detection machines and slight improvements in field protocols.
Including model covariates
Because the ϕ(2b t/t) p(3a ./t/t) model had the minimal QAICc, we considered it to be the most suitable age structure for both the survival and recapture sub-models to examine the inclusion of environmental variables and population parameters as covariates. We use this age structure with constant fledgling or adult survival [ϕ(2b ./t) p(3a ./t/t) or ϕ(2b t/.) p(3a ./t/t), respectively] as constant base models for introducing covariates. We also examined the introduction of covariates on the alternative model structure ϕ(2a t/t) p(3a ./t/t), and found the qualitative results to be the same.
On the basis of this, fledgling and yearling survival was most closely associated with the RETURN part of their journey in August and September between 30 and 50°E and explained 19.3% of the variance (Figs. 1d, 3a; Table 2a). The model incorporating the total ICE AREA during the WINTER months of May, June and July between 15 and 30°E (Figs. 1c, 3b; Table 2a) was the second most supported covariate and explained 15.8% of the variance. There was no evidence to suggest that including the terms as a quadratic function improved model fit. Various other environmental variables included as covariates improved model fit compared with the constant base model (all parameters not shown in bold in Table 2a). Breeding success was the only population parameter that improved model fit from the constant base model (Table 2b). In isolation, this parameter explained 7.3% of the temporal variability in survival (Table 2b). None of the covariates included in the fledgling and yearling survival model explained as much of the temporal variability as the fully time-dependent model.
Apparent survival estimates from the fully time-dependent model plotted against the two most influential covariates for each age class with lines indicating model predictions. Most influential covariates for fledgling and yearling survival were a total ice area during the return part of their winter migration, and b total ice area during winter, and for sub-adult and adult survival, c winter foraging ice area, and d outbound March ice area
The association between environmental variables and sub-adult and adult survival (2+ year olds) was more apparent than for fledgling survival (Table 3a). Most environmental variables improved model fit compared with the base model (variables not shown in bold in the Δ(cst-co) column in Table 3a). In particular, the quadratic relationship with WINTER FORAGING ICE AREA had as much support as the fully time-dependent model (ΔQAICc < 2; Table 3a) and explained 81.3% of adult survival temporal variability (Figs. 1c, 3c). The second most supported environmental variable was a quadratic relationship with OUTBOUND MARCH ICE AREA between 50 and 65°E during the penguin’s outbound journey (Table 3a; Figs. 1a, 3d). In both cases, there was clear statistical evidence that a quadratic term improved model fit over the linear model. Neither the SAM nor the SOI were associated strongly with adult survival. Including population parameters as covariates did not explain any of the temporal variability associated with adult survival (Table 3b).
Inclusion of multiple covariates
Including multiple covariates increased the percent deviance explained for fledgling and yearling survival but did not improve model support for sub-adult and adult survival (Table 4a, b). The subsequent survival of the fledglings was reduced in years with reduced breeding success in conjunction with extensive ice during their return journey (Table 4a; Fig. 4a). This model explained 34.2% of the deviance associated with fledging and yearling survival (Table 4a) and was substantially better supported than other models including two covariates or the covariates in isolation (ΔQAICc = 6.2 between that model and the next most supported model; Table 2). The next two models (RETURN ICE AREA and OUTBOUND APRIL ICE AREA and RETURN ICE AREA and WINTER ICE AREA) had equal support (ΔQAICc = 0.8 between the models) and explained 27.7 and 26.9% of the deviance, respectively (Table 4a). For both models, extensive ice during their return journey coupled with extensive ice during either April or the winter months resulted in reduced penguin survival (Fig. 4b, c).
Model estimates of fledgling and yearling survival as a function of a breeding success and return ice area, b return ice area and April outbound ice area, and c return ice area and winter ice area. Models explained 34, 28 and 27% of the deviance associated with temporal variability in survival, respectively
In contrast, the most supported model for sub-adult and adult survival included WINTER FORAGING ICE AREA as a quadratic term (Table 4b; Fig. 5a). This model was better supported than any other, including models with two covariates (ΔQAICc = 15.9 between that model and the next most supported model; Table 4b). The next most supported model included WINTER FORAGING ICE AREA and OUTBOUND MARCH ICE AREA and explained 63.7% of the deviance (Table 4b; Fig. 5b).
Discussion
Adélie penguin survival is broadly considered to be driven by processes occurring during the Antarctic winter (Davis et al. 1996; Ainley 2002), although in some locations summer-time predation is also apparent (Penney and Lowry 1967; Ainley and DeMaster 1980; Ainley 2002; Hall-Aspland and Rogers 2004). Without direct observations, however, the precise timing and cause of mortality is difficult to assess. With this caveat in mind, we divided the Béchervaise Island Adélie penguin winter migration into an outbound component, a winter foraging period and a return to the colony journey in order to improve our understanding of environmental influences on penguin survival during the inter-breeding period. On this basis, the sea-ice environment some 1,500 km away from their breeding site (Clarke et al. 2003b) during the winter period of rapid northward sea-ice expansion was most critical to the survival of the older birds (Fig. 1c). In contrast, younger penguin survival was more variable between years and, while driven to some extent by the sea-ice environment and conditions in the breeding season prior to fledging, a large amount of the variability associated with their survival remains unexplained.
In line with expectations, younger Adélie penguins had reduced survival compared with older penguins which was probably because of their lack of experience in foraging or predator avoidance (Ainley 2002). While leopard seals hunt young Adélie penguins as they leave some breeding sites (Penney and Lowry 1967; Hall-Aspland and Rogers 2004; Ainley et al. 2005), this has not been observed at Béchervaise Island over the last 20 years despite leopard seals being in the waters in and around the coastline. The Béchervaise Island survival estimates are comparable to other Eastern Antarctic populations for which direct estimates have been made (Jenouvrier et al. 2006; Ballerini et al. 2009; Lescroel et al. 2009). However, survival of the younger birds at Béchervaise Island (BI) is higher than at Edmonson Point (EP) and these populations have higher survival for the older birds than at Point Géologie (PG) and Cape Crozier (CC) [BI: 70% (or 56% from model 2) and 86% for young and older (>2) age classes respectively; EP: 34 and 85%, respectively (Ballerini et al. 2009); CC: 71% for breeding birds (Lescroel et al. 2009); PG: 76% for adults (Jenouvrier et al. 2006)]. Whether these differences are due to different field protocols or modelling frameworks is unclear as is the ecological relevance of these differences. The Cape Crozier estimates may also be reduced as a consequence of deleterious effects from flipper banding (Lescroel et al. 2009).
Our expectation that younger birds would be more susceptible to fluctuations in the physical environment than the older birds (as discussed in Ainley 2002) was unfulfilled for the environmental variables considered here. The higher relative temporal variability of survival associated with the younger birds (CV = 16% for the younger birds cf. 8% for the sub-adults and adults), however, potentially indicates a greater responsiveness to driving factors. While including covariates improved models for this age group, a large proportion of the temporal variability in survival remained unaccounted for (around 65%). The large inter-annual variability in fledgling and yearling survival most closely mirrored the sea-ice environment at the time of year when ice is at maximal extent. Extensive ice at this time of year may be associated with a northward extension of consolidated ice which limits access to open water (e.g. Wilson et al. 2001) or could mediate predation of young, inexperienced penguins by predators such as the leopard seal. Telemetry studies off Dronning Maud Land suggest that a portion of the adult leopard seal population may spend the winter in the open ocean close to the ice edge (Nordøy and Blix 2009) and hence any northward extension of ice at this time could increase the spatial overlap between penguins and leopard seals. Extensive ice when associated with reduced air temperatures and stronger winds may also indicate harsher conditions on the ice to which penguins are likely to be vulnerable. A lack of experience may therefore be particularly crucial for the younger penguins during years with extreme conditions. In conjunction with extensive ice, our models predict reduced younger bird survival in years when breeding success is also low. It may be that reduced breeding success indicates continued difficult conditions immediately after the austral summer or poor chick condition at the end of the breeding season.
In stark contrast to the vagaries associated with fledgling and yearling survival, sub-adult and adult survival was strongly associated with sea-ice extent during winter. A quadratic relationship between survival and ice extent between 15 and 80% sea-ice concentration explained 82% of the temporal variability in survival. This form of ice is considered suitable foraging and resting habitat for the Adélie penguin (Wilson et al. 2001). In this case, sub-adult and adult penguin survival was higher when it was not at either extreme of too little or too much of this sea-ice concentration. This survival/sea-ice response is similar to the Edmonson Point Adélie penguin population in relation to sea-ice extent anomalies in the Ross Sea (Ballerini et al. 2009), but differs from the Cape Crozier study where there was no significant association with environmental conditions (Lescroel et al. 2009). In contrast to the Ross Sea, where extensive ice moves foraging activities north of the southern boundary of the Antarctic Circumpolar Current (SB-ACC) (Wilson et al. 2001; Ballerini et al. 2009) where prey is expected to be less available (Nicol et al. 2000), satellite images in extensive sea-ice years (e.g. 2003) in the Béchervaise Island penguin winter foraging region indicate a sea-ice edge clearly south of the SB-ACC. In fact, the northerly extent of sea-ice in the year with the lowest survival was not much further north than in other years, with the notable difference being a much larger component of the sea-ice between 15 and 80% SIC. Reduced survival for the Béchervaise Island penguin population in these conditions may be due to a lack of stable ice from which to forage, escape predators and to rest and that this is reflected in the large component of sea-ice of this concentration. There is also likely to be an interaction between ice concentration and predator distribution and time lags between ice and alternative prey items impacting penguin survival (Jenouvrier et al. 2006).
An “optimal” intermediate relationship between sea-ice and penguin demography, in particular population growth rate, is proposed as the underlying principle explaining contrasting trends for Adélie penguin populations (Smith et al. 1999). This type of relationship is also important for krill (Euphausia superba) productivity (Weidenmann et al. 2009), a major food source for Adélie penguins. However, such a relationship is not necessarily intuitively obvious and may only be apparent because different underlying processes engender different responses at the ends of the environmental variables’ range. For example, if some sea-ice is required as a platform to rest between foraging bouts or as a reliable location of prey, but extensive sea-ice limits access to food because fewer gaps are in the ice in which to forage, then an optimal intermediate relationship might exist (Smith et al. 1999; Ainley 2002; Croxall and Nicol 2004; Forcada et al. 2006). Furthermore, the range of environmental conditions observed in studies such as this are likely to be less extreme than the sea-ice reduction predicted to generate dramatic declines in Emperor penguin populations (Jenouvrier et al. 2009) or the heavier ice predicted to negatively affect Adélie penguin survival (Lescroel et al. 2009). The degree to which relationships between penguin demography and the ice environment hold to an optimal intermediate pattern may only be obvious with additional data associated with more extreme environmental conditions.
A variety of environmental parameters reportedly influence penguin survival although details of which aspects are important differ for different populations (Wilson et al. 2001; Jenouvrier et al. 2006; Le Bohec et al. 2008; Ballerini et al. 2009). For example, the Point Géologie Adélie penguin survival is linked to the broad environmental index SOI (Jenouvrier et al. 2006), but the Edmonson Point (Ballerini et al. 2009) and Béchervaise Island (this study) populations are not. Broader scale climatic processes are also most likely to influence seabirds through their influence on, or teleconnections with, the local scale environment (Grosbois et al. 2008) and, because of this, we have tried here to ascertain the level of collinearity between different aspects of the environment to better understand the factors that are important for penguin survival. For example, the area of ice between the Antarctic coastline and the ice edge was strongly correlated with the distance to the 80 and 15% ice edges and the area of each of these ice concentrations. Hence, the ice area measure could reflect other aspects of the sea-ice environment such as ice concentration or distance to the ice edge, which could also influence penguin demography. Furthermore, environmental features which can influence penguin demography, such as the number, size and location of ice cracks, are readily accessible only from recent satellite images but not for the entire period of this study. The dependencies between various aspects of the sea-ice environment or broader climatic indices need to be kept in mind when interpreting results from studies such as these.
By linking penguin survival to the ice condition encountered during the period when mortality is thought to occur, our survival estimates reflect an assumed immediate interaction between the physical environment and penguins. However, the physical environment also influences the marine environment through primary production (Loeb et al. 1997; Weidenmann et al. 2009), and multi-year time-lags exist between oceanographic variability, Antarctic krill recruitment and growth, and the subsequent impact on predator populations (Murphy et al. 2007). Recent work indicates an area of high primary productivity northwest of Syowa Station (Arrigo et al. 2008) with higher Antarctic krill densities in the eastern limb of the Weddell Gyre (Jarvis et al. 2010) near the Béchervaise Island penguins’ presumed winter foraging grounds. In this region, Antarctic krill have an extensive north–south distribution, from the shelf break to at least 62°S (Jarvis et al. 2010). This area is also south of the SB-ACC where krill biomass is highest (Nicol et al. 2000). It is possible that the Béchervaise Island Adelie penguin population is deliberately seeking this area as a winter foraging ground because of its high productivity. It then stands to reason that their very survival is likely to be responsive to changes in the environment in that area and, certainly, the interplay between the ice edge and the location of the SB-ACC (Wilson et al. 2001; Ainley 2002; Jenouvrier et al. 2006). Where possible, time lags between primary productivity, its drivers and the subsequent influence on penguins as well as their response to their winter environment need to be incorporated into studies on penguin survival.
References
Ainley DG (2002) The Adélie penguin - bellwether of climate change. Columbia University Press, New York
Ainley DG, DeMaster DP (1980) Survival and mortality in a population of Adélie Penguins. Ecology 61:522–530
Ainley DG, Ballard G, Karl BJ, Dugger KM (2005) Leopard seal predation rates at penguin colonies of different size. Antarct Sci 17:335–340
Ainley DG, Russell J, Jenouvrier S, Woehler E, Lyver POB, Fraser WR, Kooyman GL (2010) Antarctic penguin response to habitat change as Earth’s troposphere reaches 2C above preindustrial levels. Ecol Monogr 80:49–66
Arrigo KR, van Dijken GL, Bushinsky S (2008) Primary production in the Southern Ocean, 1997–2006. J Geophys Res 113:C08004. doi:08010.01029/02007JC004551
Ballerini T, Tavecchia G, Olmastroni S, Pezzo F, Focardi S (2009) Nonlinear effects of winter sea ice on survival probabilities of Adélie penguins. Oecologia 161:253–265
Barbraud C, Weimerskirch H, Hinke J, Bost C-A, Forcada J, Trathan PN, Ainley DG (2008) Are king penguin populations threatened by Southern Ocean warming? Proc Natl Acad Sci USA 105:E38
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burnham KP, Anderson DR, White GC, Brownie C, Pollock KH (1987) Design and analysis methods for fish survival experiments based on release-recapture. Bethesda, Maryland
Cavalieri D, Parkinson C, Gloersen P, Zwally HJ (1996) Sea ice concentrations from Nimbus-7 SMMR and DMSP SSM/I passive microwave data, [1990–2007]. In. Boulder, CO, USA: National Snow and Ice Data Center
Clarke J, Kerry K (1998) Implanted transponders in penguins: implantation, reliability, and long term effects. J Field Ornithol 69:149–159
Clarke J, Kerry K, Irvine L, Phillips B (2002) Chick provisioning and breeding success of Adélie penguins at Béchervaise Island over eight successive seasons. Polar Biol 25:201–230
Clarke J, Emmerson L, Townsend A, Kerry K (2003a) Demographic characteristics of the Adélie Penguin population at Béchervaise Island after 12 years of study. CCAMLR Sci 10:53–74
Clarke J, Kerry K, Fowler C, Lawless R, Eberhard S, Murphy R (2003b) Post-fledging and winter migration of Adélie penguins Pygoscelis adeliae in the Mawson region of East Antarctica. Mar Ecol Prog Ser 248:267–278
Costa DP, Crocker DE (1996) Marine mammals of the Southern Ocean. Foundations for ecological research west of the Antarctic Peninsula. Antarct Res Ser 70:287–301
Crawley MJ (2002) Statistical computing: an introduction to data analysis using S-plus. Wiley, West Sussex
Croxall JP, Nicol SC (2004) Management of Southern Ocean fisheries: global forces and future sustainability. Antarct Sci 16:569–584
Croxall JP, Prince PA (1980) Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol J Linn Soc 14:103–131
Croxall JP, Trathan PN, Murphy EJ (2002) Environmental change and Antarctic Seabird populations. Science 297:1510–1514
Davis LS, Boersma PD, Court GS (1996) Satellite telemetry of the winter migration of Adélie penguins (Pygoscelis adeliae). Polar Biol 16:221–225
Davis LS, Harcourt RG, Bradshaw CJA (2001) The winter migration of Adelie penguins breeding in the Ross Sea sector of Antarctica. Polar Biol 24:593–597
Emmerson LM, Southwell CJ (2008) The effect of sea ice on Adelie penguin reproductive performance. Ecology 89:2096–2102
Forcada J, Trathan PN (2009) Penguin responses to climate change in the Southern Ocean. Glob Change Biol 15:1618–1630
Forcada J, Trathan PN, Reid K, Murphy EJ, Croxall JP (2006) Contrasting population changes in sympatric penguin species in association with climate warming. Glob Change Biol 12:411–423
Fraser WR, Trivelpiece WZ (1996) Factors controlling the distribution of seabirds: winter-summer heterogeneity in the distribution of Adélie penguin populations. Foundations for ecological research west of the Antarctic Peninsula. Antarct Res Ser 70:257–272
Grosbois V, Giminez O, Gaillard J-M, Pradel R, Barbraud C, Clobert J, Moller AP, Weimerskirch H (2008) Assessing the impact of climate variation on survival in vertebrate populations. Biol Rev 83:357–399
Hall-Aspland SA, Rogers TL (2004) Summer diet of leopard seals (Hydrurga leptonyx) in Prydz Bay, Eastern Antarctica. Polar Biol 27:729–734
Jarvis T, Kelly N, Kawaguchi S, van Wijk E, Nicol S (2010) Acoustic characterisation of the broad-scale distribution and abundance of Antarctic krill (Euphausia superba) off East Antarctica (30–80 E) in January–March 2006. Deep-Sea Res II doi:10.1016/j.dsr2.2008.06.013
Jenouvrier S, Barbraud C, Weimerskirch H (2006) Sea ice affects the population dynamics of Adelie penguins in Terre Adelie. Polar Biol 29:413–423
Jenouvrier S, Caswell H, Barbraud C, Holland M, Stroeve J, Weimerskirch H (2009) Demographic models and IPCC climate projections predict the decline of an emperor penguin population. Proc Natl Acad Sci USA 106:1844–1847
Kato A, Coudert YR, Naito Y (2002) Changes in Adélie penguin breeding populations in Lützow-Holm Bay, Antarctica, in relation to sea-ice conditions. Polar Biol 25:934–938
Kerry KR, Clarke JR, Else GD (1993a) Identification of sex of Adélie penguins from observation of incubation birds. Wildl Res 29:725–732
Kerry KR, Clarke JR, Else GD (1993b) The use of an automated weighing and recording system for the study of the biology of Adélie penguins (Pygoscelis adeliae). NIPR Symp Polar Biol 6:62–75
Kerry KR, Clarke JR, Else GD (1995) The foraging range of Adélie Penguins at Bechervaise Island, Mac.Robertson Land, Antarctica as determined by satellite telemetry. In: Dann P, Norman I, Reilly P (eds) The Penguins. Surrey Beatty, Australia, pp 216–243
Kerry KR, Irvine L, Beggs A, Watts J (2009) An unusual mortality event among Adélie penguins in the vicinity of Mawson Station, Antarctica. In: Kerry KR, Riddle M (eds) Health of Antarctic wildlife: a challenge for science and policy, pp 107–112
Kwok R, Comiso JC (2002) Southern ocean climate and sea ice anomalies associated with the southern oscillation. J Clim 15:487–501
Le Bohec C, Durant JM, Gauthier-Clerc M, Stenseth NC, Park Y-H, Pradel R, Grémillet D, Gendner J-P, Le Maho Y (2008) King penguin population threatened by Southern Ocean warming. Proc Natl Acad Sci USA 105:2493–2497
Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modelling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Lefebvre W, Goosse H (2005) Influence of the Southern Annular Mode on the sea ice-ocean system: the role of the thermal and mechanical forcing. Ocean Sci 1:145–157
Lescroel A, Dugger KM, Ballard G, Ainley D (2009) Effects of individual quality, reproductive success and environmental variability on survival of a long-lived seabird. J Anim Ecol 78:798–806
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Murphy EJ, Trathan PN, Watkins JL, Reid K, Meredith MP, Forcada J, Thorpe SE, Johnston NM, Rothery P (2007) Climatically driven fluctuations in Southern Ocean ecosystems. Proc R Soc Lond B 274:3057–3067
Nicol S, Pauly T, Bindoff NL, Wright S, Thiele D, Hosie G, Strutton PG, Woehler E (2000) Ocean circulation off east Antarctica affects ecosystem structure and sea-ice extent. Nature 406:504–507
Nordøy ES, Blix AS (2009) Movements and dive behaviour of two leopard seals (Hydrurga leptonix) off Queen Maud Land, Antarctica. Polar Rec 32:263–270
Parkinson CL (2002) Trends in the length of the Southern Ocean sea-ice season, 1979–1999. Ann Glaciol 34:435–440
Parkinson CL (2004) Southern Ocean sea ice and its wider linkages: insights revealed from models and observations. Antarct Sci 16:387–400
Penney RL, Lowry G (1967) Leopard seal predation on Adélie penguins. Ecology 48:879–882
SC-CAMLR (1997) CCAMLR ecosystem monitoring program: standard methods for monitoring studies. CCAMLR, Hobart
Siniff D, Stone S, Reichle D, Smith T (1980) Aspects of leopard seals (Hydrurga leptonyx) in the Antarctic pack ice. Antarct J U S 15:160–161
Smith RC, Ainley D, Baker K, Domack E, Emslie S, Fraser B, Kennett J, Leveter A, Mosley-Thompson E, Stammerjohn S, Vernet M (1999) Marine ecosystem sensitivity to climate change. Bioscience 49:393–404
Weidenmann J, Creswell KA, Mangel M (2009) Connecting recruitment of Antarctic krill and sea ice. Limnol Oceanogr 54:799–811
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46(Supplement):120–139
Williams TD, Croxall JP (1990) Is chick fledging weight a good index of food availability in seabird populations. Oikos 59:414–416
Wilson D (2009) Causes and benefits of chick aggregations in penguins. Auk 126:688–693
Wilson PR, Ainley DG, Nur N, Jacobs SS, Barton KJ, Ballard G, Comiso JC (2001) Adélie Penguin population change in the Pacific sector of Antarctica: relation to sea-ice extent and the Antarctic circumpolar current. Mar Ecol Prog Ser 213:301–309
Acknowledgments
We thank the Béchervaise Island field teams, in particular Judy Clarke, Megan Tierney and Knowles Kerry who established monitoring at Béchervaise Island. Angela Bender created the maps and Ben Raymond provided daily sea-ice data. Rob Massom and Alex Fraser provided suggestions regarding sea-ice data interpretation and Mark Bravington provided statistical advice. Andy Townsend developed the database and various engineers constructed, maintained and improved detection gateways and tag readers. Steve Nicol and Jemery Day provided useful suggestions for the manuscript. This project was supported by the AAD ASAC project #2722. Attachment of electronic tags to penguins and other procedures were with approval from the Australian Antarctic Division’s Animal Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Markku Orell.
Rights and permissions
About this article
Cite this article
Emmerson, L., Southwell, C. Adélie penguin survival: age structure, temporal variability and environmental influences. Oecologia 167, 951–965 (2011). https://doi.org/10.1007/s00442-011-2044-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2044-7