Abstract
Replacement of a native species by a nonnative can have strong effects on ecosystem function, such as altering nutrient cycling or disturbance frequency. Replacements may cause shifts in ecosystem function because nonnatives establish at different biomass, or because they differ from native species in traits like foraging behavior. However, no studies have compared effects of wholesale replacement of a native by a nonnative species on subsidies that support consumers in adjacent habitats, nor quantified the magnitude of these effects. We examined whether streams invaded by nonnative brook trout (Salvelinus fontinalis) in two regions of the Rocky Mountains, USA, produced fewer emerging adult aquatic insects compared to paired streams with native cutthroat trout (Oncorhynchus clarkii), and whether riparian spiders that depend on these prey were less abundant along streams with lower total insect emergence. As predicted, emergence density was 36% lower from streams with the nonnative fish. Biomass of brook trout was higher than the cutthroat trout they replaced, but even after accounting for this difference, emergence was 24% lower from brook trout streams. More riparian spiders were counted along streams with greater total emergence across the water surface. Based on these results, we predicted that brook trout replacement would result in 6–20% fewer spiders in the two regions. When brook trout replace cutthroat trout, they reduce cross-habitat resource subsidies and alter ecosystem function in stream-riparian food webs, not only owing to increased biomass but also because traits apparently differ from native cutthroat trout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence is mounting that nonnative species have effects on ecosystem function that are different than native species. Nonnative species often exclude native species, but can also alter processes that control ecosystem function (Parker et al. 1999; Hooper et al. 2005). Nonnative plants have been reported to alter nutrient cycling, geomorphology, and hydrology, and increase fire frequency and intensity (Levine et al. 2003; Gordon 2008). Invasive mollusks can increase nutrient concentrations, shift the assemblage of primary producers, and dominate macroinvertebrate secondary production (Strayer et al. 1999; Carlsson et al. 2004; Hall et al. 2006). However, despite these examples, explicit comparisons have been made only in a few cases to measure the magnitude of these effects, especially among vertebrate animals.
The magnitude of these effects of nonnative species on ecosystems depends on the outcome of the invasion, and on species-specific traits that can alter ecosystem function. Invaders may add to, or replace, native species (Sax and Gaines 2003), and if they replace them, they may do so at higher, equal, or lower density or biomass. Moreover, even if the nonnative species replaces a native species at the same density, the individuals may have a greater, equal, or lesser effect owing to differences in species-specific traits such as rates of nutrient cycling or feeding behavior (Parker et al. 1999). Understanding whether the consequences following species replacement result from, for example, greater density (or biomass) or different traits is critical for those who manage invasions. Eradication of established nonnatives is difficult and expensive (Simberloff 2003), so if a nonnative species replaces a native at the same density, and only species identity is changed but ecosystem function is not altered, then managers or the public may ask whether such efforts are worthwhile (e.g., Quist and Hubert 2004).
Salmonid fishes offer an ideal opportunity to compare effects of closely-related and apparently similar native and nonnative vertebrates on ecosystem function. Salmonids have been introduced worldwide for fish culture and recreational angling, which has resulted in widespread invasions on several continents (Rahel 2002; McDowall 2006; Fausch 2008). Recent research shows that they alter the flux of emerging insects from aquatic ecosystems to riparian zones, which is an important ecosystem function that supports riparian consumers, such as spiders, lizards, birds, and bats (Nakano and Murakami 2001; Baxter et al. 2005). For example, trout introduced to historically fishless lakes apparently reduce emerging insects which feed amphibian and avian predators (Finlay and Vredenburg 2007; Epanchin et al. 2010). Likewise, Baxter et al. (2004) found that, when nonnative rainbow trout (Oncorhynchus mykiss) were added to a stream community with native Dolly Varden charr (Salvelinus malma) in a field experiment, the trout indirectly decreased the emergence of adult aquatic insects by causing the charr to forage more on their benthic larvae, and thereby indirectly reduced spider density in the adjacent riparian area. However, we know of no studies to date measuring the effects of total replacement of one fish species by another on this emergence flux to riparian consumers, or the magnitude of these effects.
Brook trout (S. fontinalis) have undergone the most widespread salmonid invasion in the western USA and are now the most abundant nonnative fish (Schade and Bonar 2005). When introduced, they quickly invade most stream reaches without barriers throughout central and southern Rocky Mountain watersheds, and replace the native cutthroat trout (O. clarkii; Gresswell 1988; Peterson et al. 2004; Fausch 2008). Although the two species are very similar, at least three traits that differ between them may cause changes in emergence flux, and thus alter this ecosystem function. First, brook trout can achieve greater density, biomass, and production than the cutthroat trout they replace (Benjamin and Baxter 2010). Second, brook trout more often forage directly on benthic insects by picking them from the substrate, whereas cutthroat trout feed predominantly on drifting insects which often include a substantial proportion of terrestrial insects that fall into streams (Griffith 1974; Forrester et al. 1994; Lepori, Benjamin, Fausch, and Baxter, unpublished data). Third, brook trout spawn in the fall, whereas cutthroat trout spawn in spring. Hence, brook trout fry emerge earlier in summer, and are likely to consume a broader size range of benthic invertebrates for a longer period during their first summer because of their larger size (Griffith 1972; Dunham et al. 2000). All three traits may allow brook trout to exert a stronger top-down effect on benthic insects, reduce adult aquatic insect emergence from streams to riparian areas, and, in turn, reduce consumers that rely on this resource subsidy. Finally, these effects may also vary by region, because of differences in brook trout invasion success (Adams et al. 2002; Peterson et al. 2004) or the composition or response of invertebrate assemblages, so these differences must also be accounted for in any analysis.
In this study, we compared emergence flux and riparian spiders along streams inhabited by native cutthroat trout versus those where they were replaced by nonnative brook trout. We conducted the study in two regions, and addressed two main predictions. First, we predicted that density of emerging adult aquatic insects would be lower from streams where brook trout have replaced cutthroat trout, because of differences in salmonid biomass, foraging behavior, or life history. We also assessed whether this prediction held after adjusting for differences in trout biomass, which would suggest differences in other species-specific traits. Second, because insect emergence is an important resource subsidy to riparian consumers, we predicted that there would be fewer spiders in the riparian zones of streams receiving lower total insect emergence flux. Finally, we assessed whether effects on emergence or spiders differed between the two regions.
Materials and methods
Study design
We tested our predictions using a comparative study of pairs of streams where native cutthroat trout were present, or had been replaced by brook trout, in the two regions. Comparative studies (also known as natural experiments) have at least three advantages for this purpose (Diamond 1986). First, they can incorporate larger spatial and temporal scales than most experiments (Power et al. 1998). An experiment conducted simultaneously in long reaches of replicate streams would be logistically difficult even in one region. Moreover, detecting effects of brook trout on stream–riparian food webs may require a longer time period than is feasible for a field experiment. Second, comparative studies achieve greater realism and generality by incorporating natural conditions (e.g., wild populations of nonnative species and long-term effects of disturbances like floods and wildfire; Polis et al. 1998). Third, comparative studies are among the only means to study biological invasions, which are generally unethical to create experimentally given the risk of escape (Sax et al. 2007).
Study area
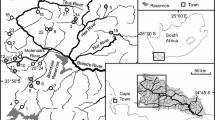
The study was conducted in 20 streams, 5 pairs in both the central and southern Rocky Mountain regions (On-line resource Figure S1). We chose small, montane streams typical of those currently inhabited by native cutthroat trout. One stream in each pair supported a wild population of native cutthroat trout and in the other nonnative brook trout had invaded and replaced them. Brook trout were prevented from invading cutthroat trout streams by physical barriers (waterfalls or diversion dams). However, where brook trout surmount such barriers, they invade rapidly and can replace cutthroat trout within about 5 years (Peterson et al. 2004; Fausch et al. 2009). The three subspecies of cutthroat trout studied (Yellowstone cutthroat trout O. c. bouvieri in the north, and greenback O. c. stomias or Colorado River cutthroat trout O. c. pleuriticus in the south) are members of the same clade, diverged recently in the last glacial period, and are considered ecological equivalents (Behnke 1992).
Streams in the central Rocky Mountain region were in Idaho and Wyoming, west of Grand Teton National Park (On-line resource Figure S1). Those in the southern region were north and south of Rocky Mountain National Park, Colorado. All streams were relatively small in wetted width [3.4 ± 0.4 m (mean ± 1SE)] and discharge at baseflow (0.10 ± 0.03 m3/s). They were shallow (15.2 ± 1.2 cm) coldwater streams (10.3 ± 0.5°C in midsummer), of moderate gradient (about 1–3%), with substantial volume in pools (35.4 ± 3.1%), extensive overhead riparian cover (66.7 ± 4.3%), and a moderate density of large woody debris (57 ± 8 pieces/150 m study reach; see below). Stream channels had predominately riffle, run, and pool morphology with cobble or gravel as the dominant substrate. Pairs of streams were in close proximity and had similar physical characteristics (elevation, geomorphology, and habitat structure). However, elevation at study reaches was lower in the central Rocky Mountain region (2,095 ± 24 m) than the southern region (2,804 ± 65 m). Of the physical characteristics measured, there were no significant differences within stream pairs (P > 0.19, paired t test) or between regions (P > 0.11, t test), except for the regional difference in elevation (P < 0.001). Vegetation was also similar in the two regions. Upland vegetation was primarily lodgepole pine (Pinus contorta), Douglas fir (Pseudotsuga menziesii) or subalpine fir (Abies lasiocarpa), and spruce (Picea spp.). Riparian vegetation was dominated by willow (Salix spp.) and dogwood (Cornus spp.) or alder (Alnus spp.).
Measurement of insect emergence, spiders, fish, and physical characteristics
In each stream, we selected one representative 150-m study reach (mean length = 151.2 ± 1.7 m) and divided it into adjacent sections for sampling emerging aquatic insects (100 m) and spiders (50 m). The riparian zone of the spider section was left undisturbed until they were counted. Adult insects emerging from the water surface were collected twice in each reach, once during the last 2 weeks of July and once during the first 2 weeks of August 2006. In the central Rocky Mountain region, emergence was collected for a longer period, 5–7 times from mid-June through mid-September 2006. We placed one floating emergence trap (0.33 m2 area, 0.2 mm mesh; Malison et al. 2010) in each of five pools dispersed throughout the 100-m study section. Pools were selected because Iwata (2006) reported a greater flux of aquatic insects from the surface of pools than riffles. Emerging insects were removed from traps after 4 days using an aspirator, and preserved in 95% ethanol. Adult aquatic insects were identified to family, dried (60°C for at least 24 h), and weighed to the nearest 0.1 mg.
Spiders of the family Tetragnathidae build horizontal orb webs in riparian zones and their diets consist mostly of emergent adult aquatic insects (Sanzone et al. 2003; Iwata 2006). Tetragnathids are relatively mobile and can track variation in aquatic insect emergence at the stream-reach scale (Gillespie 1987; Power et al. 2004). All juvenile and adult tetragnathids were counted in the 50-m undisturbed section during late July or early August 2006 at night (starting about 2200 hours under complete darkness), when they are most active (Kato et al. 2003). Counts were made in stream pairs on the same or successive nights. Two observers worked upstream, side-by-side, counting all spiders above the active channel and along the riparian zone within 1 m of the stream edge, up to a maximum height of 2.5 m. Double-observer sampling (Nichols et al. 2000) in a randomly-located 5-m reach of the spider section in each of the 10 Colorado streams yielded a median detection probability of 0.91, indicating that most spiders were detected by our sampling method (Fausch, unpublished data). Sixteen trials in 5-m reaches in Mahogany Creek, Idaho, yielded a similar detection probability (median = 0.93; Benjamin, Lepori, Baxter, unpublished data). Spiders were identified to family on sight, which is straightforward based on web and body morphology (Ubick et al. 2005). Voucher specimens were collected to confirm field identification (identified by Dr. Paula Cushing, and curated in the Denver Museum of Nature and Science, Denver, CO, USA). Tetragnathid spiders in both regions were all Tetragnatha versicolor, except a few T. extensa and T. laboriosa were also found in Meadow Creek, CO.
Fish were sampled in the entire 150-m study reach during baseflow in August 2006, after all emergence and spider sampling was complete. Study reaches were enclosed using 4.8-mm-mesh nets secured to the bed with rocks, and fish were removed in three or four upstream passes using a backpack electrofisher (model 12B or LR24; Smith-Root, Vancouver, WA, USA). Fish were held in live baskets by pass until all sampling was completed, and fork length (nearest mm) and mass (0.1 g) were measured for all trout. Only brook trout and cutthroat trout were captured in all streams, with two exceptions. In Teton Creek, two sculpin (Cottus spp.) and one rainbow trout were captured. In the lower reach of George Creek, pilot sampling of a 33-m reach revealed only brook trout (4.8 g/m2) at the beginning of the study, but the final sampling revealed only a small biomass of brown trout (0.5 g/m2; Salmo trutta), either because of seasonal movement or displacement (Fausch and White 1981). However, we included this reach because brook trout affected the stream food web for at least a portion of the study, and brook trout and brown trout have similar diets (Nyman 1970) and are likely to have similar effects on benthos (Lepori, Benjamin, Fausch, and Baxter, unpublished data). Fish were grouped into age classes (0, 1, and 2 and older) using length–frequency histograms. Density was estimated for each age class using the software programs CAPTURE (White et al. 1982) and MARK (White and Burnham 1999). Biomass was estimated by multiplying population estimates by the mean weight of fish in each age class.
Physical characteristics of study reaches were measured during late July and early August 2006 at baseflow, for use as covariates that might influence emerging insects and spiders and to assess differences in habitat for paired sites. Thermographs (StowAway Tidbit; Onset Computer, Pocassett, MA, USA) recorded temperature (±0.2°C) every 30–60 min. These were averaged for a 24-day period (24 July to 16 August 2006) during which all streams were monitored, which included most of the study period and encompassed the warmest days of the summer. Stream wetted widths (m) were measured at 10-m intervals and averaged. Most spiders attached webs to branches (Power et al. 2004; Laeser et al. 2005), so as a measure of this habitat we classified branch density within 2.5 m above the stream surface into categories (0, 1–5, 6–25, 26–50, and >50 branches) in each 2-m segment along the 50-m spider section. We included live and dead branches that were <5 cm diameter at their base and >50 cm in length. We estimated total branch density in the spider section by summing the midpoints of the categories for each segment; we used 75 as a conservative estimate for the midpoint of the largest category.
Data analyses
Model selection
We fit two different sets of models to assess whether replacement of cutthroat trout by brook trout reduced the density of adult aquatic insect emergence, and whether riparian spiders were reduced in streams with less total insect emergence flux. For both, we fit linear mixed models that included a random effect for stream pair (Littell et al. 2006), nested within region. We also wanted to assess whether other plausible factors influenced emergence and spiders, so we used an information-theoretic approach for model selection (Burnham and Anderson 2002). This is a powerful tool for comparing multiple alternate hypotheses and for inference from multiple models when several factors may combine to explain an effect.
We developed a suite of 16 a priori candidate models to predict emergence density, including combinations of covariates for region, trout species, trout biomass, stream temperature, and a region-by-trout species interaction (i.e., the effect of brook trout could vary by region). In addition to the predicted effect of trout species, we reasoned that on a per habitat area basis higher trout biomass could reduce emergence density via stronger predation, and higher stream temperature might increase productivity and hence emergence.
The same a priori candidate models were used to predict emergence density separately from the longer dataset for the central Rocky Mountain region to assess whether results changed with greater temporal scale (12 vs. 4 weeks). Trout species and biomass were strongly correlated in this dataset (F = 26.03, P = 0.007; Benjamin and Baxter 2010), so the two variables were not considered together in candidate models (except the global model).
To predict counts of tetragnathid spiders, we developed a suite of 15 a priori candidate models, with combinations of covariates for region, total emergence flux, branch habitat, and stream temperature. Total emergence flux was calculated as emergence density times surface area of the spider section (estimated as length times mean width of the reach), because spiders respond to the total flux of emerging prey that crosses the stream-riparian boundary, not the average flux per unit area across the stream surface (Gratton and Vander Zanden 2009). Thus, total emergence flux accounts for the effect of trout on emergence density, as well as differences in stream width among study reaches that also affect this prey resource. We reasoned that greater total emergence flux, branch habitat, or stream temperature (through its effects on productivity) could increase spider abundance.
Model selection under the information-theoretic approach identifies the best approximating model, produces a weight of evidence for each model, and provides a method for model averaging to achieve inference based on multiple models. We first evaluated the fit of global models by examining residual plots and using a likelihood ratio test of the deviance (Littell et al. 2006) and found no evidence of heterogeneous variance. We used Akaike’s information criterion adjusted for small sample size (AICc) to rank each set of candidate models, calculated Akaike weights (w i) to assess the relative plausibility of each model given the data, and report a confidence set of models which includes those with relative likelihood at least 1/8 that of the best approximating model (Burnham and Anderson 2002). We also used the Akaike weights to calculate a weighted average for model parameters (i.e., model averaging), and calculated an unconditional standard error for each parameter estimate by averaging over all models (the most conservative approach), which included variance inflation factors for both model selection variance and small sample size (Burnham and Anderson 2002). This adjusted standard error was used to calculate confidence intervals for the model-averaged parameter estimates. Finally, the importance of each parameter was estimated by summing Akaike weights of each model containing that parameter. Initial analysis showed that the model with the region-by-trout species interaction contributed almost no weight in the emergence model set (w i = 0.01), and the parameter estimate cannot be model-averaged, so this variable was subsequently excluded. In each model set, we included all one-variable models, and most or all two- and three-variable models, along with the global model, which produces a more balanced set and allows unbiased estimation of importance weights (Doherty et al. 2011).
Assemblage composition of emergence
Differences in the structure (based on relative biomass) of emerging insect assemblages between brook trout and cutthroat trout streams and between regions were examined using non-metric multidimensional scaling (NMDS) with a Sorensen distance matrix (McCune and Grace 2002). This method derives the dominant gradients in insect assemblage structure among streams along two or more multivariate axes, and allows comparing assemblages in different streams based on their proximity in this multivariate space. Afterwards, a blocked, multi-response permutation procedure (MRPP), a nonparametric procedure for comparing a priori groups (McCune and Grace 2002), was used to test for differences in emergence assemblage composition between streams with different trout species and in different regions.
Projecting effects of species replacement on emergence and spiders
Comparing spider counts directly for pairs of streams with brook trout versus cutthroat trout was not appropriate, because even small differences in stream width cause substantial differences in total emergence flux. Therefore, we used the linear models described above in sequence to first predict the change in total emergence flux in cutthroat streams after brook trout invasion and replacement, and then to predict the change in spider counts from this total emergence flux. We used cutthroat streams of average width as the baseline, and analyzed each region separately.
Results
Trout biomass
Brook trout biomass was, on average, 71% greater than cutthroat trout biomass in the central Rocky Mountain region (3.09 ± 0.62 vs. 1.81 ± 0.24 g/m2), and 106% greater in the southern region (7.72 ± 2.79 vs. 3.74 ± 0.91 g/m2). There was some evidence for these differences between the species (P = 0.07 by mixed model ANOVA), but the relatively high variation among stream pairs reduced power to detect them. Trout biomass (regardless of species) was greater in the southern region than the central region (P = 0.04), but no interaction between trout species and region was detected (P = 0.33).
Insect emergence
The density of emerging insects was lower in stream reaches where brook trout had replaced cutthroat trout, supporting our first prediction. The mean difference in emergence between paired streams with cutthroat trout versus brook trout was 4.5 mg m−2 day−1 (SE = 1.63) and the 95% confidence interval (CI) did not overlap zero (1.28–7.66 mg m−2 day−1), indicating that the effect was relatively precise. This difference represents a 36% lower emergence density in streams invaded by brook trout (8.0 mg m−2 day−1), based on the average emergence flux from cutthroat trout streams (12.5 mg m−2 day−1). This comparison is based on the model with trout species alone, which was the most plausible model, accounting for 35% of the total weight (Table 1). Moreover, four of five models in the confidence set included trout species, and this variable had the highest cumulative weight (w i = 0.74; Table 2).
Insect emergence density was also lower in streams with brook trout than cutthroat trout after adjusting for differences in trout biomass and other covariates. Trout biomass was included in the second and third ranked models (Table 1) and was also an important variable (cumulative w i = 0.46). Trout biomass had a negative effect on emergence density in all models, as expected, although the effect was small and not precise (Table 2). In contrast, there was little evidence for an effect of region or stream temperature on emergence density (cumulative w i = 0.17). Model averaging allowed estimating the reduction in emergence density in streams with brook trout, after adjusting for differences in trout biomass, and showed that it was 3.0 mg m−2 day−1 lower than in streams with cutthroat trout. Moreover, the 95% CI did not overlap zero and the sign of this parameter was consistent across all eight models in which it appeared. This represents a 24% lower emergence density in streams invaded by brook trout, after accounting for trout biomass, region, and temperature.
Similar results were found when emergence density was analyzed over the longer period in the central Rocky Mountain region. The most plausible model included trout species alone (AICc = 75.3, w i = 0.67), and the model-averaged estimate for emergence was 2.8 mg m−2 day−1 lower from brook trout streams than cutthroat trout streams, although the effect was not precise owing to the small sample size (95% CI: −1.10 to 6.73 mg m−2 day−1).
Although we found differences in emergence density between brook trout and cutthroat trout streams, emergent insect assemblage composition revealed no clear patterns in two-dimensional ordination space owing to trout species or region. The lack of separation of emerging insects in ordination space was further confirmed via permutation tests for trout species (MRPP: A = −0.01, P = 0.51) and region (A = −0.02, P = 0.73). Similarly, there was no difference detected in emergence assemblages between streams with cutthroat trout versus brook trout for the longer data set from the central Rocky Mountain region (A = −0.01, P = 0.52).
Riparian spiders
The number of tetragnathid spiders counted was lower along streams with lower total emergence flux, supporting our second prediction, and was lower in the central than southern Rocky Mountain region. The model with region and total emergence flux accounted for 56% of the model weight, and three of the four top models in the confidence set included both variables (Table 1). The 95% confidence intervals for neither variable overlapped zero, and both were important predictors (cumulative w i = 0.77–0.92; Table 2). In contrast, the effects of temperature and branch habitat for spiders were small, imprecise, and had little support (w i = 0.13).
Spider counts increased linearly with total emergence flux in both regions, although the relationship was apparently stronger for the central Rocky Mountain region (Fig. 1). The estimate based on model averaging showed that, on average, 190 fewer spiders were counted along streams in the central than southern region. Additionally, we coupled our models to project the effects of replacement of cutthroat trout by brook trout on total emergence flux from a 50-m reach, and spider counts. A reduction of emergence density of 4.5 mg m−2 day−1 owing to brook trout invasion (i.e., including the effect of their higher biomass) would reduce total emergence flux from a 50-m reach of a cutthroat stream by 31% in the central Rocky Mountain region (889 mg day−1) and 30% in the southern region (636 mg day−1), based on the mean stream widths. In turn, this would result in a 20% decrease in spider counts along cutthroat trout streams in the central region (27 spiders/50-m reach) where spiders were less abundant, and a 6% decrease in the southern region (20 spiders/50-m reach) where they were more abundant.
Discussion
We found that when nonnative brook trout invaded and replaced native cutthroat trout in central and southern Rocky Mountain streams, the flux of aquatic insect emergence to the riparian zone, a key ecosystem function, was 36% lower than in streams where the native trout persisted. Moreover, even after adjusting for the higher biomass of brook trout, which was similar to other streams in the region (Benjamin and Baxter 2010), emergence was still 24% lower from streams with brook trout than cutthroat trout, indicating differences in traits of the two species that affect this flux. In turn, counts of riparian spiders that depend on this emergence for prey were lower along streams with lower total emergence flux. The consequence is that, when brook trout do invade and sustain higher biomass, they apparently reduce total emergence flux from stream reaches by about a third, which is predicted to result in 6–20% fewer riparian spiders in the adjacent habitat, depending on the region.
These findings are consistent with other studies that report nonnative trout reduce the flux of emerging insects from aquatic ecosystems to riparian zones, with cascading effects to riparian consumers. Baxter et al. (2004) found that nonnative rainbow trout reduced the biomass of emerging insects by 35% when added to stream reaches with native Dolly Varden charr in an experiment, a reduction similar to our results here, and this reduced riparian spiders by 65%. Similarly, other studies reported that salmonids introduced into fishless lakes, including brook trout, apparently reduced insect emergence up to 92%, which led to 90% fewer frogs and 82% fewer birds (Finlay and Vredenburg 2007; Epanchin et al. 2010). We also found a similar reduction in emergence from our central Rocky Mountain streams when we analyzed samples from a 3-month period, a longer temporal scale than in other studies. Taken together, this evidence suggests that the negative effect of nonnative trout on the key ecosystem function of emergence flux is a general one. Moreover, even when brook trout replace native trout, rather than adding to their biomass or invading fishless ecosystems, and even after adjusting for their greater biomass, they still had important effects.
We assume that the main species-specific trait causing lower emergence density from streams where nonnative brook trout replaced native cutthroat trout was differences in foraging behavior. Several investigators have reported that brook trout have a greater propensity to pick insects directly from the stream bed (Griffith 1974; Forrester et al. 1994; Lepori, Benjamin, Fausch, and Baxter, unpublished data), and this behavior has been reported for other congeneric charr (e.g., Nakano et al. 1992; Fausch et al. 1997). There is also evidence from other systems that the strength of direct and indirect effects depends on species-specific differences in predator foraging behavior. For example, benthic-feeding stream fish caused a greater reduction in benthic invertebrates and increase in periphyton biomass than drift-feeding fish within fish enclosures (Dahl 1998). Similarly, different hunting methods practiced by two different spider species altered the behavioral response of their grasshopper prey, which, in turn, altered plant diversity and primary production (Schmitz 2008).
We calculated that replacement of cutthroat trout by brook trout would result in about 30% lower emergence flux in each region, which should have strong consequences for riparian consumers that depend on this subsidy. Moreover, a recent model for linked aquatic–terrestrial ecosystems indicates that it is the total emergence flux across their boundaries that is relevant to terrestrial communities, rather than the density of emergence across the water surface (Gratton and Vander Zanden 2009). This is especially true for riparian spiders along small streams, nearly all of which build webs within a meter of the bank to capture emerging prey, because this food source declines quickly with distance from the stream (Sanzone et al. 2003; Power et al. 2004). Therefore, this is why we used total emergence flux to project changes in spider counts after trout species replacement. Experimental reductions in emergence using mesh shields or greenhouses caused large reductions in spider abundance (Kato et al. 2003; Baxter et al. 2004; Marczak and Richardson 2007), lizard abundance and growth (Sabo and Power 2002a, b), and bat foraging (Fukui et al. 2006), confirming the importance of this subsidy for riparian consumers. We found that tetragnathid spiders increased linearly with total emergence flux, which allowed us to predict that brook trout invasion would result in a 6–20% decrease in spider abundance along cutthroat trout streams (greater in the central Rocky Mountain region where spider abundance was lower). We found no evidence that branch habitat for web support explained variation in spider numbers, probably because it was plentiful [660 ± 67 per 50-m reach (mean ± 1SE)]. We also found no evidence that stream temperature, a strong driver of productivity, influenced spider numbers, though this inference is restricted to similar coldwater mountain streams.
There was a large effect of region on tetragnathid spiders, resulting in over twice the spider counts at the southern versus central Rocky Mountain streams. This was not likely caused by different effects of brook trout on emergence in the two regions, because there was no interactive effect of trout species and region on emergence density. Likewise, we found no difference in assemblage structure of emerging insects at the family level owing to trout species or region based on ordination, suggesting that any difference caused by brook trout was not detectable at this taxonomic resolution. However, two alternate hypotheses are plausible. First, some other aspect of climate or habitat than we measured created more favorable conditions for spiders in the southern region. Second, because streams in the southern region were at higher elevation, spider counts conducted at the same time may have been at an earlier stage in the phenology of spider life history, which would result in higher counts if fewer juveniles had died (Marczak and Richardson 2008).
We were careful in the design and analysis of this study to address limitations of comparative studies and select conservative analyses, to ensure that the effects we report are as robust as possible. For example, a criticism of natural experiments like ours is that some other factor that favored brook trout invasion could also have caused lower insect emergence. However, four lines of evidence discount this alternative. First, brook trout have invaded nearly every stream reach to which they have gained access throughout these regions (Fausch 2008), so there is apparently nothing inherently different between streams inhabited by the two species. Second, the effect of nonnative brook trout on emergence density was evident over many sites in two regions, and over a range of variation in other variables, such as trout biomass and stream temperature (cf., Diamond 1986). Third, the effect persisted even after accounting for variation in these other variables using statistical models. Fourth, the effect on emergence was important even though throughout our analysis we used the most conservative approach for model averaging, and used variance inflation factors to account for small samples size and model selection uncertainty (Burnham and Anderson 2002). Finally, our research was based on a strong a priori biological mechanism, generated from previous and ongoing research on effects of nonnative trout in other ecosystems (e.g., Griffith 1974; Baxter et al. 2004; Lepori, Benjamin, Fausch, and Baxter, unpublished data).
Native species are being excluded by nonnative invaders across a diverse array of taxa (e.g., Holway et al. 2002; Levine et al. 2003; Olden et al. 2006), producing different scenarios of relative density and biomass. Our study provides the first empirical evidence that the wholesale replacement of native trout by nonnative trout alters ecosystem function by reducing insect emergence that provides prey subsidies to riparian consumers. In the ecosystem we studied, this was apparently caused not only by the higher biomass of the nonnative trout after invasion but also by species-specific differences in traits like foraging behavior. Thus, making such predictions for other taxa will likely require knowing not only the relative difference in density or biomass after replacement but also how the species differ in behavior, ecology, and life history. Moreover, our results argue for the value of restoration of native trout in these ecosystems, where possible (cf., Quist and Hubert 2004), because both types of differences can drive reduced emergence and potentially affect native riparian predators. The generality of these findings should be studied not only for other nonnative fish invasions in different biomes but also in other ecosystems subject to nonnative species invasions.
References
Adams SB, Frissell CA, Rieman BE (2002) Changes in distribution of nonnative brook trout in an Idaho drainage over two decades. Trans Am Fish Soc 131:561–568
Baxter CV, Fausch KD, Murakami M, Chapman PL (2004) Non-native stream fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85:2656–2663
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol 50:201–220
Behnke RJ (1992) Native trout of western North America. American Fisheries Society, Bethesda
Benjamin JR, Baxter CV (2010) Do nonnative salmonines exhibit greater density and production than the natives they replace? A comparison of nonnative brook trout to native cutthroat trout. Trans Am Fish Soc 139:641–651
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach. Springer-, New York
Carlsson NOL, Bronmark C, Hansson L (2004) Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85:1575–1580
Dahl J (1998) Effects of a benthivorous and a drift-feeding fish on a benthic stream assemblage. Oecologia 116:426–432
Diamond J (1986) Overview: laboratory experiments, field experiments, and natural experiments. In: Diamond J, Case T (eds) Community ecology. Harper and Row, New York, pp 3–22
Doherty PF, White GC, Burnham KP (2011) Comparison of model building strategies when all possible model structures cannot be constructed. J Ornithol (in press, available online)
Dunham JB, Rahn ME, Schroeter RE, Breck SW (2000) Diets of sympatric Lahontan cutthroat trout and nonnative brook trout: implications for species interactions. Western N Am Nat 60:304–310
Epanchin P, Knapp R, Lawler S (2010) Nonnative trout impact an alpine-nesting bird by altering aquatic insect subsidies. Ecology 91:2406–2415
Fausch KD (2008) A paradox of trout invasions in North America. Biol Invasions 10:685–701
Fausch KD, White RJ (1981) Competition between brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta) for positions in a Michigan stream. Can J Fish Aquat Sci 38:1220–1227
Fausch KD, Nakano S, Kitano S (1997) Experimentally induced foraging mode shift by sympatric charrs in a Japanese mountain stream. Behavioral Ecol 8:414–420
Fausch KD, Rieman BE, Dunham JB, Young MK, Peterson DP (2009) The invasion versus isolation dilemma: tradeoffs in managing native salmonids with barriers to upstream movement. Conserv Biol 23:859–870
Finlay JC, Vredenburg VT (2007) Introduced trout sever trophic connections in watersheds: consequences for a declining amphibian. Ecology 88:2187–2198
Forrester GE, Chace JG, McCarthy W (1994) Diel and density-related changes in food consumption and prey selection by brook charr in a New Hampshire stream. Environ Biol Fish 39:301–311
Fukui D, Murakami M, Nakano S, Aoi T (2006) Effect of emergent aquatic insects on bat foraging in a riparian forest. J Anim Ecol 75:1252–1258
Gillespie RG (1987) The mechanism of habitat selection in the long-jawed orb-weaving spider Tetragnatha elongata (Araneae, Araneidae). J Arachnol 15:81–90
Gordon DR (2008) Effects of invasive, non-indigenous plant species on ecosystem processes: lessons from Florida. Ecol Appl 8:975–989
Gratton C, Vander Zanden MJ (2009) Flux of aquatic insect productivity to land: comparison of lentic and lotic ecosystems. Ecology 9:2689–2699
Gresswell RE (ed) (1988) Status and management of interior stocks of cutthroat trout. American Fisheries Society Symposium 4, Bethesda
Griffith JS (1972) Comparative behavior and habitat utilization of brook trout (Salvelinus fontinalis) and cutthroat trout (Salmo clarki) in small streams in northern Idaho. J Fish Res Bd Can 29:265–273
Griffith JS (1974) Utilization of invertebrate drift by brook trout (Salvelinus fontinalis) and cutthroat trout (Salmo clarki) in small streams in Idaho. Trans Am Fish Soc 3:440–447
Hall RO Jr, Dybdahl MF, VanderLoop MC (2006) Extremely high secondary production of introduced snails in rivers. Ecol Appl 16:1121–1131
Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad J, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Iwata T (2006) Linking stream habitats and spider distribution: spatial variations in trophic transfer across a forest-stream boundary. Ecol Res 22:619–628
Kato C, Iwata T, Nakano S, Kishi D (2003) Dynamics of aquatic insect flux affects distribution of riparian web-building spiders. Oikos 103:113–120
Laeser SL, Baxter CV, Fausch KD (2005) Riparian vegetation loss, stream channelization and web-weaving spiders in northern Japan. Ecol Res 20:646–651
Levine JM, Vila M, Antonio CM D, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond B 270:775–781
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for Mixed Models, 2nd edn. SAS Institute, Cary
Malison RL, Benjamin JR, Baxter CV (2010) Measuring adult insect emergence from streams: the influence of trap placement and a comparison with benthic sampling. J North Am Benthol Soc 29:647–656
Marczak LB, Richardson JS (2007) Spider and subsidies: results from the riparian zone of a coastal temperate rainforest. J Anim Ecol 76:687–694
Marczak LB, Richardson JS (2008) Growth and development rates in a riparian spider are altered by asynchrony between the timing and amount of a resource subsidy. Oecologia 156:249–258
McCune B, Grace JB (2002) Analysis of ecological communities. MJM Software Design, Gleneden Beach
McDowall RM (2006) Crying wolf, crying foul, or crying shame: alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev Fish Biol Fish 16:233–422
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98:166–170
Nakano S, Fausch KD, Furukawa-Tanaka T, Maekawa K, Kawanabe H (1992) Resource partitioning between sympatric populations of bull charr, Salvelinus confluentus, and cutthroat trout, Oncorhynchus clarki, in a mountain stream in Montana, USA. Jpn J Ichthyol 39:211–217
Nichols JD, Hines JE, Sauer JR, Fallon FW, Fallon JE, Heglund PJ (2000) A double-observer approach for estimating detection probability and abundance from point counts. Auk 117:393–408
Nyman OL (1970) Ecological interaction of brown trout, Salmo trutta L., and brook trout, Salvelinus fontinalis (Mitchill), in a stream. Can Field Nat 84:343–350
Olden JD, McCarthy JM, Maxted JT, Fetzer WW, Vander Zanden MJ (2006) The rapid spread of rusty crayfish (Oronectes rusticus) with observations on native crayfish declines in Wisconsin (USA) over the past 130 years. Biol Invasions 8:1621–1628
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Peterson DP, Fausch KD, White GC (2004) Population ecology of an invasion: effects of brook trout on native cutthroat trout. Ecol Appl 14:754–772
Polis GA, Wise DH, Hurd SD, Sanchez-Pinero F, Wagner JD, Jackson CT, Barnes JD (1998) The interplay between natural history and field experimentation. In: Resetarits WJ Jr, Bernardo J (eds) Experimental ecology: issues and perspectives. Oxford University Press, New York, pp 254–280
Power ME, Dietrich WE, Sullivan KO (1998) Experiment, observation, and inference in river and watershed investigations. In: Resetarits WJ Jr, Bernardo J (eds) Experimental ecology: issues and perspectives. Oxford University Press, New York, pp 113–132
Power ME, Rainey WE, Parker MS, Sabo JL, Smyth A, Khandwala S, Finlay JC, McNeely RC, Marsee K, Anderson C (2004) River-to-watershed subsidies in an old-growth conifer forest. In: Polis GA, Power ME, Huxel (eds) Food webs at the landscape level. University of Chicago Press, Chicago, pp 217–240
Quist MC, Hubert WA (2004) Bioinvasive species and the preservation of cutthroat trout in the western United States: ecological, social, and economic issues. Environ Sci Policy 7:303–313
Rahel FJ (2002) Homogenization of freshwater faunas. Annu Rev Ecol Syst 33:291–315
Sabo JL, Power ME (2002a) River-watershed exchange: effects of riverine subsidies on riparian lizards and their terrestrial prey. Ecology 83:1860–1869
Sabo JL, Power ME (2002b) Numerical response of lizards to aquatic insects and short-term consequences for terrestrial prey. Ecology 83:3023–3036
Sanzone DM, Meyer JL, Marti E, Gardiner EP, Tank JL, Grimm NB (2003) Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia 134:238–250
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends Ecol Evol 18:561–566
Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, O’Connor MI, Rice WR (2007) Ecological and evolutionary insights from species invasion. Trends Ecol Evol 22:465–471
Schade CB, Bonar SA (2005) Distribution and abundance of nonnative fishes in streams of the western United States. N Am J Fish Manag 25:1386–1394
Schmitz OJ (2008) Effects of predator hunting mode on grassland ecosystem function. Science 319:952–954
Simberloff D (2003) How much information on population biology is needed to manage introduced species? Conserv Biol 17:83–92
Strayer DL, Caraco NF, Cole JJ, Findlay S, Pace ML (1999) Transformation of freshwater ecosystems by bivalves. Bioscience 49:19–27
Ubick D, Paquin P, Cushing PE, Roth V (eds) (2005) Spiders of North America: an identification manual. American Arachnological Society
White GC, Burnham KP (1999) Program MARK: survival estimations from populations of marked animals. Bird Study (Supplement) 24:S13–S120
White GC, Anderson DR, Burnham KP, Otis D (1982) Capture-recapture and removal methods for sampling closed populations. Los Alamos National Laboratory Report, LA-8787-NERP, Los Alamos, New Mexico, USA
Acknowledgments
Field and laboratory assistance were provided by S. Bourdon, C. Cusack, M. Dodrill, R. Dritz, G. Gillette, A. Hansen, M. Lamb, J. Malison, R. Malison, L. Morales, V. Sednek, J. Timchak, and L. Young. J. Giersch, B. Kondratieff, and C. Young provided insect identification expertise. K. Kehmeier, M. Lien, L. Mabey, B. Rosenlund, and R. Van Kirk provided valuable information regarding study sites. We thank L. Bailey, K. Burnham, P. Chapman, and T. Peterson for advice on statistical analysis, and F. Lepori, L. Marczak, and two anonymous reviewers for comments that improved the manuscript. This research was supported by the National Science Foundation (DEB-0516133 to KDF, and DEB-0516136, EPS-0447689 and EPS-0814387 to CVB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Leon Barmuta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benjamin, J.R., Fausch, K.D. & Baxter, C.V. Species replacement by a nonnative salmonid alters ecosystem function by reducing prey subsidies that support riparian spiders. Oecologia 167, 503–512 (2011). https://doi.org/10.1007/s00442-011-2000-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2000-6