Abstract
Ecosystem processes of northern peatlands are largely governed by the vitality and species composition in the bryophyte layer, and may be affected by global warming and eutrophication. In a factorial experiment in northeast China, we tested the effects of raised levels of nitrogen (0, 1 and 2 g m−2 year−1), phosphorus (0, 0.1 and 0.2 g m−2 year−1) and temperature (ambient and +3°C) on Polytrichum strictum, Sphagnum magellanicum and S. palustre, to see if the effects could be altered by inter-specific interactions. In all species, growth declined with nitrogen addition and increased with phosphorus addition, but only P. strictum responded to raised temperature with increased production of side-shoots (branching). In Sphagnum, growth and branching changed in the same direction, but in Polytrichum, the two responses were uncoupled: with nitrogen addition there was a decrease in growth (smaller than in Sphagnum) but an increase in branching; with phosphorus addition growth increased but branching was unaffected. There were no two-way interactions among the P, N and T treatments. With increasing temperature, our results indicate that S. palustre should decrease relative to P. strictum (Polytrichum increased its branching and had a negative neighbor effect on S. palustre). With a slight increase in phosphorus availability, the increase in length growth and production of side-shoots in P. strictum and S. magellanicum may give them a competitive superiority over S. palustre. The negative response in Sphagnum to nitrogen could favor the expansion of vascular plants, but P. strictum may endure thanks to its increased branching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Northern hemisphere peatlands play an important role in the global carbon (C) budget, containing about 30% of the world terrestrial soil carbon (Rydin and Jeglum 2006). They represent the result of long-term C sequestration, but it is currently debated if their role as C sinks is at risk because of global warming and anthropogenic eutrophication (Berendse et al. 2001; Vasander and Kettunen 2006; Dorrepaal et al. 2009). Being located largely in areas with cold climates and characterized by nutrient shortage, peatlands are generally considered to be particularly vulnerable to environmental perturbations.
Sphagnum is the dominant plant group in boreal and temperate peatlands. Vast areas of ombrotrophic bogs are covered by Sphagnum, and the mere existence of many bogs is due to the growth of Sphagnum, creating an acid, waterlogged and largely anoxic substrate, with a litter that is resistant to decay (Verhoeven and Liefveld 1997; Aerts et al. 1992; Freeman et al. 2002). Hence, peatland ecosystem processes may be severely affected by environmental changes that either directly reduce the growth of Sphagnum, or disfavor Sphagnum in competition with vascular plants or other bryophytes with less peat-producing ability. Absorbing nutrient and water over their whole surface, this bryophyte is also more directly sensitive to environmental changes, such as nitrogen deposition or raised surface temperature.
In peatlands, particularly bogs, mineralization rates of nitrogen (N) and phosphorus (P) are so slow that rainwater is the most important inorganic source for Sphagnum nutrition. Low concentration of nitrogen deposition may facilitate Sphagnum growth (Gunnarsson and Rydin 2000), but long-term low levels or short-term high levels of nitrogen deposition negatively affect Sphagnum, and higher levels may lead to a shift to phosphorus limitation (Aerts et al. 1992; Bragazza et al. 2004; Gerdol et al. 2007). Following N deposition, Sphagnum fallax has expanded in western Europe at the expense of other Sphagnum species, but Limpens et al. (2003) suggested that further expansion and dominance may instead be limited by P availability. Even though S. fallax is favored by nitrogen deposition, Mitchell et al. (2002) found that it was overtopped by Polytrichum strictum with a nitrogen addition of 3 g m−2 year−1. Similarly, Bubier et al. (2007) found that N addition led to an increase in abundance of P. strictum and a decrease in Sphagnum species. In combination with phosphorus, the increase in P. strictum was smaller, but the decrease in Sphagnum was also larger. In contrast, P addition seems to facilitate growth of Sphagnum (Limpens et al. 2004), especially in P-limited peatlands (cf. Güsewell 2004).
On a global scale, the annual temperature is the main environmental factor explaining differences in Sphagnum productivity as long as water is available (Gunnarsson 2005), and the importance of water availability is also underpinned by microcosm experiments (Robroek et al. 2009). A direct effect of global warming may therefore be an increased Sphagnum growth (Dorrepaal et al. 2003, Lang et al. 2009), but it seems more likely that in the long run the indirect negative effects of desiccation and water availability will be stronger (Weltzin et al. 2001; Dorrepaal et al. 2003; Gerdol et al. 2008).
The response of a bryophyte species to changed conditions could be measured as length increment (height growth), biomass production or increased abundance in the community (for example by clonal expansion), and these need not be coupled. For instance, Rincon and Grime (1989) found inverse relationships between length increment and biomass production in four bryophytes. Dorrepaal et al. (2003) measured a strong increase in length growth in Sphagnum fuscum in a warming experiment. However, the increase in mass growth was small, and the result was a looser hummock structure with poor water holding which may reduce production in the long run. Uncoupling between growth and production of side-shoots in bryophytes could indicate a trade-off between allocation to elongation to acquire light resource and lateral branching to expand clonally (Rydin 2009).
In addition to direct effects of environmental perturbations, we focus on the more rarely studied mechanism—that vegetation development could also be influenced by altered competitive abilities of the species. Robroek et al. (2007) noted that the changes in abundance of the competing Sphagnum species were correlated with the differences in length growth (r = 0.75–0.94). In a greenhouse experiment, the growth of S. balticum in monoculture increased with temperature, but less so in pots where it grew with other sphagna (Breeuwer et al. 2008), indicating that a positive growth response does not always confer competitive ability. On the other hand, there may also be cases with facilitation. For instance, with water stress, a desiccation-resistant species may have positive effects on its neighbors (e.g., Rydin 1985; Groeneveld et al. 2007).
In the regions of Daxing’an, Xiaoxing’an and Changbai Mountains in northeast China, the areas with virgin peatlands have been much reduced since the 1960s because of drainage, reclamation and peat exploitation (Chai 1990). The degraded peatlands are generally characterized by a species shift from Sphagnum to a dominance of P. strictum Menz. ex Brid. or Aulacomnium palustre (Hedw.) Schwägr. (Lang et al. 1999; Bu et al. 2005). These vegetation changes may result directly from environmental changes but also indirectly from altered competitive abilities. The general aim of this study was to experimentally test both direct effects of warming and increased nutrient availability on P. strictum and the dominant Sphagnum species (S. palustre L. and S. magellanicum Brid.), and how these effects may be altered by interactions among species.
Generally, P. strictum occupies positions on hummocks high above the water table, and might by its internal water transport ability be favored by increased temperature causing surface desiccation (Wyatt and Derda 1997; Groeneveld et al. 2007). Based on the above-mentioned studies of Polytrichum–Sphagnum relationships, we expected that Polytrichum should be favored by increased N availability in competition with S. palustre and S. magellanicum. In Hani Peatland, chemical measurements indicate that all the three bryophytes are P-limited, but the N:P ratio is lower for P. strictum (about 21) than for S. palustre and S. magellanicum (26 and 29, respectively; unpublished data).
Our incentives for the study were (1) studies covering both direct and competition-mediated effect of environmental factors are unusual, (2) growth is often used as the only response, but we argue that it should be combined with estimates of clonal expansion in studies of competition, and (3) the results can be widely generalized since the studied species are common in the whole northern hemisphere.
Specifically, we test the following hypotheses: (1) warming will cause increased growth in the three bryophytes but particularly Polytrichum; (2) nitrogen deposition will negatively influence Sphagnum but not Polytrichum; (3) phosphorus addition will be more favorable for Sphagnum than for Polytrichum; and (4) the response to environmental changes will depend on the inter-specific surrounding of the bryophytes.
Materials and methods
Study site and species
The study site Hani Peatland (42°13′N, 126°31′E.) in the Changbai Mountains, northeast China, with an area of 1,678 ha, is located in the temperate zone at an altitude of 900 m a.s.l. The climate of the region is continental with long and cold winters and warm and humid summers. Precipitation is 757–930 mm year−1. Mean temperature for January and July are −15.7 and 22.5°C, respectively. The peat layer is generally ca. 4 m thick and at most 9.6 m (Qiao 1993).
The peatland is minerotrophic with pH about 5.4. The vegetation is differentiated into forested and non-forested parts. The forested community is dominated by the tree Larix olgensis A. Henry, the dwarf shrubs Betula fruticosa Pall. var. ruprechtiana Trautv., Rhododendron tomentosum Harmaja (=Ledum palustre), Potentilla fruticosa L. and Vaccinium uliginosum L., the graminoids Carex lasiocarpa Ehrh., Eriophorum polystachion L. and Phragmites australis (Clav.) Trin., the herbs Smilacina japonica A. Gray and Sanguisorba parviflora (Maxim.) Takeda and the bryophytes S. magellanicum, S. capillifolium (Ehrh.) Hedw., S. fallax (H. Klinggr) H. Klinggr. and Polytrichum strictum. In open areas, the same vascular plants dominate, but among the bryophytes S. palustre and S. fallax dominate.
Polytrichum strictum, S. magellanicum and S. palustre were chosen as study species. The two Sphagnum species are globally important peat-forming species. They generally occur in hummocks but S. magellanicum has a wider realized niche in relation to water table depth and water chemistry than S. palustre (Bragazza 1997). In Hani Peatland, S. palustre dominates in open areas while S. magellanicum is mainly distributed in the forested margin. Generally, P. strictum occupies positions on hummocks high above the water table (Chen et al. 2009).
Experimental design
In August 2007, 0.8 × 0.8 m plots were laid out on S. palustre hummocks in the open area of Hani Peatland. We selected hummocks with as little cover of vascular plants as possible. We used a full factorial design with two levels of temperature (T 0, T 1), three levels of nitrogen (N0, N1, N2) and three levels of phosphorus (P0, P1, P2) in four randomized complete blocks, i.e. a total of 72 plots.
Open top chambers (1.1 × 1.1 m at the bottom and 0.9 × 0.9 m at the top) with transparent plastic walls (0.5 m high) were set up around plots to raise the temperature. Air temperature 10 cm above bryophyte surface was generally about 3°C higher than ambient (measure 1–2 days per month during the growing season). The chambers were removed in winter (November–April). Nitrogen was applied as NH4NO3 and phosphorus as NaH2PO4·2H2O dissolved in distilled water. The amounts added were 0, 1 and 2 g N m−2 year−1 and 0, 0.1 and 0.2 g P m−2 year−1, respectively, divided into one addition per month (300 ml per plot) during the growing season (three additions in 2007, five in 2008).
Polytrichum strictum, S. magellanicum and S. palustre monoliths with a diameter of 6.3 cm were collected in their typical monospecific stands far from the plots. After being cut to 9 cm, the monoliths were inserted in natural density into PVC pots with 6.3 cm inner diameter and 9 cm height, with dense holes both in wall and bottom to guarantee water and air exchange between bryophyte monolith inside and mat outside (Sun et al. 2005). To accurately measure growth and production of side-shoots of the bryophytes, we inserted a marked shoot bundle into each monolith. The shoot bundle was composed of ten vigorous shoots in length of 9.0 cm without side-shoots or multiple capitula. Marked with thin nylon strings, the shoots bundles were inserted into the bryophyte pots after removing a corresponding number of shoots to retain original density. Each plot contained six pots: the three monospecific pots (bundles with ten shoots inserted into pots with the same species) and the three two-species mixtures (ten shoots of each species inserted in pots with a mixture of the same species). In total, 432 bryophyte pots with marked shoot bundles were prepared. The pots were embedded in the bryophyte mat in each plot. The plants were collected from several patches, and the samples were randomly allocated to the experimental plots. In September 2008 (after 13.5 months), we harvested the pots and extracted the shoot bundles. Final length was measured and the number of side-shoots produced per bundle was counted.
Data analysis
The experiment is three-way factorial with four replicates (blocks), with the effect of neighboring species (treatment ‘Neighbor’) tested within each plot. Analysis of variance was run with Proc Mixed in SAS ver 9 (SAS Institute 2004). The four-way interaction was used as a random effect representing the effect of plot. Residual plots showed a good fit to a normal distribution for all responses. We analysed the environment and neighbor effects on each species separately, since more complex models with ‘species’ as a factor led to high-level interactions that were hard to interpret.
Results
Overall growth and production of side-shoots (branching)
The average length growth was lowest in S. palustre [S. palustre: 25.4 mm (SEM = 1.64); S. magellanicum 34.4 mm (2.55); P. strictum: 33.6 mm (1.53)] and it also produced fewer side-shoots per bundle than the other species [S. palustre: 5.7 (SEM = 0.37); S. magellanicum: 8.4 (0.61); P. strictum: 7.0 (0.41)]. There was a positive correlation between length growth and production of side-shoots which was stronger in Sphagnum (Pearson correlations: S. magellanicum, r = 0.69, P < 0.001; S. palustre, r = 0.61, P < 0.001) than in P. strictum (r = 0.42, P < 0.001).
Effects of phosphorus, nitrogen and temperature
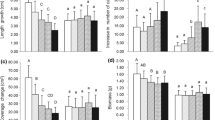
In all species, length growth increased gradually with increasing phosphorus addition (Fig. 1, Table 1a). The increase was strongest in S. magellanicum (150% higher growth at P2 than at P0), intermediate in S. palustre (85%) and weakest in P. strictum (64%). Growth decreased with nitrogen addition but the effects were only seen at the highest dose (compared to N0 the decrease at N2 was 22% in P. strictum, 56% in S. magellanicum, and 58% in S. palustre).
Main effects of phosphorus (upper panels) and nitrogen (lower panels) on a, c length increment and b, d side-shoots in Polytrichum strictum (Ps), Sphagnum magellanicum (Sm) and S. palustre (Sp). Side-shoots is the number of side-shoots produced per ten shoots. Bars represent means over all levels of the other treatments (n = 72). Vertical bars show Tukey Least Significant Difference for the comparison of treatments based on standard errors of the differences between means within each species (α = 0.05)
In Sphagnum, branching by side-shoot production responded similarly to growth, but in P. strictum branching instead increased with nitrogen addition, whereas it was indifferent to phosphorus addition (Fig. 1, Table 1b).
The only main effect of temperature was that branching increased in P. strictum from an average of 5.7 side-shoots produced per bundle at ambient temperature to 8.3 at raised temperature (P = 0.0083; Table 1b). There were no two-way interactions among the P, N and T treatments.
Effects of neighboring species and interactions with P, N and T
There was only one significant main effect of the neighboring species: the length growth of P. strictum was higher when growing with S. palustre than when growing in monospecific pots or with S. magellanicum (P = 0.0195, Table 1a).
Yet, there were several cases with interactions between the effects of neighbors and P or T (but none with N) (Table 1; Fig. 2). The effect of adding P depended on the identity of neighbors for length growth in Polytrichum and for both growth and branching in S. magellanicum. In these cases, the positive effect of phosphorus appeared at a lower dose with S. palustre as a neighbor than with any of the other species as neighbors, or, in other words, the positive effect of P1 did not occur when the neighbor was P. strictum or S. magellanicum.
Interaction diagrams showing significant interactions between neighbor and a–c phosphorus or d–e temperature. Abbreviations indicate the identity of the neighboring species in a pot: Ps Polytrichum strictum, Sm Sphagnum magellanicum and Sp S. palustre. Side-shoots is the number of side-shoots produced per ten shoots. Vertical bars show Tukey Least Significant Difference for the comparison of neighboring species within each level of phosphorus or temperature based on standard errors of the differences between means (α = 0.05). Note that the y-axes do not start at zero
Only in S. palustre was there a T × Neighbor interaction: S. palustre responded positively to high temperature when it was growing in monospecific pots, but was negatively affected when it grew with P. strictum (Fig. 2).
Discussion
The effects were more complex than stated in our hypotheses. Not only Sphagnum but also Polytrichum showed reduced growth (to a lesser degree) when N was added. The most important results were that with N addition branching increased in Polytrichum, whereas it decreased in Sphagnum. In contrast, with P addition both Sphagnum and Polytrichum showed increased growth but only Sphagnum had a concomitant increase in branching.
Direct effects of nitrogen, phosphorus and temperature
It has been suggested that the critical load for Sphagnum growth in bogs corresponds to a nitrogen deposition at or slightly above 1 g N m−2 year−1 (e.g., Vitt et al. 2003; Bragazza et al. 2004; Granath et al. 2009). Even though the response may be species-specific (e.g., van der Heijden et al. 2000), our study shows a strong effect on growth at 2 g N m−2 year−1, which fits well with this notion, as well as with the suggested critical load of nitrogen deposition (1–2 g N m−2 year−1) suggested for the Changbai Mountains region (Duan et al. 2002). The negative effect of N on Sphagnum growth appeared after a shorter time than in experiments on Swedish bogs (Gunnarsson and Rydin 2000; Gunnarsson et al. 2004). The reason is probably that Hani Peatland is minerotrophic, and, as also indicated by the rather dense vascular plant cover between the Sphagnum hummocks, with higher N availability and high N:P ratio. There are no deposition data available, but we assume that the industrial cities of Baishan (about 500,000 inhabitants; 30 km from Hain) and Jilin (almost 2 million inhabitants and with petrochemical industries; 170 km from Hani) represent considerable N sources.
The assumption has been made that Sphagnum growth in peatlands is naturally N-limited, but that N deposition gradually leads to increasing phosphorus limitation. This is sometimes inferred from geographical comparisons (Bragazza et al. 2004), but the strongest evidence comes from the experiments by Aerts et al. (1992) comparing the effects of N and P additions in regions with high and low N deposition. P limitation in Hani Peatland could explain why all the three bryophytes showed positive response to P addition even without N-fertilization. In an experiment, a shift from N to P limitation ought to lead to a statistical N × P interaction, which we did not detect in any of the species. Another field experiment showing an effect of P independent of N on Sphagnum growth was performed in the Italian Alps (Gerdol et al. 2007). In Scotland, Carfrae et al. (2007) obtained results that corroborate our findings: addition of N reduced side-shoot production and length growth in S. capillifolium, and these effects were counteracted when P was added.
Peatland ecosystems in the northern hemisphere are generally located in areas with low temperature and a short growing season, with temperature as a strong predictor of Sphagnum productivity (Gunnarsson 2005). However, effects on Sphagnum growth from field experiments with open top chambers range from positive (Dorrepaal et al. 2003) to negative (Gunnarsson et al. 2004). Our experiment did not show any effect on growth, indicating that even if there are direct positive effects they may be outbalanced by an indirect negative effect through desiccation (Weltzin et al. 2001; Dorrepaal et al. 2003; Gerdol et al. 2008). P. strictum was able to increase its branching with increasing temperature. The reason may be that the vascular system for water transport in Polytrichum stems enables it to avoid desiccation in the high-temperature treatment. This is also in line with the observation that Polytrichum is relatively independent of climatic humidity (Callaghan et al. 1978; Vitt 1990).
Inter-specific interactions under environmental changes
We did not expect the negative effect of N on P. strictum growth, and indeed the reduction was only 22% compared to almost 60% in Sphagnum. We used NH4NO3 in our experiments, and if nitrification is poor the results are in line with the observation by Paulissen et al. (2005) who showed that P. commune was less sensitive than S. contortum and S. squarrosum to ammonium. While P. strictum had a growth rate that was intermediate between S. magellanicum and S. palustre at the lower N doses, it outgrew S. magellanicum by 9 mm and S. palustre by 14 mm at 2 g N m−2 year−1. This result is similar to the relationship between P. strictum and S. fallax found by Mitchell et al. (2002), and was even more accentuated the effect of N on branching—positive in Polytrichum and negative in Sphagnum. This gives Polytrichum a double competitive advantage in the high-N treatment, where it could both overtop Sphagnum and expand laterally. Also, this result resembles the positive effect on shoot density in P. strictum and negative in S. fallax found by Mitchell et al. (2002).
As expected, Sphagnum reacted positively to phosphorus. Even though Polytrichum also increased its growth, it failed to increase its branching (in contrast to Sphagnum). Hence, the relative uncoupling of length growth and branching in P. strictum works to its advantage when N is added, but to its disadvantage when P is added. Because of such uncoupling in responses, community effects of environmental changes cannot be safely predicted solely from measurements of growth. Plant–plant interaction studies often focus on growth, ignoring other responses such as fecundity (review by Aarssen and Keogh 2002). In closed bryophytes mats establishment by sexual reproduction is rare, but our findings illustrate the weakness of using growth as the only response variable, and highlight the importance of, in addition to growth, also measuring the potential for asexual, clonal expansion when evaluating how inter-specific interactions are altered by environmental changes.
The experiments were made in S. palustre hummocks, and one might therefore have expected that S. palustre should have a general advantage. It did increase its growth rate at the intermediate dose of P, but both P. strictum and S. magellanicum performed better when growing with S. palustre than in monospecific pots, indicating that S. palustre is the weakest competitor for phosphate (perhaps by less efficient P uptake). S. magellanicum has earlier been identified as a strong competitor (Mulligan and Gignac 2002). The reasons for the difference in branching response between Polytrichum and Sphagnum are not clear. In Sphagnum, the probability of creating a side-shoot is related to shoot size (Haig 1989), which leads to a rather strong correlation between branching and growth. In Polytrichum, it appears that a reduced growth is linked to a weaker apical dominance in the shoots.
Implications for vegetation development
Both competition and facilitation are important factors structuring plant communities (Bruno et al. 2003; Brooker et al. 2008), and a large body of research has shown that facilitation may be more common than competition in bryophytes because of the positive effects on shoot water balance (review in Rydin 2009). For instance, P. strictum often grows in a matrix of Sphagnum, and its short-term competitive advantage in our fertilization experiment may well be disadvantageous in the long run if it is dependent on Sphagnum as substrate. However, the direction of interspecific interaction may shift into facilitation following disturbances that reduce bryophyte cover and open up patches with bare peat. In such situations, P. strictum can establish quickly and then act as a nursing plant for Sphagnum re-establishment by creating a suitable microclimate (Groeneveld et al. 2007). With recurrent disturbances, long-term species coexistence will then depend on the balance between facilitation in the regenerative stage and both competition and facilitation in the established stage (cf. Grubb 1977).
With increasing temperature, our results (the neighbor effect of Polytrichum and the increased branching in Polytrichum) indicate that S. palustre should decrease relative to P. strictum. With a slight increase in phosphorus availability, our results indicate that the higher growth potential of P. strictum and S. magellanicum (both in length growth and in branching) will give them a competitive superiority over S. palustre. With increasing nitrogen availability, the negative growth response of all three species implies that the bryophytes may give way to a dominance of vascular plants (Berendse et al. 2001).
In mixed communities, indirect effects, mediated by altered inter-specific interaction among species, may lead to altered species composition. Mixed peatland bryophyte communities, which are often established following re-colonization of disturbed patches (Soro et al. 1999), are not rare in the northern hemisphere. This suggests that effects mediated by altered interspecific interaction may be common and should be acknowledged when assessing community and ecosystem effects of environmental changes.
References
Aarssen LW, Keogh T (2002) Conundrums of competitive ability in plants: what to measure? Oikos 96:531–542
Aerts R, Wallén B, Malmer N (1992) Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. J Ecol 80:131–140
Berendse F, van Breemen N, Rydin H, Buttler A, Heijmans M, Hoosbeek MR, Lee JA, Mitchell E, Saarinen T, Vasander H, Wallén B (2001) Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Global Change Biol 7:591–598
Bragazza L (1997) Sphagnum niche diversification in two oligotrophic mires in the Southern Alps of Italy. Bryologist 100:507–515
Bragazza L, Tahvanainen T, Kutnar L, Rydin H, Limpens J, Hájek M, Grosvernier P, Hájek T, Hájkova P, Hansen I, Iacumin P, Gerdol R (2004) Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen deposition in Europe. New Phytol 163:609–616
Breeuwer A, Heijmans MMPD, Robroek BJM, Berendse F (2008) The effects of temperature on growth and competition between Sphagnum species. Oecologia 156:155–167
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JMJ, Anthelme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire F, Quiroz CL, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R (2008) Facilitation in plant communities: the past, the present and the future. J Ecol 96:18–34
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Bu Z, Yang Y, Dai D, Wang X (2005) Age structure and growth pattern of Polytrichum juniperinum populations in a mire of Changbai Mountains. Chin J Appl Ecol 16:44–48
Bubier JL, Moore TR, Bledzki LA (2007) Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog. Global Change Biol 13:1–19
Callaghan TV, Collins NJ, Callaghan CH (1978) Photosynthesis, growth and reproduction of Hylocomium splendens and Polytrichum commune in Swedish Lapland. Oikos 31:73–88
Carfrae JA, Sheppard LJ, Raven JA, Leith ID, Crossley A (2007) Potassium and phosphorus additions modify the response of Sphagnum capillifolium growing on a Scottish ombrotrophic bog to enhanced nitrogen deposition. Appl Geochem 22:1111–1121
Chai X (1990) Peatland science. Geological Publishing House, Beijing
Chen X, Bu Z, Wang S, Li H, Zhao H (2009) Niches of seven bryophyte species in Hani Peatland of Changbai Mountains. Chin J Appl Ecol 20:574–578
Dorrepaal E, Aerts R, Cornelissen JHC, Callaghan TV, van Logtestijn RSP (2003) Summer warming and increased winter snow cover affect Sphagnum fuscum growth, structure and production in a sub-arctic bog. Global Change Biol 10:93–104
Dorrepaal E, Toet S, van Logtestijn R, Swart E, van de Weg M, Callaghan T, Aerts R (2009) Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460:616–619
Duan L, Hao J, Xie S, Zhou Z (2002) Estimating critical loads of sulfur and nitrogen for Chinese soils by steady state method. Environ Sci 23:7–12
Freeman C, Ostle N, Kang HJ (2002) An enzymic ‘latch’ on a global carbon store. Nature 409:149
Gerdol R, Petraglia A, Bragazza L, Iacumin P, Brancaleoni L (2007) Nitrogen deposition interacts with climate in affecting production and decomposition rates in Sphagnum mosses. Global Change Biol 13:1810–1821
Gerdol R, Bragazza L, Brancaleoni L (2008) Heatwave 2003: high summer temperature, rather than experimental fertilization, affects vegetation and CO2 exchange in an alpine bog. New Phytol 179:142–154
Granath G, Strengbom J, Breeuwer A, Heijmans MMPD, Berendse F, Rydin H (2009) Photosynthetic performance in Sphagnum transplanted along a latitudinal nitrogen deposition gradient. Oecologia 159:705–715
Groeneveld EVG, Massé A, Rochefort L (2007) Polytrichum strictum as a nurse-plant in peatland restoration. Restor Ecol 15:709–719
Grubb PJ (1977) The maintenance of species richness in plant communities: the importance of the regeneration niche. Biol Rev 52:107–145
Gunnarsson U (2005) Global patterns of Sphagnum productivity. J Bryol 27:267–277
Gunnarsson U, Rydin H (2000) Nitrogen fertilisation reduces Sphagnum production in Swedish bogs. New Phytol 147:527–537
Gunnarsson U, Granberg G, Nilsson M (2004) Growth, production and interspecific competition in Sphagnum: effects of temperature, nitrogen and sulphur treatments on a boreal mire. New Phytol 163:349–359
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Haig ETW (1989) Individual interactions in Sphagnum populations. PhD dissertation, University of London
Lang H, Zhao K, Chen K (1999) Wetland vegetation in China. Science Press, Beijing
Lang SI, Cornelissen JHC, Hölzer A, ter Braak CJF, Ahrens M, Callaghan TV, Aerts R (2009) Determinants of cryptogam composition and diversity in Sphagnum-dominated peatlands: the importance of temporal, spatial and functional scales. J Ecol 97:299–310
Limpens J, Tomassen HBM, Berendse F (2003) Expansion of Sphagnum fallax in bogs: striking the balance between N and P availability. J Bryol 25:83–90
Limpens J, Berendse F, Klees H (2004) How phosphorus availability affects the impact of nitrogen deposition on Sphagnum and vascular plants in bogs. Ecosystems 7:793–804
Mitchell EAD, Buttler A, Grosvernier P, Rydin H, Siegenthaler A, Gobat J-M (2002) Contrasted effects of increased N and CO2 supply on two keystone species in peatland restoration and implications for global change. J Ecol 90:529–533
Mulligan R, Gignac D (2002) Bryophyte community structure in a boreal poor fen II: interspecific competition among five mosses. Can J Bot 80:330–339
Paulissen MPCP, Besalú LE, de Bruijn H, van der Ven PJM, Bobbink R (2005) Contrasting effects of ammonium enrichment on fen bryophytes. J Bryol 27:109–117
Qiao S (1993) A preliminary study on Hani peat mire in the west part of the Changbai Mountain. Sci Geogr Sin 13:279–286
Rincon E, Grime JP (1989) An analysis of seasonal patterns of bryophyte growth in a natural habitat. J Ecol 77:447–455
Robroek BJM, Limpens J, Breeuwer A, Crushell PH, Schouten MGC (2007) Interspecific competition between Sphagnum mosses at different water tables. Funct Ecol 21:805–812
Robroek BJM, Schouten MGC, Limpens J, Berendse F, Poorter H (2009) Interactive effects of water table and precipitation on net CO2 assimilation of three co-occurring Sphagnum mosses differing in distribution above the water table. Global Change Biol 15:680–691
Rydin H (1985) Effect of water level on desiccation of Sphagnum in relation to surrounding Sphagna. Oikos 45:374–379
Rydin H (2009) Population and community ecology of bryophytes. In: Shaw AJ, Goffinet B (eds) Bryophyte biology. Cambridge University Press, Cambridge, pp 393–444
Rydin H, Jeglum JK (2006) The biology of peatlands. Oxford University Press, Oxford
SAS Institute (2004) SAS OnlineDoc 9.1.3. SAS Institute, Cary, NC
Soro A, Sundberg S, Rydin H (1999) Species diversity, niche width and species associations in harvested and undisturbed bogs. J Veg Sci 10:549–560
Sun Q, Bu Z, Wang S, Meng X (2005) Preliminary study on density dependence of Sphagnum imbricatum population in Hani oligotrophic mire. Wetl Sci 3:116–120
van der Heijden E, Jauhiainen J, Silvola J, Vasander H, Kuiper PJC (2000) Effects of elevated atmospheric CO2 concentration and increased nitrogen deposition on growth and chemical composition of ombrotrophic Sphagnum balticum and oligo-mesotrophic Sphagnum papillosum. J Bryol 22:175–182
Vasander H, Kettunen A (2006) Carbon in boreal peatlands. In: Wieder K, Vitt DH (eds) Boreal peatland ecosystems. Springer, Berlin, pp 165–194
Verhoeven JTA, Liefveld WM (1997) The ecological significance of organochemical compounds in Sphagnum. Acta Bot Neerl 46:117–130
Vitt DH (1990) Growth and production dynamics of boreal mosses over climatic, chemical and topographic gradients. Bot J Linn Soc 104:35–59
Vitt DH, Wieder K, Halsey LA, Turetsky M (2003) Response of Sphagnum fuscum to nitrogen deposition: a case study of ombrogenous peatlands in Alberta, Canada. Bryologist 106:235–245
Weltzin JF, Harth C, Bridgham SD, Pastor J, Vonderharr M (2001) Production and microtopography of bog bryophytes: response to warming and water-table manipulations. Oecologia 128:557–565
Wyatt R, Derda S (1997) Population biology of the Polytrichaceae. Adv Bryol 6:265–296
Acknowledgments
This study was funded by the Natural Science Foundation of China (contract No. 30700055 and No. 40971036), The National Grand Fundamental Research 973 Program of China (No. 2009CB426305), the Training Fund of NENU’S Scientific Innovation Project (contract No. NENU-STB07002) and the Swedish Research Council Formas. We thank Xiangjun Meng and Gaolin Zhao for open top chamber preparation, Yuxin Jiao and Gaolin Zhao for bryophyte samples preparation, Lihong Jiang, Yuan Tang, Chunquan Wang, Jinbin Xu, Yi Han and Luwu Xie for fertilization, and Zhiwei Xu, Meijuan Zhou and others for the laboratory work. Lennart Norell, Wei Gao and Lei Shi helped us with the statistical analysis. Urban Gunnarsson, Joachim Strengbom and Sebastian Sundberg kindly commented on the manuscript. The experiment complies with all laws of the People’s Republic of China, where it was performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernhard Schmid.
Rights and permissions
About this article
Cite this article
Bu, ZJ., Rydin, H. & Chen, X. Direct and interaction-mediated effects of environmental changes on peatland bryophytes. Oecologia 166, 555–563 (2011). https://doi.org/10.1007/s00442-010-1880-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1880-1