Abstract
Plants experience unique challenges due to simultaneous life in two spheres, above- and belowground. Interactions with other organisms on one side of the soil surface may have impacts that extend across this boundary. Although our understanding of plant–herbivore interactions is derived largely from studies of leaf herbivory, belowground root herbivores may affect plant fitness directly or by altering interactions with other organisms, such as pollinators. In this study, we investigated the effects of leaf herbivory, root herbivory, and pollination on plant growth, subsequent leaf herbivory, flower production, pollinator attraction, and reproduction in cucumber (Cucumis sativus). We manipulated leaf and root herbivory with striped cucumber beetle (Acalymma vittatum) adults and larvae, respectively, and manipulated pollination with supplemental pollen. Both enhanced leaf and root herbivory reduced plant growth, and leaf herbivory reduced subsequent leaf damage. Plants with enhanced root herbivory produced 35% fewer female flowers, while leaf herbivory had no effect on flower production. While leaf herbivory reduced the time that honey bees spent probing flowers by 29%, probing times on root-damaged plants were over twice as long as those on control plants. Root herbivory increased pollen limitation for seed production in spite of increased honey bee preference for plants with root damage. Leaf damage and hand-pollination treatments had no effect on fruit production, but plants with enhanced root damage produced 38% fewer fruits that were 25% lighter than those on control plants. Despite the positive effect of belowground damage on honey bee visitation, root herbivory had a stronger negative effect on plant reproduction than leaf herbivory. These results demonstrate that the often-overlooked effects of belowground herbivores may have profound effects on plant performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Belowground interactions between plants and other organisms influence, and are influenced by, interactions above the soil surface (Bardgett and Wardle 2003; Wardle et al. 2004; van der Putten et al. 2009). Although our understanding of plant–herbivore interactions is derived largely from studies of leaf herbivory (van der Putten et al. 2001), a rapidly growing body of research demonstrates that underground herbivory can have as great or greater impacts on plant fitness (e.g., Strong et al. 1995), community structure (Brown and Gange 1989, 1990; Gange and Brown 2002), and ecosystem function (Bardgett et al. 2005; De Deyn and van der Putten 2005). Damage to roots may affect chemical defense levels in leaves and vice versa, altering the likelihood of later herbivore attack (Bezemer and van Dam 2005; Kaplan et al. 2008). Because belowground herbivory is more difficult to quantify and manipulate than leaf herbivory, we are only beginning to understand its effects on plant fitness. These fitness effects could be driven both by direct effects on plants and by altering interactions with mutualists, such as pollinators.
Pollinators are essential for the successful reproduction of many plants, including at least 90 major crops in the USA (Allen-Wardell et al. 1998; Kearns et al. 1998; Kremen et al. 2002), and pollinator behavior can be mediated by herbivory. Leaf damage can reduce floral display, leading to fewer pollinator visits (Strauss et al. 1996; Steets et al. 2006; Adler 2008). However, plants may respond to herbivory by increasing male flower production, leading to higher male reproductive success (Strauss et al. 2001). Surprisingly, the effect of belowground herbivores on pollinators has only been examined in two systems. Poveda et al. (2003, 2005a) reported that wild mustard (Sinapis arvensis) attacked by root herbivores actually attracted more pollinators than plants without root herbivory, although this effect disappeared in plants that also suffered early-season leaf damage. However, in butternut squash (Cucurbita moschata), root damage had no effect on pollinator visitation (Hladun and Adler 2009). Thus, root damage may reduce fitness less than aboveground damage if it increases pollinator attraction, but the effect will depend on the aboveground community of both pollinators and herbivores.
Cucumber (Cucumis sativus: Cucurbitaceae), a widely-cultivated annual herb, is ideal for studying the effects of above- and belowground herbivores and pollinators on plant performance. The striped cucumber beetle (Acalymma vittatum: Chrysomelidae), a specialist on Cucurbitaceae, frequently attacks cucumber both above- and belowground. Adult beetles feed on leaves, stems, and flowers, while larvae feed on roots for 10–20 days before pupation; multiple generations may occur per year (York 1992). In our system, larvae are present from mid-June until the end of the growing season. Cucumber is monoecious and reliant on pollinators for fertilization. Both male and female flowers remain open for a single day and are visited by a variety of generalist pollinators. Total flower production varies according to the growing conditions, but male flowers usually outnumber females by at least tenfold. Female flowers produce more nectar than male flowers, but the nectar in male flowers has a higher sugar content (Collison 1973). Pollination may limit reproduction in Cucurbitaceae (Stanghellini et al. 1997; Gingras et al. 1999; Kremen et al. 2002; Strauss and Murch 2004), and both cucumber fruit size and yield are significantly correlated with the number and cumulative duration of pollinator visitors to cucumber flowers (Stanghellini et al. 1997, 1998; Gingras et al. 1999). We conducted a manipulative field study aimed at exploring how leaf herbivory, root herbivory, and pollination affect plant growth, floral display, pollinator preference, and reproduction.
Methods
Experimental treatments
On 11 June 2007, we planted cucumber seeds (Marketmore 76; Southern Exposure Seed Exchange, Mineral, VA) in Metromix 360 soil (Sun Gro, Bellevue, WA). On 19 June, 96 seedlings at the cotyledon stage were transplanted to 0.6-L pots filled with Metromix 360 soil.
We manipulated leaf herbivory, root herbivory, and pollination (2 levels of each) in a factorial design for a total of eight treatment combinations (n = 12 plants/combination). At the seedling stage, potted plants were separated into blocks of eight and randomized so that one treatment combination was applied to one plant in each block. Damage treatments were applied to all plants in a block on the same days so that plants within a block were exposed to herbivores for the same amount of time. Leaf herbivory treatments began in the greenhouse on 25 June, when plants were at the one-leaf stage, by placing three field-collected beetles enclosed in a mesh bag on each leaf. Beetles were placed on fully expanded leaves and removed after 50% of each leaf was consumed; this is well within the range of natural herbivory since young plants can be completely defoliated and killed by beetles. (R. Hazzard, personal communication). This was repeated for the second and third leaves when each fully expanded. Control plants received mesh bags without beetles, and bags were removed at the same time for both control and damaged plants. A. vittatum overwinter as adults and damage young plants aboveground before laying eggs that hatch into root-feeding larvae (Marsh 1910); consequently, we conducted root herbivory treatments after leaf herbivory to mimic natural damage patterns. Root herbivory treatments began in the greenhouse on 11 July when all leaf herbivory treatments were complete. Plants with root herbivory received 50 A. vittatum eggs placed in agar next to the stem at the soil surface. Because female beetles can lay this many eggs in a single night, this is a relatively low density of eggs per plant (R. Smyth, personal communication). We obtained eggs by mating field-collected beetles in the laboratory. As no attempt was made to deter leaf or root herbivory once plants were put in the field, the treatments represent an augmentation of natural damage levels.
On 19 July, plants were transplanted into an agricultural field (Hampshire Farm, Amherst, MA) in two rows (48 plants/row) with 1.2 m between plants and rows. Planting in rows mimics the normal layout of crop cucumbers, but we did use wider spacing than that normally found in commercial agricultural fields to allow us to distinguish individual plants during the entire season. Plants within blocks were planted adjacent to each other within a row to account for any variation in abiotic conditions along the row, such as differences in water or light availability. To determine if cucumber plants were pollen limited, we enhanced pollination to all female flowers produced on plants in the supplemental pollination treatment by applying supplemental pollen with a paintbrush 5 days/week beginning when the first female flowers appeared on 25 July. We obtained pollen from extra non-experimental field plants. Hand-pollination has been shown previously to increase fruit biomass in cucumber (Thomson et al. 2003). Plants in both the supplemental and natural pollination treatments were always exposed to natural pollinator visits.
Growth and herbivory
We assessed plant growth and subsequent leaf herbivory on three dates (26 July, 6 August, 13 August). We counted the total number of leaves per plant and measured the length and width of the three youngest fully expanded leaves on one haphazardly chosen vine per plant. Leaf area was calculated as length × width and averaged to create one value per plant per date. We also estimated by eye the percentage damage to each of these three leaves. We measured plant growth at the end of the experiment by harvesting each plant, dividing plants at the soil surface into aboveground and belowground portions, drying these at 40°C, and weighing them.
Flowers and pollination
When flowering began (17 July), we counted all male and female flowers 5 days/week until the end of the experiment (7 September). To measure floral display, in mid-August we measured the length and width of one petal on up to three male and three female flowers on each plant. We observed pollinator behavior in August by following individual pollinators and recording on voice recorders (1) pollinator taxa, (2) which plants they visited, (3) how many flowers were probed per plant, and (4) the duration of each flower probe. Plants were observed between 1000 and 1400 hours when flowers were open, and only on sunny days. The total observation time was 14 person-hours across nine different dates between 1 and 21 August.
Reproduction
We collected and weighed all cucumber fruits produced once they reached 18 cm in length; beyond this length, fruits began to turn yellow and decay. Some fruits did not reach this length and were collected when they began to turn yellow, which indicated maturity. To determine reproductive success and provide a further measure of pollen limitation, we measured seed production on the first three fruits produced on each plant. We cut cucumbers in half lengthwise, counted the number of visible developed seeds on each half, and averaged this value first within fruit and then within plant (as in Gingras et al. 1999). The reproduction responses we analyzed were total number of fruits produced per plant, average fruit weight per plant, seed production, and fruit set (proportion of female flowers that produced fruit).
Analyses
We analyzed results with general linear models (PROC GLM, SAS 9.1; SAS Institute, Cary, NC) with type III sums of squares; independent variables were the three treatments and all interactions. Block was included in models to account for possible spatial variation in measured responses or variation due to the timing of leaf and root herbivory treatments. We treated block as a fixed factor, following the recommendation of Newman et al. (1997) for situations when blocks are arbitrarily defined units. Treating block as a random factor did not qualitatively change results (not shown). Response variables were averaged within each plant so that plant was the unit of replication. For responses measured on multiple dates (leaf count, leaf size, and subsequent damage), we used repeated measures analysis. The total number of visits to each plant was analyzed for all pollinators combined and separately for the two most common pollinators, bumble bees (Bombus spp.) and honey bees (Apis mellifera). For these two taxa, we also analyzed the proportion of open flowers on a plant that were probed per visit and the time spent per flower probe. Visits to male and female flowers were not distinguished. We transformed response variables to meet assumptions of normality and homogeneity of variance. Number of leaves, subsequent leaf damage, and leaf and root weights were square root transformed; fruit set was arcsine square root transformed.
Results
Growth and herbivory
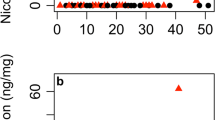
Both root and leaf herbivory affected plant growth, but only leaf herbivory affected subsequent leaf damage (Fig. 1). Enhanced root damage significantly reduced the number of leaves per plant (Table 1). This effect was most pronounced at the last census, when leaf number was reduced by 15%, suggesting that effects of early root damage increased rather than attenuated over time (Fig. 1a). Leaf number was not affected by enhanced leaf damage, and leaf area was not affected by leaf or root herbivory (Table 1). Root herbivory caused a marginally significant reduction in aboveground biomass (Table 2, Fig. 1b). There was a significant leaf × root herbivory interaction effect, such that enhanced leaf herbivory reduced aboveground biomass only on plants that did not receive enhanced root herbivory [Fig. 1b, data given as mean ± standard error (SE); natural leaf herbivory with (1) natural root herbivory, 24.28 ± 2.05 g, and (2) enhanced root herbivory, 15.46 ± 1.58 g, Tukey HSD α = 0.05, P = 0.005; enhanced leaf herbivory with (1) natural root herbivory, 16.10 ± 1.89 g, and (2) enhanced root herbivory, 18.19 ± 1.93 g, Tukey HSD α = 0.05, P = 0.864]. Aboveground biomass also varied among blocks. Leaf damage reduced final root biomass by 12% (Table 2, Fig. 1d), but root biomass was unaffected by other treatments including root herbivory. Plants with enhanced leaf herbivory received less subsequent leaf damage in the first two surveys (Table 1, Fig. 1c). Pollination treatments had no effects on plant growth or leaf damage (Tables 1, 2).
Herbivory treatment effects on number of leaves (a), aboveground biomass (b), subsequent leaf damage (c), and belowground biomass (d). Herbivory treatments: NT No treatment (natural leaf and root herbivory), L enhanced leaf herbivory (with natural root herbivory), R enhanced root herbivory (with natural leaf herbivory), L + R enhanced leaf and root herbivory. Values in all panels are given as back-transformed least square means ± standard error (SE). See text for details of the statistical analyses
Flowers and pollination
Enhanced root herbivory, but not leaf herbivory, reduced the total number of female flowers. Plants with root damage produced 35% fewer female flowers than control plants (Table 3, Fig. 2a). Treatments did not affect the number of male flowers produced (overall mean 53.13 ± 1.70 flowers, all F < 3.09, P > 0.12). Leaf herbivory reduced petal width on female flowers (mean ± SE: control 9.64 ± 0.33 mm; enhanced herbivory 8.69 ± 0.40 mm; F 1,37 = 5.42, P = 0.031), but there were no other treatment effects on this or other floral measurements (all F < 1.96, P > 0.17).
Herbivory treatment effects on total female flower production (a), fruit production (b), fruit weight (c), and number of seeds per fruit (d). Values are given as untransformed least square means ± 1 SE. See Fig. 1 for herbivory treatment abbreviations
We observed 249 individual pollinators probe 968 flowers. The majority of pollinators (51.8%) were bumble bees (Bombus spp.), followed by honey bees (Apis mellifera; 26.1%). Small numbers of butterflies (Lepidoptera: Pieridae), skippers (Lepidoptera: Hesperiidae), hoverflies (Diptera: Syrphidae), and sweat bees (Hymenoptera: Halictidae) also visited flowers. The total number of pollinator visits to plants was not significantly affected by treatments (Table 4). Both leaf and root herbivory affected the length of time that honey bees probed flowers, but in opposite directions: honey bees spent 28% less time per flower on plants with enhanced leaf herbivory, but 119% more time per flower on plants with enhanced root herbivory (Table 4, Fig. 3). There was a significant interaction between leaf herbivory and pollination treatment on the number of honey bee visits because hand pollination marginally significantly increased visits on plants with natural leaf herbivory (mean ± SE; natural leaf herbivory with (1) natural pollination, 1.09 ± 0.26, and (2) enhanced pollination, 2.27 ± 0.46, Tukey HSD, α = 0.05, P = 0.053; enhanced leaf herbivory with (1) natural pollination, 1.40 ± 0.47, and (2) enhanced pollination, 1.24 ± 0.26, Tukey HSD, α = 0.05, P = 0.922). Bumble bee probe duration was not affected by treatments, although there was a trend for root herbivory to reduce total bumble bee visits (Table 4).
Herbivory treatment effects on honey bee probe duration per flower. Values are given as the means ± 1 SE. See Fig. 1 for herbivory treatment abbreviations
Reproduction
Both number of fruit and average fruit weight were significantly reduced by enhanced root herbivory, but unaffected by leaf herbivory and pollination treatments. Mirroring the effects on female flower production, plants with enhanced root herbivory produced 38% fewer fruits (Table 3, Fig. 2b) that were, on average, 25% lighter (Table 3, Fig. 2c) compared to plants with natural levels of root herbivory. Leaf herbivory increased the number of seeds per fruit, while seeds per fruit was reduced by root herbivory (Fig. 2d). Enhanced pollination had a marginally significant positive effect on number of seeds per fruit, and there was a significant pollination × root herbivory interaction such that hand pollination erased the negative effect of root damage (Table 3) [mean ± SE; natural pollination with (1) natural root herbivory, 38.96 ± 3.25, and (2) enhanced root herbivory, 24.01 ± 3.19, Tukey HSD, α = 0.05, 0.016; enhanced pollination with (1) natural root herbivory, 36.21 ± 3.03, and (2) enhanced root herbivory, 37.10 ± 3.24, Tukey HSD, α = 0.05, P = 0.997]. There were no main effects of enhanced leaf or root damage on fruit set, but the leaf × root herbivory interaction was significant (Table 3). However, post-hoc tests revealed no significant differences between different leaf and root herbivory treatment combinations [mean ± SE; natural root herbivory with (1) natural leaf herbivory, 0.53 ± 0.07, and (2) enhanced leaf herbivory, 0.91 ± 0.13; enhanced root herbivory with (1) natural leaf herbivory, 0.65 ± 0.09, and (2) enhanced leaf herbivory, 0.53 ± 0.08, Tukey HSD, α = 0.05, all P > 0.3].
Discussion
Herbivory reduced plant growth in our experiment, which is hardly surprising. However, we found that above- and belowground growth were mainly influenced by herbivores on the opposite side of the soil surface. Enhanced root damage by beetle larvae reduced the number of leaves and had a marginally significant negative effect on total aboveground biomass at the end of the growing season, but it did not affect final root biomass. Correspondingly, early leaf damage reduced final root biomass, but only affected aboveground biomass when plants did not receive enhanced root damage (Fig. 1b). It is possible this pattern is due to a growth–defense tradeoff (Herms and Mattson 1992) wherein resources are allocated to defending the portion of the plant under attack at a growth expense to other plant parts. However, our evidence for a growth–defense tradeoff is limited to the aboveground portion of this experiment because root damage is extremely difficult to measure. We might expect stronger reductions in both above- and belowground biomass when leaf and root damage are enhanced simultaneously, but this was not observed. Under severe stress (such as combined root and leaf attack), these plants may have a limit as to how much resources they will reallocate away from growth. Quantification of chemical defenses in both root and leaf tissues following attack would help address this question.
Early-season leaf damage was found to reduce the likelihood of leaf damage later in the season, consistent with results from many previous studies (Karban and Baldwin 1997; Agrawal 1999) and suggesting that folivory induced a defensive response. In spite of the economic importance of cucurbits and their specialized cucumber beetle herbivores (Metcalf and Metcalf 1992; USDA 2002), we are not aware of other studies examining how cucumber beetle herbivory affects subsequent resistance to beetle damage. This question is particularly interesting because cucurbitacins, the major defensive compound known in cucurbits, are phagostimulants to these herbivores (Chambliss and Jones 1966; Metcalf et al. 1980). The induction of cucurbitacins in cucumber has been demonstrated only in response to damage by generalist spider mites, which increased resistance to later mite attack (Agrawal et al. 1999). If cucumber beetle damage also increased cucurbitacins, we would expect more subsequent herbivory by cucumber beetles. That we found less damage suggests the mechanism of resistance in cucumbers may differ between generalist and specialist induction, as has been suggested previously for cucurbits (Tallamy and Krischik 1989; Tallamy and McCloud 1991) or that damage induces other defensive changes that affect cucumber beetles more than increased levels of cucurbitacins.
Interestingly, leaf and root herbivory had opposite effects on pollinator preference. Plants with enhanced leaf damage received significantly shorter honey bee flower probes (Fig. 2). This reduced attractiveness to pollinators following folivory is consistent with results reported from numerous previous studies (Strauss et al. 1996; Lehtilä and Strauss 1997; Strauss and Armbruster 1997; Mothershead and Marquis 2000; Hambäck 2001; reviewed in Adler 2008). The effects of leaf herbivory on pollinator preference could be mediated by flower size, since leaf herbivory reduced floral display due to narrower petals on female flowers. Surprisingly, honey bees spent significantly more time on plants with enhanced root herbivory. To the best of our knowledge, the effect of root herbivory on pollinators has only been examined in two other systems. One study found no effect of cucumber beetle damage on pollinator behavior in butternut squash (Hladun and Adler 2009). In the other system, results were remarkably consistent with ours: root damage by wireworms (Agriotes sp.) in wild mustard consistently enhanced attraction of pollinators, which were primarily honey bees (Poveda et al. 2003, 2005a). In both of these studies, there was no effect of root damage on any measure of floral size (Poveda et al. 2005b; Hladun and Adler 2009) or on nectar production or anther length in wild mustard (Poveda et al. 2005b). It is possible that root damage changed other unmeasured attractiveness traits, such as floral scent or nectar composition, which often affect pollinator behavior (Dobson et al. 2005; Nicolson 2007). Although root herbivory altered flower sex ratios by reducing female flower production, this is unlikely to cause the observed changes in honey bee behavior because female flowers are more rewarding than male flowers (Collison 1973). In the confamilial C. moschata, bee flower probes to female flowers were twice as long as those to male flowers (K. Hladun and L. Adler, unpublished data). If this pattern holds in cucumber, then we would expect the altered sex ratio due to root damage to reduce rather than increase probe duration. In our study, enhanced root herbivory also had a tendency to reduce bumblebee visits, suggesting that root herbivory may induce changes that affect closely related pollinators differently. Further studies should examine induced changes in floral traits, including scent and nectar production, in response to root herbivory, and how different pollinators may respond to induction.
Root herbivory had a greater effect than leaf herbivory on reproduction. Plants with enhanced root damage produced fewer female flowers (Fig. 3a). Despite the positive effects of root herbivory on honey bee probe duration, root herbivory reduced fruit production, mean fruit weight, and seed production (Fig. 3b–d). This contrasts with recent findings in the confamilial C. moschata in which leaf herbivory by adult A. vittatum reduced reproduction more than larval root damage (Hladun and Adler 2009). Under enhanced root herbivory, seed production was pollen-limited, as shown by the significant interaction between the hand-pollination and root herbivory treatments (Table 3, Fig. 2d). This is surprising given the positive effect of root herbivory on honey bee pollination behavior. However, honey bees may be inefficient pollinators, especially compared to bumble bees, which have been shown to visit more flowers per unit time and deposit more pollen per flower visit than honey bees (Stanghellini et al. 1997, 2002). Since root herbivory tended to reduce the number of bumble bee visits, this effect may outweigh any benefits of increased time spent per flower by honey bees. Enhanced leaf damage unexpectedly caused greater seed production in spite of reducing pollinator attraction. This may also be due to the poor efficiency of honey bees as pollinators. Leaf herbivory treatments reduced honey bee probe duration, so more efficient bumble bees may have accounted for a greater proportion of successful pollinations. If wild bumble bees are more efficient pollinators than domestic honey bees, these results may underscore the importance of healthy populations of native pollinators that could help plants overcome limitations due to antagonists (Strauss and Murch 2004).
In conclusion, while both above- and belowground herbivory by A. vittatum affected cucumber plants, root herbivory more consistently reduced reproductive measures, such as fruit production, fruit size, and seed production. These results may have important implications not only for our understanding of plant–herbivore interactions in natural systems, but also for managed systems. In agroecosystems, the importance of belowground herbivory is recognized only when herbivores have dramatic impacts (e.g., Ellis et al. 1999; Felkl et al. 2005; van Dam et al. 2005). However, when root damage is cryptic and does not cause mortality, the impacts of root herbivory on yield may still be substantial but go undetected.
References
Adler LS (2008) Selection by pollinators and herbivores on attraction and defense. In: Tilmon KJ (ed) Specialization, speciation, and radiation. University of California Press, Berkeley, pp 162–173
Agrawal AA (1999) Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80:1713–1723
Agrawal AA, Gorski PM, Tallamy DW (1999) Polymorphism in plant defense against herbivory: constitutive and induced resistance in Cucumis sativus. J Chem Ecol 25:2285–2304
Allen-Wardell G, Bernhardt P, Bitner R, Burquez A, Buchmann S, Cane J, Cox PA, Dalton V, Feinsinger P, Ingram M, Inouye D, Jones CE, Kennedy K, Kevan P, Koopowitz H, Medellin R, Medellin-Morales S, Nabhan GP, Pavlik B, Tepedino V, Torchio P, Walker S (1998) The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv Biol 12:8–17
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol 20:634–641
Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20:617–624
Brown VK, Gange AC (1989) Differential effects of above ground and below ground insect herbivory during early plant succession. Oikos 54:67–76
Brown VK, Gange AC (1990) Insect herbivory belowground. Adv Ecol Res 20:1–58
Chambliss OL, Jones CM (1966) Cucurbitacins: specific insect attractants in Cucurbitaceae. Science 153:1392–1393
Collison CH (1973) The interrelationships of honey bee activity, foraging behavior, climatic conditions and flower in the pollination of pickling cucumbers, Cucumis sativus L. PhD thesis. Michigan State University, East Lansing
De Deyn GB, Van der Putten WH (2005) Linking aboveground and belowground diversity. Trends Ecol Evol 20:625–633
Dobson HEM, Raguso RA, Knudsen JT, Ayasse M (2005) Scent as an attractant. In: Dafni A, Kevan PG, Husband BC (eds) Practical pollination biology. Enviroquest, Cambridge, pp 197–230
Ellis PR, Pink DAC, Barber NE, Mead A (1999) Identification of high levels of resistance to cabbage root fly, Delia radicum, in wild Brassica species. Euphytica 110:207–214
Felkl G, Jensen EB, Kristiansen K, Andersen SB (2005) Tolerance and antibiosis resistance to cabbage root fly in vegetable Brassica species. Entomol Exp Appl 116:65–71
Gange AC, Brown VK (2002) Soil food web components affect plant community structure during early succession. Ecol Res 17:217–227
Gingras D, Gingras J, De Oliveira D (1999) Visits of honeybees (Hymenoptera: Apidae) and their effects on cucumber yields in the field. J Econ Entomol 92:435–438
Hamback PA (2001) Direct and indirect effects of herbivory: feeding by spittlebugs affects pollinator visitation rates and seedset of Rudbeckia hirta. Ecoscience 8:45–50
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Hladun KR, Adler LS (2009) Influence of leaf herbivory, root herbivory, and pollination on plant performance in Cucurbita moschata. Ecol Entomol 34:144–152
Kaplan I, Halitschke R, Kessler A, Sardanelli S, Denno RF (2008) Constitutive and induced defenses to herbivory in above- and belowground plant tissues. Ecology 89:392–406
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualisms: the conservation of plant–pollinator interactions. Ann Rev Ecol Syst 29:83–112
Kremen C, Williams NM, Thorp RW (2002) Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci USA 99:16812–16816
Lehtilä K, Strauss SY (1997) Leaf damage by herbivores affects attractiveness to pollinators in wild radish, Raphanus raphanistrum. Oecologia 111:396–403
Marsh HO (1910) Biologic notes on species of Diabrotica in southern Texas. USDA Bureau Entomol Bull 82:76–84
Metcalf RL, Metcalf ER (1992) Plant kairomones in insect ecology and control. Chapman and Hall, New York
Metcalf RL, Metcalf RA, Rhodes AM (1980) Cucurbitacins as kairomones for diabroticite beetles. Proc Natl Acad Sci USA 77:3769–3772
Mothershead K, Marquis RJ (2000) Fitness impacts of herbivory through indirect effects on plant- pollinator interactions in Oenothera macrocarpa. Ecology 81:30–40
Newman JA, Bergelson J, Grafen A (1997) Blocking factors and hypothesis tests in ecology: is your statistics text wrong? Ecology 78:1312–1320
Nicolson S (2007) Nectar consumers. In: Nicolson S, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, pp 289–342
Poveda K, Steffen-Dewenter I, Scheu S, Tscharntske T (2003) Effects of below- and above-ground herbivores on plant growth, flower visitation and seed set. Oecologia 135:601–605
Poveda K, Steffen-Dewenter I, Scheu S, Tscharntske T (2005a) Effects of decomposers and herbivores on plant performance and aboveground plant–insect interactions. Oikos 108:503–510
Poveda K, Steffen-Dewenter I, Scheu S, Tscharntske T (2005b) Floral trait expression and plant fitness in responses to below- and aboveground plant–animal interactions. Perspect Plant Ecol 7:77–83
Stanghellini MS, Ambrose JT, Schultheis FR (1997) The effects of honey bee and bumble bee pollination on fruit set and abortion of cucumber and watermelon. Am Bee J 137:386–391
Stanghellini MS, Ambrose JT, Schultheis FR (1998) Seed production in watermelon: a comparison between two commercially available pollinators. Hortic Sci 33:28–30
Stanghellini MS, Ambrose JT, Schultheis JR (2002) Diurnal activity, floral visitation and pollen deposition by honey bees and bumble bees on field-grown cucumber and watermelon. J Apic Res 41:27–34
Steets JA, Hamrick JL, Ashman T-L (2006) Consequences of vegetative herbivory for maintenance of intermediate outcrossing in an annual plant. Ecology 87:2717–2727
Strauss SY, Armbruster WS (1997) Linking herbivory and pollination—new perspectives on plant and animal ecology and evolution. Ecology 78:1617–1618
Strauss SY, Murch P (2004) Towards an understanding of the mechanisms of tolerance: compensating for herbivore damage by enhancing a mutualism. Ecol Entomol 29:234–239
Strauss SY, Conner JK, Rush SL (1996) Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. Am Nat 147:1098–1107
Strauss SY, Conner JK, Lehtilä KP (2001) Effects of foliar herbivory by insects on the fitness of Raphanus raphanistrum: damage can increase male fitness. Am Nat 158:496–504
Strong DR, Maron JL, Connors PG, Whipple A, Harrison S, Jefferies RL (1995) High mortality, fluctuation in numbers, and heavy subterranean insect herbivory in bush lupine, Lupinus arboreus. Oecologia 104:85–92
Tallamy DW, Krischik VA (1989) Variation and function of cucurbitacins in Cucurbita: an examination of current hypotheses. Am Nat 133:766–786
Tallamy DW, McCloud ES (1991) Squash beetles, cucumber beetles, and inducible cucurbit responses. In: Tallamy DW, Raupp MJ (eds) Phytochemical induction by herbivores. Wiley, New York, pp 155–182
Thomson VP, Cunningham SA, Ball MC, Nicotra AB (2003) Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia 134:167–175
USDA (2002) Census of Agriculture, vol 1 chapter 2: US State Level Data, Table 29. Vegetables and melons harvested for sale, 2002 and 1997. USDA, Washington D.C.
van Dam NM, Raaijmakers CE, van der Putten WH (2005) Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomol Exp Appl 115:161–170
Van der Putten WH, Vet LEM, Harvey JA, Wäckers FL (2001) Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16:547–554
Van der Putten WH, Bargett RD, de Ruiter PC, Hol WHG, Meyer KM, Bezemer TM, Bradford MA, Christensen S, Eppinga MB, Fukami T, Hemerik L, Molofsky J, Schädler M, Scherber C, Strauss SY, Vos M, Wardle DA (2009) Empirical and theoretical challenges in aboveground–belowground ecology. Oecologia 161:1–14
Wardle DA, Bardgett RD, Klioronomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:16–1629
York A (1992) Pests of Cucurbit crops: marrow, pumpkin, squash, melon and cucumber. In: McKinlay RG (ed) Vegetable crop pests. CRC Press, Boca Raton, pp 139–161
Acknowledgments
We thank Nancy Hansen and Hampshire College Farm for providing a field site and assisting with cultivation, and J. Calderon-Ayala, A. Roehrig, and N. Scalfone for field assistance. The manuscript benefitted from helpful comments by S. Gillespie, N. Soper Gorden, and two anonymous reviewers. This research was partially supported by USDA NRI 2008-02346 and USDA/CSREES MAS00931. All the work described in this manuscript complies with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alice Winn.
Rights and permissions
About this article
Cite this article
Barber, N.A., Adler, L.S. & Bernardo, H.L. Effects of above- and belowground herbivory on growth, pollination, and reproduction in cucumber. Oecologia 165, 377–386 (2011). https://doi.org/10.1007/s00442-010-1779-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1779-x