Abstract
Although trade-offs between reproductive effort and other fitness components are frequently documented in wild populations, the underlying physiological mechanisms remain poorly understood. Parasitism has been suggested to mediate reproductive trade-offs, yet only a limited number of parasite taxa have been studied, and reproductive effort-induced changes in parasitism are rarely linked to trade-offs observed in the same population. We conducted a brood size manipulation experiment in blue tits (Cyanistes caeruleus) infected with malaria (Plasmodium) parasites, and used quantitative PCR to measure changes in parasitaemia. In one of two years investigated, parasitaemia increased as a result of brood enlargement, and was also positively associated with two other indicators of reproductive effort: clutch size and single parenthood. These associations between both experimental and naturally varying reproductive effort and parasitaemia suggest that immune control of chronic malaria infections can be compromised when parents are working hard. Brood size manipulation significantly affected the number of independent offspring produced, which was maximised when brood size was unchanged. Moreover, when parents were infected with one of two common Plasmodium species, the shape of this trade-off curve was more pronounced, suggesting that parasitic infection may exacerbate the trade-off between quantity and quality of offspring. Although the involvement of parasites in survival costs of reproduction has received much attention, these results suggest their role in other commonly documented reproductive trade-offs, such as that between number and quality of offspring, warrants further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elucidating the mechanisms that limit reproductive effort in wild animal populations remains a key challenge in life-history biology (Williams 1966; Stearns 1992). Manipulative studies in birds have repeatedly demonstrated that, although parents are capable of raising more offspring than their original clutch size, elevated reproductive effort often carries a cost. Negative effects of manipulated reproductive effort have been documented on offspring survival and recruitment (Dijkstra et al. 1990; Pettifor 1993a; Pettifor et al. 2001), offspring fecundity (Blondel et al. 1998), parental survival (Dijkstra et al. 1990) and future fecundity (Gustafsson and Sutherland 1988; Parejo and Danchin 2006). Although at one level such reproductive trade-offs can explain why reproductive effort is limited, the proximate physiological mechanisms underpinning such trade-offs remain elusive (Zera and Harshman 2001).
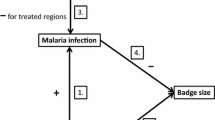
One proposed physiological mechanism, which has been widely invoked to explain survival costs of reproduction, posits that reproductive investment diminishes an individual’s ability to deal with parasitic infection (Gustafsson et al. 1994), under the assumption that parasite defence depletes resources otherwise available for reproduction (Bonneaud et al. 2003; Lochmiller and Deerenberg 2000). This hypothesis is appealing because it provides several possible routes by which reduced parasite defence could translate into fitness effects, either in the short term or over longer time-periods (Fig. 1). For example, reduced immune function could increase the likelihood of acquiring a potentially life-threatening infection, hence lowering survival. Alternatively, it could reduce an individual’s ability to control or eliminate existing infections, potentially limiting their ability to raise current offspring, or their future reproductive potential.
The potential role of parasites in reproductive trade-offs. Reproductive trade-offs may occur in the currency of either parental survival or future fecundity, or the survival or fecundity of offspring produced in the current reproductive attempt (pathway A). Current reproductive effort may increase parasitism (pathway B), and if such changes can reduce fitness (pathway C) they may mediate the observed reproductive trade-off
Studies in diverse animal taxa have documented negative effects of mating or parental effort on immune function, as well as positive effects on parasitism (Fedorka et al. 2004; Harshman and Zera 2007; Sheldon and Verhulst 1996). In the avian literature, experiments testing the effect of reproductive effort on blood parasitism are now relatively numerous, and provide support for the idea that elevated reproductive effort can increase blood parasite density (parasitaemia), as well as reduce measures of immune responsiveness (Knowles et al. 2009). Conversely, supplementary feeding experiments have shown reductions in the level of blood parasitism when the parental effort is reduced as a consequence (e.g. Wiehn and Korpimäki 1998). However, several gaps remain in our understanding of such effects and their potential importance in reproductive trade-offs. First, the majority of such studies (63%; Knowles et al. 2009) have investigated a single blood parasite genus (Haemoproteus), and it is therefore unclear how general such effects are across other parasite taxa. Second, the reasons underlying variation in the strength of trade-offs detected are not well understood, and this is difficult to assess since most studies are conducted in a single year. Since the manifestation of trade-offs may be environmentally dependent (e.g. Wilson et al. 2009), there is a need to investigate trade-offs under more than one set of conditions and ideally in multiple years within the same population. Finally, although existing studies have investigated how experimental reproductive effort affects parasitism, very few have linked these effects to observed reproductive trade-offs (but see Stjernman et al. 2004). Experimentally enhanced reproductive effort is likely to have diverse effects on physiology (Horak et al. 1998; Wiersma et al. 2004), of which an increase in parasitism may be one, but any of them could be responsible for observed trade-offs. There is a therefore a need to assess what role, if any, reproductive effort-related changes in parasitism play in mediating trade-offs, and under what circumstances such effects may be important (see Fig. 1).
To investigate the effect of reproductive effort on parasitism and its potential relevance in reproductive trade-offs, we conducted a brood size manipulation across two years in a population of blue tits (Cyanistes caeruleus) infected with malaria (Plasmodium) parasites. Since Plasmodium parasites typically occur at very low densities in wild birds and are thought to be heavily suppressed by immune mechanisms (Valkiūnas 2005), these parasites provide an ideal model system for testing whether immune control of infections can be compromised during reproduction. Because parasite quantification in these chronic infections is difficult using microscopy, we used a sensitive quantitative PCR (qPCR) assay for measuring Plasmodium parasitaemia. Follow-up of parents and their offspring in subsequent breeding seasons allowed us to assess the impact of brood size manipulation on parental fitness traits and assess whether parasitism-related effects were likely to play a role in any trade-offs detected.
Materials and methods
Field methods
In 2006 and 2007 we performed a brood size manipulation experiment in a nest-box breeding population of blue tits at Wytham Woods, a ca. 385 ha mixed deciduous woodland near Oxford, UK (51°47′N, 1°20′W). All reproductive attempts are monitored regularly to determine lay date (LD: day first egg was laid), clutch size (CS) and hatch date (HD: day first egg hatched). Manipulations were performed on day 2 post-hatch (day 0 = hatch date), when approximately one-quarter of the brood (median 27%, interquartile range 25–30%) were randomly selected and transferred from a donor (reduced) to a recipient (enlarged) nest. Pairs of nests were matched for hatch date and the number of young to be transferred, and on each day that brood size was manipulated, an approximately equal number of nests were maintained as controls, with no disturbance on day 2. Among enlarged broods, there was no significant difference in mass between transferred chicks and those hatched and reared in the same nest (F 1,867 = 1.41, p = 0.235, including nest of rearing as a random effect), suggesting foster chicks were not treated differently by parents.
Before the experiment, females were weighed to the nearest 0.1 g and their blood was sampled (under licence, by brachial venipuncture) on the expected hatch date (calculated as LD + CS + 12 days). Blood samples were stored in SET buffer (0.015 M NaCl, 0.05 M Tris, 0.001 M EDTA, pH 8.0). Males were not blood-sampled before the experiment since they are difficult to catch at this stage. On day 13–15 post-hatch, both parents were captured whilst feeding young, blood-sampled to provide a post-manipulation sample, and tarsus and mass were measured to the nearest 0.1 mm and 0.1 g, respectively. Birds were sexed according to the presence (female) or absence (male) of a brood patch, and age (yearling or older) was scored using plumage characteristics (Svensson 1992). On day 14, nestlings were ringed and weighed to the nearest 0.1 g. The number of parents present on day 13–14 at each nest was noted, based on how many parents were caught and/or how many blue tits alarm-called during the ringing of nestlings. In 2007, for additional confirmation that our manipulation effectively altered reproductive effort, nest visitation rate of both parents was estimated at a subset of nests on day 15 (n = 90). For this purpose, both parents were fitted with a transponder (PIT tag) on the tarsus, and an antenna connected to a data logger was fitted to the nest-box entrance (see the Electronic supplementary material for details of nest visitation rate measurement). All nests were inspected after the breeding season to determine which nestlings had fledged. In total, 310 nests were part of the experiment, 143 in 2006 (45 reduced, 53 controls and 45 enlarged) and 167 in 2007 (52 reduced, 63 controls and 52 enlarged).
In 2007–2008, at all (non-experimental) nests, parents were captured on day 8–15 post-hatch and blood-sampled as part of standard field protocols in this population. Thus, we could assess the malaria infection status of individuals involved in the 2006 or 2007 experiments for malaria infection one year post-treatment. The number of independent offspring produced by each nest was assessed using records of breeding recruits (from 2007–2009) as well as winter catching data. During the winters of 2006–2008 (September–March), mist-nets were set up at feeding sites throughout the study site. Any offspring captured either during winter or as breeding adults during this period were classified as being independent. Although we are ultimately interested in the effect of manipulation on how many offspring survive to breed (i.e. recruitment), we use the number of independent offspring as an approximation for several reasons. First, breeding adults are only identified in this population when captured at nestboxes between days 8 and 15 of the nestling period. Thus, any recruits that breed outside the monitored nestboxes, cannot be captured, or whose breeding attempts fail early on would not be recorded as breeding. Second, studies on tits suggest that most offspring mortality occurs soon after fledging, and that those surviving the first three months post-fledging have approximately equal chances of eventually breeding (Naef-Daenzer et al. 2001; Nur 1984; Perrins 1965). Thus, including birds caught during their first winter is considered to provide a more accurate assessment of offspring fitness.
Molecular characterisation of malaria infections
Previous molecular characterisation of haemosporidian infections in this population has shown that Plasmodium infections are common during the breeding season, with a prevalence of around 25–48% (Wood et al. 2007). Two divergent cyt b clades are regularly detected, which represent two well-defined morphospecies, P. relictum and P. circumflexum (Palinauskas et al. 2007). For simplicity, we use their morphospecies classification from here on. A quantitative (q)PCR assay was employed for Plasmodium detection and quantification in this study, using the primers L9 5′-AAACAATTCCTAACAAAACAGC-3′ and NewR 5′-ACATCCAATCCATAATAAAGCA-3′, which target a 188 bp region of the mitochondrial cytochrome b gene. Genomic DNA was extracted from all blood samples using a standard ammonium acetate method, and total DNA concentration was measured using a PicoGreen assay (Quant-iT PicoGreen dsDNA Assay Kit, Invitrogen). All samples were diluted to a standard working concentration of 2 ng/μl prior to qPCR. Standard curves were created using a full-length cyt b PCR product from P. relictum (lineage pSGS1), and qPCR reactions were performed exactly as described in Knowles et al. (2010). We made use of the fact that qPCR products from P. relictum and P. circumflexum show consistently different melting temperatures (around 75.2 vs. 74.2°C respectively) to diagnose Plasmodium species in each positive sample (see the Electronic supplementary material for further details).
Statistical analyses
Examining both Plasmodium infection status (infected or uninfected) and parasitaemia (parasite density in infected individuals) allowed us to test for effects of our manipulation on two different processes: (1) changes in immune control of existing infections as indicated by chronic parasitaemia, and (2) altered susceptibility to infection gain or loss, indicated by infection status. We used ln(1 + Plasmodium copies)—which was approximately normally distributed—as a measure of parasitaemia in statistical models, to meet model assumptions.
GLMs were used to confirm that brood size manipulation (treated as a three-level factor, “Treatment”) effectively altered reproductive effort, as assessed by the number and combined mass of nestlings on day 14 as well as nest visitation rate, and that parental response to manipulation did not vary with infection status, age or sex. In the analysis of visitation rate, original clutch size (CS) and single parenthood (SP) were also included as fixed effects, since both are predicted to influence parental work rate: clutch size should positively covary with brood size and therefore provisioning rate (Perrins 1979), and single parents are expected to work harder to compensate for the lack of a partner (Alatalo et al. 1988). The effect of manipulation on measures of reproductive effort was essentially linear across treatment groups (Fig. 2a–c, Table S2). As we hypothesised that any effects of manipulation on parasite traits would be driven by manipulation-induced changes in reproductive effort, we entered manipulation as a continuous linear predictor when modelling parasitaemia and infection status (“Manipulation”, −1, 0, 1).
Effect of brood size manipulation in 2006 (filled circles, solid lines) and 2007 (open circles, dashed lines) on a number of fledglings, b total brood mass on day 14, c nest visitation rate (number of minutes present between 0630 and 1230 h on day 15) and d mean nestling mass on day 14. Means and standard errors are shown, and only nests where at least one offspring was ringed on day 14 were included in analyses
Standard GLMs were used to investigate the effect of brood size manipulation on parasitaemia, whilst generalized linear models (GLZs) with binomial errors and a logit link were used to model infection status. Original clutch size and single parenthood were included in both analyses, as these are considered non-experimental measures of reproductive effort, and they had strong effects on nest visitation rate in 2007 (see Table 1). In infection status analyses, non-experimental factors known to predict this variable in the study population (Wood et al. 2007) were also controlled for. We also used a GLZ to test whether brood size manipulation influenced susceptibility to infection gain or loss in the long term by modelling infection status one year after the experiment (during the nestling phase), including year and a Manipulation × Year interaction to allow for possible differences between years.
Reproductive trade-offs were investigated by testing for effects of brood size manipulation on five fitness-related traits. Only nests where at least one chick was ringed on day 14 (i.e. where parents experienced manipulation for the full period over which changes in parasitism were monitored) were included in these analyses. The fitness-related traits considered were: (1) offspring survival, measured as the number of independent offspring recaptured per experimental nest (see “Field methods”); (2) parental recapture probability the following breeding season (as a measure of survival); (3) clutch size and (4) lay date the following year; and (5) the combined number of independent offspring produced by both parents the year after manipulation. A standardised measure of lay date was used (calculated as [LD − annual mean LD]/annual LD standard deviation) to provide a relative measure of reproductive timing in a given year. Only females were included in the clutch size and lay date analyses, since males appear to have negligible influence on these traits in tits (Browne et al. 2007; Caro et al. 2009; Liedvogel et al. 2009). We used GLZs with binomial errors for recapture probability, Poisson errors for the number of independent offspring, and normal GLMs for clutch size and lay date. A continuous measure of manipulation was used in these models (“Manipulation”, −1, 0, 1). A quadratic effect of manipulation on the number of independent offspring is expected under the individual optimisation hypothesis (IOH; Perrins and Moss 1975), and has previously been demonstrated in several passerine populations, including blue tits and great tits in Wytham Woods (Gustafsson and Sutherland 1988; Pettifor 1993a; Pettifor et al. 2001). Because of this, and because the manner in which manipulation might affect the other fitness-related traits is uncertain, we allowed for nonlinear effects in these analyses by including quadratic manipulation terms (Manipulation2). This approach is equivalent to using an ANOVA and contrasts between treatment groups. To initially explore the evidence for trade-offs and test whether patterns differed between years, we first fitted full factorial models including year, Manipulation and Manipulation2. Where an effect of Manipulation or Manipulation2 was detected, we then tested for an interaction with parental infection status to assess whether malaria infection might modulate the trade-off. Parental infection status was included as a two-level factor, indicating whether at least one parent at a nest was Plasmodium-infected or not.
All statistical analyses were performed in JMP software v.6 (SAS Institute 2005), and overdispersion was tested and corrected for in GLZs where necessary. Models were simplified by backwards stepwise elimination, sequentially excluding terms for which p > 0.1 and finally all those with p > 0.05 to obtain the minimal model. Approximately 13% of all individuals were experimental subjects in both years; wherever data for an individual were available for both years, one year’s data was randomly excluded from the analysis.
Results
Pre-experimental conditions and effect of manipulation on reproductive effort
In 2006, blue tits bred considerably later, female body mass prior to the experiment was lower, and parasitaemia was generally higher than in 2007 (see the Electronic supplementary material for details). Thus, blue tits appear to have been physiologically more stressed in the first year of the experiment. Treatment groups were successfully randomised with respect to timing of breeding (lay date and hatch date), clutch size and, among females, pre-manipulation Plasmodium prevalence and parasitaemia (all associations with manipulation p > 0.6, Table S1). Brood size manipulation successfully altered reproductive effort, as assessed by the number of nestlings and brood mass measured on day 14 (Fig. 2a, b). There was no evidence of nonlinearity in these effects, or of differential responses of parents to the manipulation according to year or malaria infection status (Table S2). Nest visitation rate (measured in 2007 only) showed a clear positive association with brood size manipulation (Fig. 2c), and did not vary significantly with parental age, sex or Plasmodium infection status (Table 1). Original clutch size (CS), and even more so single parenthood (SP), also showed significant positive associations with nest visitation rate (Table 1). Brood enlargement negatively affected mean fledgling mass (Fig. 2d), and fledgling number and mean mass were significantly lower at single compared to two-parent nests (fledgling number F 1,251 = 20.34 p < 0.001, mean fledgling mass F 1,238 = 21.35, p < 0.001).
Effect of reproductive effort on parasitaemia
In a model including data from both years, a significant Manipulation × Year interaction term indicated that the effect of brood size manipulation on Plasmodium parasitaemia differed between the two years studied; in 2006, brood size manipulation had a positive effect on parasitaemia, with mean parasitaemia increasing approximately fourfold between the reduced and enlarged treatment groups (effect size r = 0.275), whereas in 2007 there was no significant trend in either direction (r = −0.063; Fig. 3a, Table 2). Both single parenthood and clutch size also positively predicted Plasmodium parasitaemia, though these effects did not differ significantly between years (Table 2, Fig. 3b, c). See the Electronic supplementary material for analyses of within-female changes in parasitaemia.
Effects of both manipulated and natural reproductive effort on Plasmodium parasitaemia among blue tit parents. a Brood size manipulation caused an increase in parental Plasmodium parasitaemia in 2006 (filled circles) but not 2007 (open circles). Residuals from a general linear model are plotted with standard errors, controlling for significant effects of single parenthood, clutch size and Plasmodium species (see Table 2). b and c Associations between parental parasitaemia and single parenthood and original clutch size, respectively. Means and standard errors are plotted using raw data
Effect of reproductive effort on infection status
Total (post-manipulation) Plasmodium prevalence was similar in both years (43% in 2006, n = 207, 47% in 2007, n = 234), although the prevalence of each species was more variable between years (P. relictum 14.6% in 2006, 26.5% in 2007; P. circumflexum 28.2% in 2006, 20.0% in 2007). We found no evidence that brood size manipulation affected the probability of infection by either Plasmodium species (P. relictum: Manipulation χ2 = 2.60, p = 0.107; P. circumflexum: Manipulation χ2 = 1.16, p = 0.282, Table S3); similarly, neither clutch size nor single parenthood significantly predicted infection status (Table S3). Infection status in the following year was not predicted by manipulation, either for pooled Plasmodium or for the species separately; if anything, there was a tendency for brood size enlargement to be associated with a lower probability of infection the following year (Plasmodium species pooled, Manipulation: χ2 = 3.55, p = 0.060, year: χ2 = 1.3, p = 0.255, Manipulation × Year: χ2 = 0.51, p = 0.477; Table S4).
Brood size manipulation and reproductive trade-offs
We found no evidence of parental survival costs of brood size manipulation in this population, or of altered clutch size or lay date the following year amongst females (Table 3). However, we did detect a significant quadratic effect of brood size manipulation on the number of independent offspring produced (Table 3). The turning point of this quadratic relationship was estimated at a manipulation of +0.042, which produced a mean number of 1.42 independent offspring in 2006 and 0.51 in 2007. These values are not significantly different from those at zero manipulation each year (2006: mean = 1.42, 95% CI 1.12, 1.80; 2007: mean = 0.51, 95% CI 0.37, 0.71). Thus that parents raising control broods produced more independent offspring than those rearing reduced or enlarged broods (see also the Electronic supplementary material). To test whether the quadratic effect of brood size manipulation on offspring survival was influenced by parental malaria infection, we fitted interaction terms between Manipulation2 and parental infection status. Parental infection status was modelled as a binary variable indicating whether at least one parent at a nest was infected. Considering all Plasmodium infections pooled, as well as P. circumflexum, this interaction term was non-significant (pooled Plasmodium: χ2 = 1.83, p = 0.176, n = 172; P. circumflexum: χ2 = 0.17, p = 0.684, n = 169). However, for P. relictum the interaction term was significant (χ2 = 5.28, p = 0.022, n = 169); at nests where both parents were free of P. relictum, the quadratic effect of manipulation (Fig. 4a; Manipulation2 χ2 = 0.63, p = 0.426, n = 106) was weaker than among nests where at least one parent was infected (Fig. 4b; Manipulation2 χ2 = 10.70, p = 0.001, n = 63). We found no evidence that the way parental parasitism affected the shape of the relationship between manipulation and offspring survival differed between years (all interactions between Year and Manipulation2 × parental infection status terms p > 0.35).
Effect of brood size manipulation on the number of independent offspring produced at nests where either a neither or b at least one parent was infected with P. relictum during the experiment. Data are predicted values and 95% confidence intervals from a full factorial Poisson GLZ containing effects of manipulation (as a covariate, −1, 0, 1), Manipulation2 and a binary variable denoting whether at least one parent was infected with P. relictum. Only nests where the infection status of both parents was known are included
We also detected an effect of brood size manipulation on the number of independent offspring parents produced in the year after the experiment: a quadratic effect of manipulation indicated that parents with unaltered brood size produced more offspring the following year than those for which brood size had been altered (Table 3). This quadratic effect was not significantly influenced by parental infection status (Manipulation2 × Parental infection status: pooled Plasmodium χ2 = 1.21, p = 0.272, P. relictum χ2 = 0.47, p = 0.495, P. circumflexum χ2 = 2.48, p = 0.120). Since the form of this quadratic effect resembled that of manipulation on offspring survival in the current reproductive attempt, and some individuals were subjected to manipulation in both 2006 and 2007, we tested whether there was a covariance between manipulation treatments among birds involved in the experiment in both years that could have contributed to this effect. However, this was not the case (F 1,41 = 0.01, p = 0.904). When those broods that were experimentally enlarged or reduced in the year following initial manipulation were excluded from this analysis, the quadratic effect remained (χ2 = 8.27, p = 0.004, n = 230). To assess whether this effect was driven by differential survival of young within the nest, we tested for a quadratic effect of manipulation on the number of fledglings produced in the year after manipulation. There was no evidence for such a relationship, suggesting the effect on the number of independent offspring arises post-fledging (N fledglings: Manipulation2 F 1,78 = 0.17, p = 0.677).
Discussion
We investigated the effect of brood size manipulation on malaria (Plasmodium) parasitism in blue tits and found evidence that brood enlargement led to a short-term increase in parasitaemia among infected individuals in one of the two years studied. Moreover, single parents (largely females) and parents attending naturally larger broods (those with a larger original clutch size) also had higher parasitaemia at the end of the nestling period. Single parenthood by this stage is not uncommon in this population, and probably reflects unexpected loss of a mate, for example due to predation, abandonment, or polygynous males providing care at only one nest. As both clutch size and single parenthood, like experimental brood enlargement, were associated with higher nest visitation rates, together these results support a causal link between reproductive effort and Plasmodium parasitaemia. Notably, the size of the effect on parasitaemia was similar for all three variables. Although this is the first study reporting effects of brood size manipulation on Plasmodium parasitaemia, the effects detected here (across both years combined r = 0.106; 2006: r = 0.275; 2007: r = −0.063) are of a similar magnitude to those found in previous brood size manipulation studies on related blood parasites, in particular Haemoproteus (Knowles et al. 2009; mean effect size r = 0.155). It therefore appears that increases in blood parasitaemia may be a fairly general consequence of brood size manipulation, and that the effect is not parasite taxon specific.
Parasitaemia in chronic malaria infections is thought to be suppressed by host immunity (Valkiūnas 2005), and numerous studies have demonstrated that brood size manipulation can negatively affect measures of immune responsiveness (Knowles et al. 2009 and references therein). Thus, the effects of reproductive effort on parasitaemia detected here may reflect compromised immune control of infections when parents are working hard. The generality of such an effect among blood parasites suggests that either non-specific immune mechanisms are involved, or that multiple parasite-specific immune responses can be affected by elevated reproductive effort. However, since parasitaemia is a trait determined by parasite replication as well as host immunity, parasite-driven shifts in replication strategies in response to changes in the within-host environment (Reece et al. 2009; Escobedo et al. 2005) could also play a role in these effects.
Despite controlling for known risk factors for malaria infection such as breeding location (Wood et al. 2007), we found no evidence that brood size manipulation altered the risk of becoming infected (or relapsing) across the 12-day period of the experiment or during the following year. Again, these findings concur with studies on other blood parasites (Knowles et al. 2009) and indicate that parasitaemia changes among existing infections are a more common result of altered reproductive effort than changes in susceptibility to novel infections. However, given the possibility of mortality associated with novel (acute) infection, it is perhaps unsurprising that such an effect was not detected. Generally, the extent to which prevailing environmental or physiological conditions can alter individuals’ susceptibility to gaining infection is poorly understood for many infectious diseases, including avian malaria, and would benefit from further investigation, ideally involving controlled experimental infections in semi-natural conditions.
In this study, the predicted effect of manipulated reproductive effort on Plasmodium parasitaemia was only observed in one of the two years investigated. Since there was no evidence that parents responded differently to manipulation in the two years (Fig. 2a, b, Table S2), this difference in effect size seems to reflect variation between years in how altered reproductive effort affected parasitism. Both theory and empirical studies suggest that physiological trade-offs are influenced by environmental conditions, and are more likely to occur when resources are scarce or conditions are unfavourable (Monaghan 2008; Sandland and Minchella 2003; Stearns 1992). Females in 2006 were in poorer condition prior to the experiment than in 2007, and parasitaemia was generally higher in 2006 regardless of experimental treatment (Table 2). Thus it seems that in 2006 birds may have had fewer resources available for both somatic maintenance and immune function before the experiment began. An environmental dependency of the trade-off between reproductive effort and either avian blood parasitism or immune function has been reported previously (e.g. Ardia 2005; Wiehn and Korpimäki 1998). For example, in a study on Eurasian Kestrels (Wiehn and Korpimäki 1998), a negative effect of supplementary feeding on Haemoproteus and Trypanosoma prevalence was only observed in years of low food availability and high trypanosome prevalence. Our results are reminiscent of these findings, and support the view that a trade-off between reproductive effort and parasite defence (or indeed any other trade-off) is likely to be more pronounced under unfavourable conditions. Indeed, numerous studies indicate that the costs of parasite defence (i.e. reductions in other life-history traits as a result of parasite defence) are greater under poor conditions (Hoang 2001; Moret and Schmid-Hempel 2000; Sandland and Minchella 2003).
The question of whether reproductive effort-induced increases in parasitism play any role in reproductive trade-offs has attracted much attention, although few empirical studies have addressed this directly. Stjernman et al. (2004) found negative effects of brood size manipulation on both Haemoproteus parasitaemia and parental survival, but using path analysis demonstrated that changes in parasitaemia were unlikely to be an important mechanism underlying the reproductive costs observed. We found no evidence of a survival cost of reproduction in this study. However, in line with previous work on this population of blue tits and a sympatric great tit population (Pettifor 1993a, b; Pettifor et al. 2001), offspring survival in the current reproductive attempt was affected by brood size manipulation, with the number of independent offspring being maximised when brood size was unaltered. This pattern is consistent with the individual optimisation hypothesis (IOH), which posits that individuals optimise their current reproductive efforts according to the number of offspring they can successfully raise to independence (Perrins and Moss 1975). In other words, optimal reproductive effort is—at least in part—determined by a trade-off between number and quality of offspring. Furthermore, we found some evidence that this trade-off may be influenced by parental parasitism: the quadratic effect of brood size manipulation was more pronounced when at least one parent was infected with P. relictum, one of two common Plasmodium parasites infecting this population (Fig. 4). This finding could indicate that among parasitised birds, for which resources are more limited due to the need to fight infection, even slight deviations from their original brood size lead to decreased fitness (offspring recruitment) compared to unparasitised birds. However, if this scenario is correct and parasitic infection does limit the extent to which parents can raise offspring, two questions present themselves. First, one may question why in this study P. relictum-infected females were not found to lay smaller clutches in the first place (F 1,347 = 1.59, p = 0.208); second, it remains unclear why this trade-off would be exacerbated by infection with one Plasmodium species but not another.
We also found evidence that parents subjected to brood reduction or enlargement produced fewer independent offspring from their reproductive attempts in the following year (but with no suggestion that parasites affected this relationship). This result is puzzling, since there was no evidence that clutch size the following year was influenced by manipulation (which could suggest an adjustment of reproductive effort based on previous experience), and it is difficult to envisage how brood reduction in one year could negatively affect reproductive success in the next. The sample size for this analysis is not very large owing to low recruitment rates in the years that contributed these data (n = 18 independent offspring captured from 85 breeding attempts). However, since the effect is fairly strong, it warrants testing in future brood size manipulation experiments.
At least two previous wild bird studies have documented intensified reproductive costs under conditions of greater parental parasitic infection (Descamps et al. 2009; Møller 1993), suggesting that modulation of trade-offs by parasites may be more common than hitherto realised. Associations between parasitism and expression of trade-offs could arise in a number of ways. If increases in parasitaemia arising from reproductive effort are detrimental, such effects may play a causal role in the pattern of more pronounced trade-offs among parasitised individuals (as in Fig. 1). Alternatively, if parasitized birds have fewer resources available due to the physiological demands of controlling infections, life-history trade-offs may be exacerbated just as any trade-off is predicted to be when resources are scarce (Stearns 1992). Finally, it is possible that birds in a poorer condition tend to be parasitized, and these resource-limited individuals are also those that are most likely to exhibit trade-offs for reasons unrelated to parasitism. Thus, although our findings suggest that parasitism may influence the strength with which life-history trade-offs operate in wild populations, further studies in which both parasitic infection and reproductive effort are simultaneously manipulated are needed to test this possibility definitively. If the modulation of reproductive trade-offs by parasites is a widespread phenomenon, individual variations in infection or immunological status could help explain unaccounted for discrepancies and variations in the strength of trade-offs detected within and across animal populations. Furthermore, the context dependency of trade-offs suggested by this study implies that individual optima for reproductive investment may shift between breeding attempts as individuals’ physiological or environmental conditions change, resulting in selection for facultative reproductive strategies.
References
Alatalo RV, Gottlander K, Lundberg A (1988) Conflict or cooperation between parents in feeding nestlings in the pied flycatcher Ficedula hypoleuca. Ornis Scand 19:31–34
Ardia DR (2005) Tree swallows trade off immune function and reproductive effort differently across their range. Ecology 86:2040–2046
Blondel J, Maistre M, Perret P, Hurtrez-Bousses S, Lambrechts MM (1998) Is the small clutch size of a Corsican blue tit population optimal? Oecologia 117:80–89
Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G (2003) Assessing the cost of mounting an immune response. Am Nat 161:367–379
Browne WJ, McCleery RH, Sheldon BC, Pettifor RA (2007) Using cross-classified multivariate mixed response models with application to life history traits in great tits (Parus major). Stat Model 7:217–238
Caro SP, Charmantier A, Lambrechts MM, Blondel J, Balthazart J, Williams TD (2009) Local adaptation of timing of reproduction: females are in the driver’s seat. Funct Ecol 23:172–179
Descamps S, Gilchrist HG, Bety J, Buttler EI, Forbes MR (2009) Costs of reproduction in a long-lived bird: large clutch size is associated with low survival in the presence of a highly virulent disease. Biol Lett 5:278–281
Dijkstra C, Bult A, Bijlsma S, Daan S, Meijer T, Zijlstra M (1990) Brood size manipulations in the kestrel (Falco tinnunculus)—effects on offspring and parent survival. J Anim Ecol 59:269–285
Escobedo G, Roberts CW, Carrero J, Morales-Montor J (2005) Parasite regulation by host hormones: an old mechanism of host exploitation? Trends Parasitol 21:588–593
Fedorka KM, Zuk M, Mousseau TA (2004) Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution 58:2478–2485
Gustafsson L, Sutherland WJ (1988) The costs of reproduction in the collared flycatcher Ficedula albicollis. Nature 335:813–815
Gustafsson L, Nordling D, Andersson MS, Sheldon BC, Qvarnström A (1994) Infectious diseases, reproductive effort and the cost of reproduction in birds. Philos Trans R Soc Lond Ser B 346:323–331
Harshman LG, Zera AJ (2007) The cost of reproduction: the devil in the details. Trends Ecol Evol 22:80–86
Hoang A (2001) Immune response to parasitism reduces resistance of Drosophila melanogaster to desiccation and starvation. Evolution 55:2353–2358
Horak P, Ots I, Murumagi A (1998) Haematological health state indices of reproducing great tits: a response to brood size manipulation. Funct Ecol 12:750–756
Knowles SCL, Nakagawa S, Sheldon BC (2009) Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: a meta-regression approach. Funct Ecol 23:405–415
Knowles SCL, Palinauskas V, Sheldon BC (2010) Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J Evol Biol 23:557–569
Liedvogel M, Szulkin M, Knowles SCL, Wood MJ, Sheldon BC (2009) Phenotypic correlates of Clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Mol Ecol 18:2444–2456
Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98
Møller AP (1993) Ectoparasites increase the cost of reproduction in their hosts. J Anim Ecol 62:309–322
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc Lond Ser B 363:1635–1645
Moret Y, Schmid-Hempel P (2000) Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168
Naef-Daenzer B, Widmer F, Nuber M (2001) Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J Anim Ecol 70:730–738
Nur N (1984) The consequences of brood size for breeding blue tits 2. Nestling weight, offspring survival and optimal brood size. J Anim Ecol 53:497–517
Palinauskas V, Kosarev V, Shapoval A, Bensch S, Valkiūnas G (2007) Comparison of mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites of the subgenera Haemamoeba and Giovannolaia (Haemosporida:Plasmodiidae). Zootaxa 1626:39–50
Parejo D, Danchin E (2006) Brood size manipulation affects frequency of second clutches in the blue tit. Behav Ecol Sociobiol 60:184–194
Perrins CM (1965) Population fluctuations and clutch size in the great tit, Parus major L. J Anim Ecol 34:601–647
Perrins CM (1979) British tits. Collins, Glasgow
Perrins CM, Moss D (1975) Reproductive rates in the great tit. J Anim Ecol 44:695–706
Pettifor RA (1993a) Brood manipulation experiments 1. The number of offspring surviving per nest in blue tits (Parus caeruleus). J Anim Ecol 62:131–144
Pettifor RA (1993b) Brood manipulation experiments 2. A cost of reproduction in blue tits (Parus caeruleus). J Anim Ecol 62:145–159
Pettifor RA, Perrins CM, McCleery RH (2001) The individual optimization of fitness: variation in reproductive output, including clutch size, mean nestling mass and offspring recruitment, in manipulated broods of great tits Parus major. J Anim Ecol 70:62–79
Reece SE, Ramiro RS, Nussey DH (2009) Plastic parasites: sophisticated strategies for survival and reproduction? Evol Appl 2:11–23
Sandland GJ, Minchella DJ (2003) Costs of immune defense:an enigma wrapped in an environmental cloak? Trends Parasitol 19:571–574
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stjernman M, Råberg L, Nilsson JA (2004) Survival costs of reproduction in the blue tit (Parus caeruleus): a role for blood parasites? Proc R Soc Lond B 271:2387–2394
Svensson L (1992) Identification guide to European passerines, 4th edn. Natural History Museum, Stockholm
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Wiehn J, Korpimäki E (1998) Resource levels, reproduction and resistance to haematozoan infections. Proc R Soc Lond B 265:1197–1201
Wiersma P, Selman C, Speakman JR, Verhulst S (2004) Birds sacrifice oxidative protection for reproduction. Proc R Soc Lond B 271:S360–S363
Williams GC (1966) Natural selection, the cost of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690
Wilson AJ, Pemberton JM, Pilkington JG, Clutton-Brock TH, Kruuk LEB (2009) Trading offspring size for number in a variable environment: selection on reproductive investment in female Soay sheep. J Anim Ecol 78:354–364
Wood MJ, Cosgrove CL, Wilkin TA, Knowles SCL, Day KP, Sheldon BC (2007) Within-population variation in prevalence and lineage distribution of avian malaria in blue tits, Cyanistes caeruleus. Mol Ecol 16:3263–3273
Zera AJ, Harshman LG (2001) The physiology of life history trade-offs in animals. Annu Rev Ecol Syst 32:95–126
Acknowledgments
We thank Ben Carpenter and Clare Andrews for assistance with experiments, J. Quinn, A. Gosler, S. Bouwhuis, S. Patrick and J. Carpenter for winter catching data, T. Uller for helpful comments on the manuscript and Staffan Bensch for advice with qPCR assay design. S. Knowles was funded by a NERC studentship, and M. Wood and B. Sheldon by NERC grants to BCS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pawel Koteja.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knowles, S.C.L., Wood, M.J. & Sheldon, B.C. Context-dependent effects of parental effort on malaria infection in a wild bird population, and their role in reproductive trade-offs. Oecologia 164, 87–97 (2010). https://doi.org/10.1007/s00442-010-1706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1706-1