Abstract
Understanding patterns of gene flow, selection and genetic diversity within and among populations is a critical element of predicting how long-term changes in environmental conditions are likely to affect species distribution. The intertidal mussel Perna perna consists of two distinct genetic lineages in South Africa, but the mechanisms maintaining these lineages remains obscure. We used regional oceanography and lineage-specific responses to environmental conditions as proxies for gene flow and local selection, respectively, to test how these mechanisms could shape population genetic structure. Laboratory experiments supported the field findings that mussels on the east coast (eastern lineage) are physiologically more tolerant of sand inundation and high temperatures than those on the south coast (western lineage). Temperature loggers mimicking mussel body temperatures revealed that mussels experience higher body temperatures during aerial exposure on the subtropical east coast than on the temperate south coast. Translocations showed that, on the east coast, the western lineage suffered higher mortality rates than local individuals, while on the south coast, mortality rates did not differ significantly between the lineages. Nearshore drogues showed remarkably little overlap between the trajectories of drifters released off the south coast and those released off the east coast. Physiological tolerances can thus explain the exclusion of western individuals from the east coast, but they cannot explain the exclusion of the eastern lineage from the south coast. In contrast, however, ocean dynamics may limit larval dispersal between the two lineages, helping to explain the absence of eastern individuals from the south coast. We emphasise the importance of a multidisciplinary approach in a macro-ecological context to understand fully the mechanisms promoting evolutionary divergence between genetic entities. Our results suggest that phylogeographic patterns of Perna perna may be maintained by a combination of local conditions and the isolating effect of the Agulhas Current that reduces gene exchange.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sharp breaks in the genetic structure of broad-ranging species tend to coincide with recognised biogeographic boundaries (Avise 2000). In marine ecosystems, genetic differentiation among contiguous coastal populations can be caused by barriers to dispersal (e.g., currents, upwelling) and environmental gradients through the regulation of interpopulation connectivity or natural selection (Gaylord and Gaines 2000; O’Hara and Poore 2000; Sotka et al. 2004; Teske et al. 2008). The model of Pringle and Wares (2007) indicates that genetic structure in species with high dispersal requires strong selection and/or alongshore variation in currents or habitat quality. In order to eliminate the competing hypothesis that such genetic discontinuities arise from stochastic processes, it is therefore necessary to integrate methods from different fields (genetics, physiology and oceanography) and to use different experimental procedures, such as common garden and reciprocal transplant experiments (Chown and Gaston 2008; Prada et al. 2008; Teske et al. 2008). In the study reported here, we apply such an integrated approach in an attempt to explain the phylogeographic structure exhibited by the intertidal mussel Perna perna in South Africa.
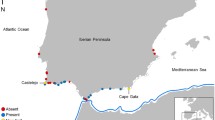
The South African coastline covers a wide range of climatic and oceanic conditions and can be divided into biogeographic regions that support different intertidal biota (Emanuel et al. 1992; Harrison 2000). Based on an analysis of rocky shore invertebrates, Emanuel et al. (1992) divided the South African coast into three zoogeographic regions: a cool-temperate Namaqua Province (from Lüderitz in Namibia to Cape Point), a warm-temperate Agulhas Province (from Cape Point eastward to East London), and a subtropical Natal Province (from East London north to Mozambique; Fig. 1). P. perna is an abundant habitat-forming species found across all three of these biogeographic regions. Although this mussel species has planktotrophic larvae with high dispersal potential, an earlier study indicated that it is genetically structured along the South African coast. In particular, analyses of mitochondrial DNA (mtDNA)sequences indicated a phylogeographic break on the south-east coast (Zardi et al. 2007). A western lineage of P. perna includes animals from both the Namibian coast and south coast of South Africa. This is striking because P. perna is absent from the upwelling-influenced Benguela region on the west coast of South Africa so that this lineage spans a lacuna in the distribution of P. perna of some 1,000 km. The second, eastern lineage includes individuals from the south-east and east coasts of South Africa. The two lineages overlap in their distributions on the south-east coast over a distance of approximately 200 km. This description of the phylogeography of P. perna is based on a single genetic marker, and different genes can have unique histories so that the mitochondrial and nuclear genome of the same individual may exhibit different patterns because of their different forms of inheritance and the effects of non-neutral processes (Gompert et al. 2008). Consequently, genetic structure can result from multiple interacting factors, and a much more robust understanding emerges from examining multiple genetic markers and multiple species (Sotka and Palumbi 2006).
Float patterns for drifters released on the south coast (blue) and on the east coast (red) of South Africa between 2002 and 2007. Dashed lines are the continental shelf bathymetry (−500 and −1,000 m). Black dots indicate the position of release, and numbers refer to drifters named in the text. Location of coastal cities are indicated by white dots
In the case of P. perna, our confidence in the validity of these lineages is strengthened by several other studies that have used a variety of genetic markers and approaches to demonstrate similar genetic clines in other organisms along this part of the coastline. These involved a range of species with different modes of reproduction, living in different marine environments, including limpets (Ridgeway et al. 1998) and a variety of crustaceans (Teske et al. 2006, 2007a, b). Importantly, phylogeographic studies of crustaceans demonstrating the separation of major groupings in the same region revealed congruence between morphology and mtDNA in the case of a crab (Edkins et al. 2007) and between mitochondrial and nuclear makers in the case of a prawn (Teske et al. 2009). Consequently, despite the potential limitations of a description of phylogeography based on a single genetic marker, evidence from other studies supports the interpretation presented here.
Multiple mechanisms may be at play in establishing the observed distribution of P. perna lineages. Phylogeography can be strongly affected by hydrodynamics, and currents can play a crucial role in regulating larval dispersal and, consequently, the degree of connectivity among local adult populations (Chiswell et al. 2003; Sotka et al. 2004). The effects of hydrodynamics are not simple, as the interaction of propagule behaviour with water movement can be extremely complex, particularly because hydrodynamics affect propagule dispersal across an enormous range of spatial scales (Pineda 2000; Shanks et al. 2003) and because different larval stages of the same species may differ in their behaviour or position in the water column (Gallager et al. 1996; Tapia and Pineda 2007). There is even evidence that conspecific larvae from different populations can behave differently (Manuel and O’Dor 1997), with the potential of altering their dispersal. Nevertheless, there appear to be robust links between scales of dispersal and the duration of the planktonic phase of the life cycle of benthic organisms (Kinlan and Gaines 2003). The warm Agulhas Current is the primary oceanographic feature on the east and south coasts of South Africa (Fig. 1). It flows to the southwest at rates of 10–20 km day−1, following the 200-m isobath of the continental shelf from Maputo (Mozambique) to the tip of the Agulhas Bank in South Africa (Lutjeharms 1998) where the current undergoes a dramatic retroflection, moving hundreds of miles away from the mainland. The inshore thermal front of this current varies both geographically and in time. On the east coast, it usually lies 14–38 km offshore, but it lies much closer inshore near the transition between the temperate and subtropical region (0–1 km offshore; Goschen and Schumann 1990), consequently influencing the along-shore transport of larvae in this area (Lutjeharms 2004). On the south coast, where the shelf is wider, the Agulhas Current still influences an extensive area of the shelf (Goschen and Schumann 1988), although wind is the main forcing function (McQuaid and Phillips 2000).
Similarly, biogeographic distribution patterns can be driven by pre- and post-settlement mortality (e.g., Suchanek et al. 1997; Braby and Somero 2006). We examined the potential roles of oceanography (primarily the Agulhas Current) and environmental factors (specifically temperature) in forming and maintaining the phylogenetic structure indicated by mtDNA in P. perna. We used manipulative experiments and the release of oceanographic drifters to test the following hypotheses: (1) the south-flowing Agulhas Current lies close inshore in the region of overlap between the two lineages and acts as a physical barrier, limiting the dispersal of larvae from west to east and carrying larvae that originate on the east coast offshore so that they cannot reach a suitable habitat; (2) the environmental conditions experienced in the different biogeographic regions (warm-temperate and subtropical provinces) help to maintain the genetic structure seen by subjecting adult P. perna populations to different selective forces.
Materials and methods
Field experiments
Transplants
Transplant experiments were run twice because during the first experiment one of the sites was heavily affected by sand inundation. Data obtained from the two experiments were treated separately. Each experiment involved three treatments, each with four replicates. The treatments were: undisturbed (U), where quadrats were marked in situ, with no disturbance of the mussels within the marked quadrat; transplant (T), in which mussels were taken from their site of origin and translocated to a second site; disturbed (D), where mussels were translocated between locations within the same site. The study sites have similar environmental conditions (i.e., same tidal range, wave exposures, same orientation towards the incoming waves, same shoreline angle), with the exception of differences in temperature.
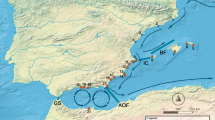
The first experiment was performed for 1 month in the summer of 2006 (November) at two locations (Fig. 1): Hougham Park (HP) on the south coast and Haga Haga (HH) on the east coast. There were two sites at each location, about 10 km apart (HP: 33°44′12″S, 25°48′24″E; 33°46′58″S, 25°43′01″E; HH: 32°46′51″S, 28°12′01″E; 32°44′58″S, 28°16′49″E). After 1 month, HP was inundated with sand due to the construction of a nearby harbour, resulting in the burial of the animals used for the experiment. Nevertheless, mortality rates were recorded at HP and used to investigate tolerance to sand stress of the two lineages. Mortality rates at HH were not recorded. As the experimental site at HP was disrupted by sand inundation, the experiment was repeated. The second experiment took place at two locations, Cape Recife on the south coast (CR: 34°01′53″S, 25°42′07″E; 34°02′28″S, 25°32′01″E) and again at HH on the east coast (Fig. 2). This experiment ran for 45 days at the end of the 2007 summer (March to April).
Cumulative mortality rates of Perna perna mussels [mean percentage + standard deviation (SD); sites pooled] at 15, 30 and 45 days after treatment initiation [disturbed (D), transplanted (T) and undisturbed (U)] in transplant experiment 2 performed at Cape Recife on the south coast (a) and Haga Haga on the east coast (b)
Mortality of mussels was assessed to be due to a failure to close the valves when disturbed and was recorded after 30 days for experiment 1 (end of the experiment) and at 15, 30 and 45 days for experiment 2. In experiment 1, mortality data were analysed using two-way analysis of variance (ANOVA) with treatment (U, D, T) as a fixed factor and sites (A, B) nested in treatment. In experiment 2, mortality data for the last measurement were analysed as above for each location separately (CR, HH).
At each site, mussels (shell length 4–5 cm) were collected from the mid-mussel zone and kept immersed in a controlled environment chamber at 19°C (±0.5°C) under a 12/12-h (light/dark) photoregime for 2 days before being returned to the field. For each D and T treatment at each site, 50 mussels were placed in five metal quadrats (n = 10 each quadrat) and covered with a coarse plastic mesh (2 cm) so that they could re-attach firmly. The temperature experienced by mussels with and without the mesh was the same, based on the results of tests using two temperature loggers (see description below), one covered and the other uncovered by mesh, for a period of 1 month (data not shown). Treatment U involved five patches of ten mussels attached on rocks at the same tidal level that were tagged in situ but otherwise left undisturbed. These mussels were tagged with a paint dot (diameter approx. 3 mm), which was fixed to the shell by covering it with superglue. Mortality for this treatment was recorded as number of survivors minus the number of mussels tagged at the beginning of the experiment.
Body temperature loggers
Temperature loggers (Tidbit V2 Dataloggers; Onset Computer Corp, Pocassett, MA) were encased in artificial mussels with a length of approximately 7 cm and made of resin in order to mimic the thermal characteristics of living mussels (as described in Helmuth et al. 2002 and Fitzhenry et al. 2004). These biomimetic loggers (robomussels) were deployed in the mid-mussel zone at each study site, adjacent to the mussels used in the transplant experiment. The robomussels were glued to the rocks using epoxy (Z-Spar A-788), and they were placed vertically within natural mussel beds to mimic the natural growth position of the surrounding mussels as closely as possible. Previous tests with the comparably sized mussel Mytilus californianus have shown that robomussel temperatures are, on average, within 2°C of the temperature of living mussels, and both are often quite different from the surrounding air temperature (Fitzhenry et al. 2004). Data from the loggers were used to estimate average aerial and total body temperatures at the two sites; rapid temperature drops in the temperature records (diagnostic of wave splash) were used as indicators of submergence and emersion (see Harley and Helmuth 2003).
Laboratory experiment
Mussels (shell length 4–5 cm) of both lineages were collected from the mid mussel zone at the study sites. Before the experiments, all individuals were acclimated in oxygenated seawater for 17 days in a controlled environment chamber at 19°C under a 12/12-h (light/dark) regime.
Mussels were then exposed to air in a controlled environment chamber set at 19°C (±0.5°C) and 60% humidity (n = 10 of each lineage at each site, four replicates). The temperature of the chamber was raised at a rate of 1°C every 10 min until 45°C was reached and then kept at this temperature for 20 min, simulating the body temperatures recorded by the temperature loggers in the field at HH (see body temperature loggers in “Results”). At the end of the experiment, the percentage of mussel mortality was checked, based on the failure to close valves when disturbed.
The experiment was also performed at 40, 35 and 19°C (for each temperature, n = 10 of each lineage at each site, four replicates). When the experimental temperature was reached, it was maintained until total exposure time at that temperature was 280 min. Mortality rates were analysed using three-way ANOVA, with lineage (western, eastern) and temperature (45, 40, 35 and 19°C) as a fixed factor and sites nested in lineage.
Oceanographic drifters
The trajectories of 17 satellite drifters released between 2002 and 2007 were used to determine circulation patterns in the surface layer of the oceans around the South African coastline. The drifters were of the holey-sock type, and design criteria met WOCE standards (Niiler et al. 1995) that minimise wind-induced slip. Ten drifters (numbered 1–10, Fig. 1) were released on the south coast between Plettenberg Bay and Port Alfred (Fig. 1); all were deployed within 10 km of the coast in less than 100 m of water, except for drifters 1 and 2, which were deployed 35 km offshore. On the east coast, seven drifters (11–17, Fig. 1) were deployed north of Durban, all beyond the shelf break in the region of Maputo Bay, except for one deployment that took place in the same region 5 km off the Sodwana Bay coast. The drifters were deployed throughout the year and communications lasted between 6 and 173 days. GPS positions were obtained through the Inmarsat Indian Ocean Region Satellite and interpolated using a cubic spline interpolation.
Results
Field experiments
Transplants
Data obtained from the two experiments were treated separately. In experiment 1, at HP (sand inundated site), mortality rates were significantly different among the three treatments (p ≤ 0.01; data not shown). Undisturbed (U) and disturbed (D) treatments showed a significantly higher mortality rate than the transplanted treatment [T; Student–Newman–Keuls (SNK) test p ≤ 0.01], but U and D mortality rates were not significantly different.
In experiment 2, there was no treatment effect (p = 0.65) at CR (Fig. 2a). At HH (Fig. 2b), the U and D treatments showed significantly lower mortality than the T treatment (SNK test p ≤ 0.01), but there were no significant differences between U and D (i.e., western lineage mussels suffered a higher mortality than eastern lineage individuals native to HH, and disturbance had no effect).
Body temperature loggers
During the 45-day experiments, maximum temperatures recorded by the loggers occurred during emersion and were higher at HH (maximum 45.09°C; Fig. 3a) than at CR (maximum 37.6°C; Fig. 3b). At CR, low tide temperatures rose above 35°C on only one occasion, while at HH temperatures peaked over 35°C on several occasions. During emersion, average body temperature was 20.25 and 18.38°C for HH and CR, respectively, while the overall average was 19.9 and 19.19°C, respectively.
Laboratory experiment
While the Western lineage had significantly greater mortality rates than the Eastern lineage at both 45 and 40°C (40 and 29% more; SNK test p < 0.001; Fig. 4), no differences were observed at 35 or 19°C (lineage × temperature interaction, p < 0.001).
Oceanographic drifters
There was remarkably little overlap between the trajectories of drifters released off the south coast and those released on the east coast (Fig. 1). Ocean-surface drifter 8 landed on the south coast in the vicinity (about 10 km) of its release point. Drifters 9 and 10 released off Port Alfred repeatedly drifted north and eastwards to Kidd’s Beach and were then caught by the Agulhas Current and moved primarily offshore and towards the southwest (Figs. 1, 5).
Drifters 1–7 flowed in the south-west direction along the continental shelf off the south coast and finally entered the Agulhas Current flux. These trajectories seem to follow the energetic, meandering and eddying flow of the Agulhas Current. None of the drifters released off the south coast between 2002 and 2007 moved close to the east coast. Surface drifters released on the east coast (11–17) were caught by the Agulhas Current, moved southwards away from the coast and never landed on the south coast. Only drifter 11 flowed relatively near (less than 10 km) the coastline north of Port Alfred.
Discussion
Although our recognition of a phylogeographic break in P. perna is based on a single mtDNA marker (Zardi et al. 2007), field and laboratory experiments have identified strong differences between the two lineages in terms of their physiological tolerances. The eastern lineage was physiologically more robust, being more tolerant than western individuals of sand burial as well as high aerial body temperatures. Nevertheless, physiological mechanisms alone are not sufficient to explain the geographic separation of eastern and western lineages, and it is necessary to invoke the effects of oceanography.
Sediments can have several adverse effects on benthic organisms (Abelson and Denny 1997). Coarse sediments, such as sand and gravel, may scour surfaces and abrade tissue from organisms or remove the organism completely from a reef. Suspended particles may interfere with filter feeding of benthic invertebrates, and the deposition of fine sediments can interfere with settlement, growth and photosynthetic activity of organisms (Airoldi 1998; Cheung and Shin 2005). Consequently, sand inundation can strongly shape intertidal ecosystems (e.g., Littler et al. 1983; McQuaid and Dower 1990; Airoldi 2003) and has been shown to affect habitat segregation among species (Marshall and McQuaid 1989; Zardi et al. 2008). Our data show that, under field conditions, the two genetic lineages of mussels studied here have a different tolerance to sand stress. In particular, the eastern lineage had lower mortality rates when subjected to sand burial on the south coast than mussels from the local population. While sand inundation is widespread on the South African and other coasts (Bally et al. 1984; Zardi et al. 2006), it is essentially site-specific.
There are two additional environmental conditions that are pervasive but which differ between these two regions: air and sea water temperature. Exposure to air involves both desiccation and exposure to high daytime temperatures and is a primary limiting factor in the distribution of intertidal organisms in general (Newell 1979) and mussels in particular (Tsuchiya 1983; Braby and Somero 2006). During low tide, we found that the maximum and average body temperatures in the regions occupied by the two lineages differ, and our results show that the western lineage is much more vulnerable to high temperatures under laboratory conditions, experiencing higher mortality rates at 40 and 45°C. In contrast, at lower temperatures, mortality rates were very low or nonexistent (a single dead mussel at 35°C) in the western lineage and not significantly different from that of the eastern lineage.
While submerged, an ectothermic invertebrate is likely to have a body temperature similar to that of the surrounding water, and studies have shown strong impacts on growth and survival in mussels (e.g., Bayne et al. 1976). However, temperature extremes experienced by intertidal organism during low tide can far exceed those experienced during submersion (Helmuth 1998), so that even though metabolic rates are lower during aerial exposure, aerial body temperature is nevertheless an important driver of selection (Harley 2008). Natural populations of Mytilus trossulus experience maximum body temperatures during periods of tidal emersion (Hofmann and Somero 1995), and mass mortalities due to thermal stress are mainly the result of temperature extremes experienced during low tide (Suchanek 1978; Tsuchiya 1983; Williams and Morritt 1995). Likewise, mass mortalities of P. perna on the study coast can follow the appearance of hot dry conditions known as berg winds (CMcQuaid personal observation). A limited food supply may also have an effect on the mussels’ ability to handle thermal stress (Braid et al. 2005; Schneider et al. 2010). It has been suggested that low tide thermal stress events may have a greater effect on survival when they occur after periods of low food availability than they would when food was abundant (Schneider 2008; Schneider et al. 2010). However, along the South African coast, the biomass of intertidal filter-feeders is not related to intertidal primary production. At a local scale, filter-feeder biomass is known to be strongly influenced by wave action, implying that the local-scale water movements over-ride any effects that large-scale gradients of primary production may have on filter-feeders (Bustamante et al. 1995). As described in "Materials and methods", the transplant sites were carefully chosen to have similar wave exposure, and therefore food probably has a less relevant role. Recent evidence suggests that physiological damage experienced during low tide can have significant consequences for metabolic and stress-related processes during high tide (Place et al. 2008). When extreme environmental conditions occur during aerial exposure at low tide, they can create such high metabolic demands that these effects continue to affect animals during high tide (Place et al. 2008). Thus, body temperatures both in the air and in water are important to growth and survival, and they may interact to drive the physiological responses of intertidal organisms (e.g., Pincebourde et al. 2008).
Analysis of the data from the body temperature loggers (robomussels) revealed that the highest body temperatures are experienced during air exposure. Our two study areas are approximately 300 km apart and, critically, maximum temperatures were significantly higher on the east than on the south coast. During air exposure, P. perna displays “gaping behaviour” (periodically opening and closing of valves; Nicastro et al. 2010), and the robomussels used in these experiments are unable to mimic this behaviour. Previous studies with Mytilus have shown, however, that despite the increase in water loss, periodic gaping does not appear to have a significant influence on body temperature through evaporative cooling (Bayne et al. 1976; Elvin and Gonor 1979; Fitzhenry et al. 2004), so that gaping is probably aimed at increasing aerobic respiration rather than evaporative cooling (Lent 1969; Moon and Pritchard 1970; Bayne et al. 1976). Results from earlier studies indicate that robomussels provide realistic measurements of body temperatures in the field (Fitzhenry et al. 2004). Therefore, while it is possible that temperatures recorded by the instruments overestimated the temperature of animals in the field, the error would have been similar at both sites.
On the east coast, transplanted mussels suffered massive mortality in the first 15 days of the experiment, probably due to extreme body temperatures (over 40°C). In particular, more than 80% of transplanted mussels died and mortality rates were significantly higher than those of local individuals, even when these had been similarly disturbed. Likewise, on the south coast, most mortality occurred during the first part of the experiment; however, mortality rates were substantially lower than on the east coast and there was no significant difference between lineages. While it is possible that slow acclimation of mussels transplanted from the temperate south coast to the subtropical east coast could have resulted in lower levels of survival, these results, coupled with the laboratory results suggest the existence of distinct physiologically differences between the two lineages.
Thus, the combined field measurements of body temperatures and laboratory results correlated well with mortality during translocation in the absence of sand inundation. The experimental design showed no artifacts due to translocation, and individuals of the eastern lineage showed good survival both in their native environment and when moved westwards onto the south coast. In contrast, western lineage individuals survived well in their home environment but showed high mortality when moved eastwards. Taken together, the results from the field and laboratory experiments suggest that extreme temperatures occurring on the east coast are responsible for excluding the western lineage from this region, while adults of the eastern lineage are capable of surviving on the cooler south coast. Critical to testing whether this indicates that the observed phylogeographic structure derives from the action of environmental selection would be an examination of survival of the two lineages in the 200-km overlap region. Despite the wider latitudinal distribution of the western lineage than the eastern lineage, it is still unknown if western lineage animals are able to extend into subtropical and tropical areas of the south coast of Angola. An extended study further up the western African coastline is needed to determine if the presence of a similar transition zone (temperate–subtropical transition) as between the South African south-east coast results in an analogous genetic cline.
Apart from the mortality measured here, environmental stressors could also have sub-lethal physiological effects that could enhance the differences between the two genetic entities. In mussels, high aerial temperatures during low tide can lead to protein damage (e.g., Helmuth and Hofmann 2001; Halpin et al. 2002; Place et al. 2008) and reduced growth (e.g., Menge et al. 2002). In addition, environmental stressors, such as high temperature, can negatively affect the ability of organisms to reproduce because animals re-allocate energy away from gamete production and towards defense and repair mechanisms (e.g., Michalek-Wagner and Willis 2001; Petes et al. 2003; Place et al. 2008). The thermal stress-response can also alter the timing of reproduction with potential consequences for asynchrony and overall decreased fertilisation and recruitment success (Walther et al. 2002; Philippart et al. 2003; Petes et al. 2007) as well as rates of feeding (Pincebourde et al. 2008). All of these factors can lead to a decreased number of propagules and thus decrease species colonisation potentials (Minchinton and Scheibling 1991; van Erkom Schuring and Griffiths 1991; Balch and Scheibling 2000). Such effects could reduce the reproductive potential of the western lineage in populations living towards its eastern limit.
Thus, correlations among environmental temperatures to which the two lineages are exposed and their laboratory and field survival rates can explain the exclusion of the western lineage from the eastern part of the coast where it shows low survival. The converse is not true, however, and eastern adults translocated to the west have a survival rate equal to that of local individuals. Of course, this ignores sub-lethal effects and the possibility that larvae or newly settled eastern juveniles cannot survive in the west, but we can also seek explanations for the failure of the eastern lineage to occur farther west in terms of larval dispersal so that oceanography also plays a role. Oceanic fronts represent sharp discontinuities of physical and biochemical variables that are likely to represent barriers to larval dispersal and hence genetic exchange between populations (e.g., Palumbi 1994; Galarza et al. 2009). We have no evidence of any such discontinuities, but drifter trajectories showed very limited connection between waters originating on the east and south coasts. Drifters dispersed hundreds of kilometres within 10 days, yet over the long-term (months) there was remarkably little overlap between the trajectories of drifters released to the north and south of our genetic cline. When overlap was observed, it occurred outside the continental shelf, hundreds of kilometres offshore. Geographic trajectories of oceanic drifters suggest that ocean currents potentially contribute to maintaining the geographical patterns of gene flow found in previous studies (e.g., Zardi et al. 2007; Teske et al. 2007a, b). Mitochondrial haplotype frequencies indicate high levels of gene flow within lineages, but very limited flow between lineages (Zardi et al. 2007). The major oceanographic influence on the east and south coasts of South Africa is the Agulhas Current. This warm, powerful current flows to the southwest along the eastern seaboard of South Africa at rates of 10–20 km day−1, following the 200-m isobath of the continental shelf from Maputo in Mozambique to the tip of the Agulhas Bank, some hundreds of kilometres offshore of the South African coast (Fig. 1; Lutjeharms 1998, 2004).
The inshore thermal front of this current varies geographically and in time (Goschen and Schumann 1990). Inshore, between the Agulhas front and the coastline, cooler pockets of water flow parallel to the coast, but in the opposite direction to the Agulhas Current (i.e., towards the north-east; Lutjeharms 2004). In the region of the genetic break, on the south-east coast, the shelf width is minimal and the Agulhas Current lies close to the coast at 0–1 km offshore (Goschen and Schumann 1990). Lutjeharms (2004) showed that the counter currents are squeezed close to the shore and are often overpowered by the Agulhas. The trajectories of two drifters (9 and 10) released on the south coast support these results. After being released offshore of Port Alfred, they remained close to the shore (1–2 km offshore). Over the course of a few days, they showed a repeated pattern of drifting north-east before stopping in the vicinity of Kidd’s Beach (Fig. 1) and returning south-westwards again. They were finally caught in the Agulhas flux and carried hundreds of kilometres offshore.
It is increasingly clear that larvae need not behave as passive particles (Sponaugle et al. 2002) and that assumptions of passive dispersal indicate potential rather than realised dispersal (Roberts 1997). Drifter trajectories make no allowance for larval behaviour, which can profoundly influence dispersal trajectories (e.g., Shanks and Brink 2005), and so cannot fully represent how planktonic larvae disperse in the nearshore region. Nonetheless, these results strongly suggest that a high proportion of larvae that approach the region of the break from both west and east become entrained in the Agulhas Current, are transported away from the coast and are unable to return to the intertidal zone. The trajectory of drifter 11 in particular supports this conclusion. It was released north of the genetic break and although it flowed southwards and close (less than 10 km) to the coastline north of Port Alfred, it was flushed offshore by the current without reaching the coast.
Data from other species of mussels also support our results. Mytilus galloprovincialis is invasive in many parts of the world, occurring in temperate regions of both hemispheres (McDonald et al. 1991; Hilbish et al. 2000). After its arrival on the west coast of South Africa, it spread eastward along the south coast for 15 years (McQuaid and Phillips 2000), but its range extension has virtually ceased in the region of genetic disjunction in P. perna (Robinson et al. 2005). While this could be coincidental, it seems probable that the factors responsible for maintaining geographic separation of the two lineages of P. perna may also limit the demographic expansion of M. galloprovincialis, an invasive species with similar dispersal potential.
In conclusion, we show that two lineages of P. perna identified using mtDNA show different tolerances of environmental conditions that are correlated with their survival when translocated among regions. The results of our laboratory and field experiments suggest that this indicates local adaptation of the two lineages. This can explain the distribution of the western lineage, but not of the eastern lineage, and our study illustrates the value of using an approach of laboratory and manipulative experiments combined with oceanographic studies as indicators of evolutionary divergence between genetic entities. It has been traditionally assumed that marine range limits result primarily from strong gradients in water properties, such as water temperature that arise at oceanographic discontinuities (e.g., Hedgpeth 1957; Hayden and Dolan 1976; Suchanek et al. 1997; but see Clarke 1993). However, this view may overlook the possibility that ocean flows themselves may generate distributional patterns (e.g., Gaylord and Gaines 2000). Because it tends to advect larvae away from the coast, the Agulhas Current may inhibit gene flow along a linear coast. We suggest that, combined with environmental clines, this current helps to shape the genetic structure of P. perna. Our study underlines the importance of an integration of laboratory and field experiments in a macrophysiological context and on a spatially explicit basis to understand fully the relationship between species distributions and the environment and possibly to forecast distributional shifts under various climatic change scenarios.
References
Abelson A, Denny M (1997) Settlement of marine organisms in flow. Annu Rev Ecol Syst 28:317–339
Airoldi L (1998) Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 79:2759–2770
Airoldi L (2003) The effects of sedimentation on rocky coast assemblages. Oceanogr Mar Biol Annu Rev 41:161–236
Avise JC (2000) Phylogeography. Harvard University Press, Cambridge
Balch T, Scheibling RE (2000) Temporal and spatial variability in settlement and recruitment of echinoderms in kelp beds and barrens off Nova Scotia. Mar Ecol Prog Ser 205:139–154
Bally R, McQuaid CD, Brown AC (1984) Shores of mixed sand and rock: an unexplored marine ecosystem. S Afr J Sci 80:500–503
Bayne BL, Bayne CJ, Carefoot TJ, Thompson RJ (1976) The physiological ecology of Mytilus californianus Conrad. Oecologia 22:211–228
Braby C, Somero GN (2006) Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J Exp Biol 209:2554–2566
Braid BA, Moore JD, Robbins TT, Hedrick RP, Tjeerdema RS, Friedman CS (2005) Health and survival of red abalone, Haliotis rufescens, under varying temperature, food supply, and exposure to the agent of withering syndrome. J Invertebr Pathol 89:219–231
Bustamante RH, Branch GM, Eekhout S, Robertson B, Zoutendyk P, Schleyer M, Dye A, Hanekom N, Keats D, Jurd M, McQuaid CD (1995) Gradients of intertidal primary productivity around the coast of South Africa and their relationships with consumer biomass. Oecologia 102:189–201
Cheung SG, Shin PKS (2005) Size effects of suspended particles on gill damage in green-lipped mussel Perna viridis. Mar Pollut Bull 51:110–801
Chiswell SM, Wilkin J, Booth JD, Stanton B (2003) Trans-Tasman sea-larval transport: is Australia a source for New Zealand rock lobsters? Mar Ecol Prog Ser 247:173–182
Chown SL, Gaston KJ (2008) Macrophysiology for a changing world. Proc R Soc B 275:1469–1478
Clarke A (1993) Temperature and extinction in the sea: a physiologist’s view. Paleobiology 19:499–518
Edkins MT, Teske PR, Griffiths CL, Papadopulos I (2007) Genetic and morphological analyses suggest that southern African crown crabs, Hymenosoma orbiculare, represent five distinct species. Crustaceana 80:667–683
Elvin DW, Gonor JJ (1979) The thermal regime of an intertidal Mytilus californianus Conrad population on the central Oregon coast. J Exp Mar Biol Ecol 39:265–279
Emanuel BP, Bustamante RH, Branch GM, Eekhout S, Odendaal FJ (1992) A zoogeographic and functional approach to the selection of marine reserves on the west coast of South Africa. S Afr J Mar Sci 12:341–354
Fitzhenry T, Halpin PM, Helmuth B (2004) Testing the effects of wave exposure, site, and behaviour on intertidal mussel body temperatures: applications and limits of temperature logger design. Mar Biol 145:339–349
Galarza JA, Carreras-Carbonell J, Macpherson E, Pascual M, Roques S, Turner GF, Rico C (2009) The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. PNAS 106:1473–1478
Gallager SM, Manuel JL, Manning DA, O’Dor R (1996) The vertical distribution of scallop larvae Placopecten magellanicus in 9 m deep mesocosms as a function of light, food and temperature. Part I. Ontogeny of vertical migration behaviour. Mar Biol 124:679–692
Gaylord B, Gaines SD (2000) Temperature or transport? Range limits in marine species mediated solely by flow. Am Nat 155:769–789
Gompert Z, Forister ML, Fordyce DA, Nice CC (2008) Widespread mito-nuclear discordance with evidence for introgressive hybridization and selective sweeps in Lycaeides. Mol Ecol 17:5231–5244
Goschen WS, Schumann EH (1988) Ocean current and temperature structures in Algoa Bay and beyond in November 1986. S Afr J Mar Sci 7:101–116
Goschen WS, Schumann EH (1990) Agulhas current variability and inshore structures off the Cape Province, South Africa. J Geophys Res 95:667–678
Halpin PM, Sorte CJ, Hofmann GE, Menge BA (2002) Patterns of variation in levels of Hsp70 in natural rocky shore populations from microscales to mesoscales. Integr Comp Biol 42:815–824
Harley CDG (2008) Tidal dynamics, topographic orientation, and temperature-mediated mass mortalities on rocky shores. Mar Ecol Prog Ser 371:37–46
Harley CDG, Helmuth BST (2003) Local and regional scale effects of wave exposure, thermal stress, and absolute vs. effective shore level on patterns of intertidal zonation. Limnol Oceanogr 48:1498–1508
Harrison TD (2000) Preliminary assessment of the biogeography of the fishes in South African estuaries. Mar Freshw Res 53:479–490
Hayden BP, Dolan R (1976) Coastal marine fauna and marine climates of the Americas. J Biogeogr 3:71–81
Hedgpeth JW (1957) Estuaries and lagoons. II. Biological aspects. In: Hedgpeth JW (ed) Treatise on marine ecology and paleoecology 1. Memoir No. 67. Geological Society of America, Boulder, pp 673–693
Helmuth B (1998) Intertidal mussel microclimates: predicting the body temperature of a sessile invertebrate. Ecol Monogr 68:29–52
Helmuth B, Hofmann GE (2001) Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol Bull 201:374–384
Helmuth B, Harley CDG, Halpin P, O’Donnell M, Hofmann GE, Blanchette C (2002) Climate change and latitudinal patterns of intertidal thermal stress. Science 298:1015–1017
Hilbish TJ, Mullinax A, Dolven SI, Meyer A, Koehn RK, Rawson PD (2000) Origin of the antitropical distribution pattern in marine mussels (Mytilus spp.): routes and timing of transequatorial migration. Mar Biol 136:69–77
Hofmann GE, Somero GN (1995) Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus. J Exp Biol 198:1509–1518
Kinlan BP, Gaines SD (2003) Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84:2007–2020
Lent CM (1969) Adaptations of the ribbed mussel, Modiolus demissus (Dillvvyn), to the intertidal habitat. Am Zool 9:283–292
Littler MM, Martz DR, Littler DS (1983) Effects of recurrent sand deposition on rocky intertidal organisms: importance of substrate heterogeneity in a fluctuating communities. Mar Ecol Prog Ser11:129–139
Lutjeharms JRE (1998) Coastal hydrography. In: Lubke R, de Moor I (eds) Eastern and southern Cape coasts. University of Cape Town Press/Grahamstown Branch of the Wildlife and Environment Society of Southern Africa, Ronde-bosch, pp 50–61
Lutjeharms JRE (2004) The coastal oceans of south-eastern Africa. Sea 14:781–832
Manuel JL, O’Dor RK (1997) Vertical migration for horizontal transport while avoiding predators: I. A tidal/diel model. J Plankton Res 19:1929–1947
Marshall DJ, McQuaid CD (1989) The influence of the respiratory response on the tolerance to sand inundation of the limpets Patella granularis (L) (Prosobranchia) and Siphonaria capensis (Q et G) (Pulmonata). J Exp Mar Biol Ecol 129:191–201
McDonald JH, Seed R, Koehn RK (1991) Allozymes and morphometric characters of three species of Mytilus in the Northern and Southern Hemispheres. Mar Biol 3:323–333
McQuaid CD, Dower KM (1990) Enhancement of habitat heterogeneity and species richness on rocky shores inundated by sand. Oecologia 84:142–144
McQuaid CD, Phillips TE (2000) Limited wind-driven dispersal of intertidal mussel larvae: in situ evidence from the plankton and the spread of the invasive species Mytilus galloprovincialis in South Africa. Mar Ecol Prog Ser 201:211–220
Menge BA, Olson AM, Dahlhoff E (2002) Environmental stress, bottom-up effects, and community dynamics: integrating molecular-physiological with ecological approaches. Integr Comp Biol 42:892–908
Michalek-Wagner K, Willis BL (2001) Impacts of bleaching on the soft coral Lobophytum compactum. I. Fecundity, fertilisation and offspring viability. Coral Reefs 19:231–239
Minchinton TE, Scheibling RS (1991) The influence of larval supply and settlement on the population structure of barnacles. Ecology 72:1867–1879
Moon TW, Pritchard AW (1970) Metabolic adaptations in vertically-separated populations of Mytilus californianus Conrad. J Exp Mar Biol Ecol 5:79–90
Newell RC (1979) Biology of intertidal animals, 3rd edn. Marine Ecological Surveys, Faversham
Nicastro KR, Zardi GI, McQuaid CD, Stephens L, Radloff S, Blatch GL (2010) The role of gaping behaviour in habitat partitioning between coexisting intertidal mussels. BMC Ecol 10:17
Niiler PP, Sybrandy AS Bi K, Poulain PM, Bitterman D (1995) Measurement of the water-following capability of holey-sock and TRISTAR drifters. Deep Sea Res I 42:1951–1964
O’Hara TD, Poore GCB (2000) Patterns of distribution for southern Australian marine echinoderms and decapods. J Biogeogr 27:1321–1335
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Syst 25:547–572
Petes LE, Harvell CD, Peters EC, Webb MAH, Mullen KM (2003) Pathogens compromise reproduction and induce melanization in Caribbean seafans. Mar Ecol Prog Ser 264:167–171
Petes LE, Menge BA, Murphy GD (2007) Environmental stress decreases survival, growth, and reproduction in New Zealand mussels. J Exp Mar Biol Ecol 351:83–91
Philippart CJM, van Aken HM, Beukema JJ, Bos OG, Cadée GC, Dekker R (2003) Climate-related changes in recruitment of the bivalve Macoma balthica. Limnol Oceanogr 48:2171–2185
Pincebourde S, Sanford E, Helmuth B (2008) Body temperature during low tide alters the feeding performance of a top intertidal predator. Limnol Oceanogr 53:1562–1573
Pineda J (2000) Linking larval settlement to larval transport: assumptions, potentials, and pitfalls. Oceanogr East Pac 1:84–105
Place SP, O’Donnell MJ, Hofmann GE (2008) Gene expression in the intertidal mussel Mytilus californianus: physiological response to environmental factors on a biogeographic scale. Mar Ecol Prog Ser 356:1–14
Prada C, Schizas NV, Yoshioka PM (2008) Phenotypic plasticity or speciation? A case from clonal marine organisms. BMC Evol Biol 8:47
Pringle JM, Wares JP (2007) The maintenance of alongshore variation in allele frequency in a coastal ocean. Mar Ecol Prog Ser 335:69–84
Ridgeway TM, Stewart BA, Branch GM, Hodgson AN (1998) Morphological and genetic differentiation of Patella granularis (Gastropoda: Patellidae): recognition of two sibling species along the coast of southern Africa. J Zool 245:317–333
Roberts CM (1997) Connectivity and management of Caribbean coral reefs. Science 278:1454–1457
Robinson TB, Griffiths CL, McQuaid CD, Rius M (2005) Marine alien species of South Africa—status and impacts. Afr J Mar Sci 27:297–306
Schneider KR (2008) Heat stress in the intertidal: comparing survival and growth of an invasive and native mussel under a variety of thermal conditions. Biol Bull 215:253–264
Schneider KR, Van Thiel LE, Helmuth B (2010) Interactive effects of food availability and aerial body temperature on the survival of two intertidal Mytilus species. J Therm Biol 35:161–166
Shanks AL, Brink L (2005) Upwelling, downwelling, and cross-shelf transport of bivalve larvae: test of a hypothesis. Mar Ecol Prog Ser 302:1–12
Shanks AL, Grantham BA, Carr MH (2003) Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl 13:S159–S169
Sotka EE, Palumbi SR (2006) The use of genetic clines to estimate dispersal distances of marine larvae. Ecology 87:1094–1103
Sotka EE, Wares JP, Barth JA, Crossberg RK, Palumbi SR (2004) Strong genetic clines and geographical variation in gene flow in the rocky intertidal barnacle Balanus glandula. Mol Ecol 13:2143–2156
Sponaugle S, Cowen RK, Shanks A, Morgan SG, Leis JM, Pineda J, Boehlert GW, Kingsford MJ, Lindeman K, Grimes C, Munro JL (2002) Predicting self-recruitment in marine populations: biophysical correlates and mechanisms. Bull Mar Sci 70:341–375
Suchanek TH (1978) The ecology of Mytilus edulis L. in exposed rocky intertidal communities. J Exp Mar Biol Ecol 31:105–120
Suchanek TH, Geller JB, Kreiser BR, Mitton JB (1997) Zoogeographic distributions of the sibling species Mytilus galloprovincialis and M. trossulus (Bivalvia: Mytilidae) and their hybrids in the North Pacific. Biol Bull 193:187–194
Tapia FJ, Pineda J (2007) Stage-specific distribution of barnacle larvae in nearshore waters: potential for limited dispersal and high mortality rates. Mar Ecol Prog Ser 342:177–190
Teske PR, McQuaid CD, Froneman PW, Barker NP (2006) Impacts of marine biogeographic boundaries on phylogeographic patterns of three South African estuarine crustaceans. Mar Ecol Prog Ser 314:283–293
Teske PR, McQuaid CD, Froneman PW, Barker NP (2007a) Impacts of marine biogeographic boundaries on phylogeographic patterns of three South African estuarine crustaceans. Mar Ecol Prog Ser 314:283–293
Teske PR, Papadopulos I, Zardi GI, McQuaid CD, Griffiths CL, Edkins MT, Barker NP (2007b) Implications of life history for genetic structure and migration rates of five southern African coastal invertebrates: planktonic, abbreviated and direct development. Mar Biol 152:697–711
Teske PR, Papadopoulos I, Newman BK, Dworschak PC, McQuaid CD, Barker NP (2008) Oceanic dispersal barriers, adaptation and larval retention: an interdisciplinary assessment of potential factors maintaining a phylogeographic break between sister lineages of an African prawn. BMC Evol Biol 8:341
Teske PR, Winker H, McQuaid CD, Barker NP (2009) A tropical/subtropical biogeographic disjunction in southeastern Africa separates two evolutionarily significant units of an estuarine prawn. Mar Biol 156:1265–1275
Tsuchiya M (1983) Mass mortality in a population of the mussel Mytilus edulis L. Caused by high temperature on rocky shores. J Exp Mar Biol Ecol 66:101–111
Van Erkom Schuring C, Griffiths CL (1991) A comparison of reproductive cycles and reproductive output in four southern African mussel species. Mar Ecol Prog Ser 76:123–134
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Williams GA, Morritt D (1995) Habitat partitioning and thermal tolerance in a tropical limpet, Cellana grata. Mar Ecol Prog Ser 124:89–103
Zardi GI, Nicastro KR, Porri F, McQuaid CD (2006) Sand stress as a non-determinant of habitat segregation of indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels in South Africa. Mar Biol 148:1031–1038
Zardi GI, McQuaid CD, Teske PR, Barker NP (2007) Unexpected genetic structure of indigenous Perna perna and invasive Mytilus galloprovincialis mussel populations in South Africa. Mar Ecol Prog Ser 337:135–144
Zardi GI, Nicastro KR, McQuaid CD, Erlandsson J (2008) Sand and wave induced mortality in invasive (Mytilus galloprovincialis) and indigenous (Perna perna) mussels. Mar Biol 153:853–858
Acknowledgments
This work is based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation with additional funding from Rhodes University and from FCT (Fundação para a Ciência ea Tecnologia; project awarded to GIZ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Tony Underwood.
G. I. Zardi and K. R. Nicastro contributed equally to the work.
Rights and permissions
About this article
Cite this article
Zardi, G.I., Nicastro, K.R., McQuaid, C.D. et al. The combination of selection and dispersal helps explain genetic structure in intertidal mussels. Oecologia 165, 947–958 (2011). https://doi.org/10.1007/s00442-010-1788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1788-9