Abstract
The relative importance of growth and defense to tree mortality during drought and bark beetle attacks is poorly understood. We addressed this issue by comparing growth and defense characteristics between 25 pairs of ponderosa pine (Pinus ponderosa) trees that survived and trees that died from drought-associated bark beetle attacks in forests of northern Arizona, USA. The three major findings of our research were: (1) xylem resin ducts in live trees were >10% larger (diameter), >25% denser (no. of resin ducts mm−2), and composed >50% more area per unit ring growth than dead trees; (2) measures of defense, such as resin duct production (no. of resin ducts year−1) and the proportion of xylem ring area to resin ducts, not growth, were the best model parameters of ponderosa pine mortality; and (3) most correlations between annual variation in growth and resin duct characteristics were positive suggesting that conditions conducive to growth also increase resin duct production. Our results suggest that trees that survive drought and subsequent bark beetle attacks invest more carbon in resin defense than trees that die, and that carbon allocation to resin ducts is a more important determinant of tree mortality than allocation to radial growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth and defense are major uses of plant carbon resources. Carbon allocation to growth promotes survival by increasing resource acquisition and storage and hence competitive ability, whereas allocation to defense promotes survival by producing substances or structures (e.g., resin, latex, spines) that deter herbivores (Herms and Mattson 1992). The allocation of carbon to defense may have important consequences for plant survival, especially during periods of stress and herbivore attacks (Lorio 1986; Mattson and Haack 1987; Lorio et al. 1990; Herms and Mattson 1992). Many conifers are subject to injury and death due to insect attack and many characteristics of growth (Phipps and Whiton 1988; Leblanc 1990; Ogle et al. 2000; Suarez et al. 2004) and defense (Vité and Wood 1961; Hodges et al. 1979; Raffa and Berryman 1982; Reeve et al. 1995; Strom et al. 2002) have been related to this mortality. Few studies, however, have compared the importance of both growth and defense to tree survival during insect attacks.
Oleoresin production and composition are important defenses of conifers against bark beetles (Vité and Wood 1961; Hodges et al. 1979; Strom et al. 2002). However, these resin characteristics have not been widely associated with tree mortality, perhaps because they are difficult and expensive to measure over ecologically meaningful temporal and spatial scales. Alternatively, measures of defense structures, such as vertical resin ducts in xylem, can provide surrogate, retrospective data on tree carbon allocation to defense at an annual resolution and with less effort than direct measurements of resin flow and chemical composition. Resin ducts may be important surrogate measures of tree investment in defense because they are responsible for the production and storage of oleoresin (Hodges et al. 1985, Franceshi et al. 2005). While results are not consistent, some studies have shown a relationship between xylem-based resin ducts and oleoresin flow in conifers of varying sizes (Blanche et al. 1992; Lombardero et al. 2000).
Ponderosa pine (Pinus ponderosa C. Lawson var. scopulorum Engelm.) is a good model organism for examining relationships between tree mortality and carbon allocation to growth and defense. Ponderosa pine is a long-lived dominant tree present throughout much of the western United States (Oliver and Ryker 1990), and has evolved in the presence of tree-killing bark beetles (e.g., Dendroctonus spp., Ips spp.) (Negron and Popp 2004; Breece et al. 2008; Negron et al. 2009). Vertical resin ducts in the xylem of ponderosa pine can be quantified retrospectively with annual resolution using standard dendrochronological techniques. Ponderosa pine stands in northern Arizona currently contain a mixture of live trees and recently dead trees attributed to severe drought in 2002 (65% of normal precipitation) and subsequent bark beetle attacks (Negron et al. 2009).
We compared growth and resin duct characteristics between live and dead ponderosa pine trees following a recent drought and subsequent bark beetle attack in forests of northern Arizona. Additionally, we developed tree mortality models to determine which parameters of radial growth and resin duct characteristics were best associated with tree mortality. We also investigated relationships between temporal variation in annual tree radial growth and vertical resin duct production.
Materials and methods
Study site
This study was conducted within ponderosa pine forests on the Coconino National Forest in northern Arizona, USA. We sampled pairs of live and dead trees from six plots, ranging in elevation from 2,300 to 2,600 m, that were included in a previous study of ponderosa pine mortality (Negron et al. 2009). Recent tree mortality in these plots ranged from 14 to 62% and was attributed to recent drought and attacks by bark beetles, especially Ips spp. (Negron et al. 2009). The six plots were selected based on the occurrence of at least four live and dead tree pairs. A total of 25 pairs were sampled over all plots. For all dead trees sampled, we confirmed the presence of at least one bark beetle sign or symptom, including exit holes, galleries, and the fungal associate, Ophiostoma spp., in the sapwood. The dead tree in each pair was selected based on close location and similar size to the live tree. The close proximity (within 15 m) controlled for differences in soil, aspect, slope, competition, and other microsite differences between trees. The diameter at breast height (dbh, 1.37 m above ground) of all sampled trees was between 15 and 51 cm and trees in all pairs were within 2 cm dbh. Sampled live trees had less than 25% dieback of the canopy and contained little evidence of dwarf mistletoe infection. All sampled dead trees had intact bark and sound outer xylem.
Tree growth and defense measures
All trees were cored once at approximately breast height using a large diameter (12 mm) increment borer. Samples were taken to the laboratory and allowed to dry for 2 weeks, after which all cores were mounted on wood blocks and progressively sanded with 80- to 400-grit sandpaper on a belt sander. We created a high-resolution image (1,200 dpi) by scanning each core and entering all images into WinDendro© (Regent Instruments 2009) to assign annual ring boundaries and measure ring widths (mm year−1). All ring width series from live trees were then cross-dated, and COFECHA (Grissino-Mayer 2001) was used to assess the strength of cross-dating. Once a chronology was developed for the live samples, we cross-dated the ring width series of each dead tree to determine the year of the last ring formed. We assumed that the last formed ring corresponded to the year of death of the dead tree, although death may have occurred in the following year that had no radial growth. All dead trees sampled died after the year 2000, with 70% dying between 2001 and 2004.
In addition to measuring ring width, we also calculated basal area increment (cm2 year−1) using both ring width data and tree-specific cross-sectional radii (inside bark) with the assumption that tree rings represent concentric circles. Measures of defense structures included resin duct production (no. annuli−1), resin duct density (no. mm−2), resin duct width (mm), and resin duct area (%). All defense measures of the two trees in each pair were made along a 10-mm-wide core section spanning 20 years of growth including the last ring formed in dead trees. Resin duct production was measured as a count of resin ducts per annual ring observed. Resin duct production is the mean number of resin ducts present within an annual ring, unadjusted for the area sampled, and represents a relative measure of tree carbon investment in resin ducts. We calculated resin duct density by dividing the resin duct production for a given year by the area of xylem sampled (10 mm multiplied by the ring width of the year measured). Resin duct density is the mean number of resin ducts present per unit area of radial growth and measures tree carbon investment to resin ducts relative to the amount of radial growth. We also estimated resin duct size as the average latitudinal width (mm) of all resin ducts counted in each annual ring. Individual resin duct areas were determined by calculating the area of a circle based on the measured resin duct width. For simplicity, we assumed all resin ducts were perfect circles although many were slightly elliptical. Percent resin duct area is the proportion of resin duct area relative to the area of radial growth, and was calculated for each year (i.e., tree ring) by multiplying the resin duct production by the average resin duct area, dividing the product by the area of annual growth observed, and then multiplying the quotient by 100. Mean values of radial growth and resin duct properties were calculated annually and for 5-, 10-, and 20-year growth periods.
Statistical analysis
We used paired t tests to statistically compare measures of growth and defense between live and recently dead trees. A Bonferroni correction factor (α = 0.05/3 = 0.017) was made for all growth periods within the same measurement type (e.g., 5-, 10-, and 20-year means for basal area increment) to reduce the risk of making Type I errors (Sokal and Rohlf 1995).
Probability of tree mortality was modeled using logistic regression on 50 samples (25 living, 25 dead). Model evaluation and selection were determined using an information theoretic approach. We calculated Akaike’s Information Criterion with a second-order bias correction (AICc) to better estimate model performance where there are many estimated parameters and small sample sizes (Burnham and Anderson 1998). Based on AICc values for each model, we calculated ∆AICc and Akaike weights (w i ), where ∆AICc represents the difference in AICc values between the model of interest and the best performing model and w i is the proportionate likelihood that a particular model is the best. AIC values are valuable for model selection because models are penalized as the number of parameters increase. Models with lower AICc and higher w i values represent stronger and more parsimonious models (Burnham and Anderson 1998).
Individual models were selected by first calculating all single-variable models for both growth and defense measures, with the inclusion of each growth period (i.e., 5-, 10-, and 20-year). We then chose the top five defense variables and top two growth measures based on AICc values for inclusion in the multivariable models considered. Multivariable model generation consisted of all possible combinations of parameters up to a five-variable model. To address potential issues of multicollinearity, we did not include the same variable with overlapping growth periods (i.e., 10- and 20-year basal area increment). Additionally, we calculated variance inflation factors (VIF) and selected models with variable combinations having VIF values <10 (Hosmer and Lemeshow 2000). Receiver operating curves (ROC) were developed to provide a threshold-independent measure of model discrimination (area under the curve), where values greater than 0.8 suggest excellent discrimination (Hosmer and Lemeshow 2000). In all, we compared 54 models and report the top 20 models (∆AICc < 6.0). The proportion of correctly classified dead and live trees was used to internally validate each model.
We compared relationships between measures of annual radial growth (ring width and basal area increment) and resin duct characteristics (resin duct production, resin duct density, and resin duct area), spanning 27 years (1981–2007), using correlation analysis for live, dead, and all trees. All analyses were conducted using SPSS (PASW Statistics 2009).
Results
Live and dead tree comparisons
All measures of radial growth were not significantly different between live and dead trees. Average ring widths for the 5-, 10-, and 20-year periods ranged between 0.49 and 0.68 mm year−1 for dead trees and 0.59–0.79 mm year−1 for live trees, and were consistently 9–16% greater for live trees (Table 1). Similarly, mean basal area increment ranged between 4.25–5.63 cm2 year−1 for dead trees and 4.80–6.31 cm2 year−1 for live trees, and was 7–11% greater for live trees. Both mean ring width and basal area increment increased for live and dead trees by more than 30% as the growth period measured increased from 5 to 20 years (Table 1).
In contrast to growth, all resin duct measures differed significantly between live and dead trees for most growth periods. Percent resin duct area differed most strongly and varied by about twofold (2–4% of the total ring area) across all samples (Table 1). On average, live trees had 51–66% greater resin duct area than dead trees. Additionally, resin duct production for the 5-, 10-, and 20-year periods was 3.64, 3.94, and 4.33 no. year−1, for live trees and 2.37, 2.75, and 3.36 no. year−1 for dead trees, respectively (Table 1). On average, resin duct production of live trees was 29–54% greater than dead trees. Resin duct density differed only for the 10-year growth period with live trees having a 30% greater density than dead trees. Resin duct width was consistently 13% greater in live trees than dead trees across all growth periods and did not vary across all time periods within live or dead trees (Table 1).
Factors of tree mortality
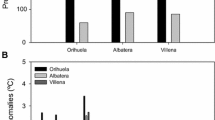
Measures of resin ducts were more important in models of tree mortality than measures of growth. Specifically, percent resin duct area (RDA10-, RDA20-year) and resin duct production (RDP5-, RDP10-year) were regularly included in the models selected (Table 2). Of the growth parameters measured, only RW5- and BAI20-year were included in the top-ranked models, however, neither were included in the top seven models. The two resin duct variables included in the best model (w i = 0.149) were RDP5- and RDA10-year (Fig. 1). The probability of mortality (P m) equation for this model is:
Most of the mortality models presented in Table 2 performed well in classifying both live and dead trees from the sample provided, ranging from 66 to 84% accuracy. On average, models classified dead trees 9% better than living trees (Table 2).
Predictions of ponderosa pine (Pinus ponderosa) mortality from the best model in Table 2 based on resin duct production averaged over 5 years and percent resin duct area averaged over 10 years
Patterns and relationships between radial growth and resin duct characteristics
Many of the bivariate correlations of temporal variation in annual radial growth rates and resin duct characteristics for years 1981–2007 were significant; however, the strength and direction of the relationship was not consistent. Growth rate measures were positively correlated to resin duct production across live, dead, and all trees (Table 3). Ring width had the strongest positive relationship to resin duct production for dead trees (r = 0.72, P < 0.001). Basal area increment was also positively correlated with resin duct production, but to a slightly lesser extent (Table 3). Conversely, resin duct density and percent resin duct area were negatively correlated or had no relationship with growth measures. For live trees, these correlations were consistently negative for all comparisons (P < 0.05). For dead trees and all trees, resin duct density or resin duct area was not related to any growth measure (Table 3).
Mean basal area increment and resin duct production ranged considerably over the 26-year time period; 0.9 and 9.4 cm year−1 and 0.5 and 9.2 ducts year−1, respectively. Both basal area increment and resin duct production recovered more after recent droughts (1996, 2002) for live trees than dead trees (Fig. 2). Increases in basal area increment in the first year after drought were 18–21% greater for live than dead trees. Increases in resin duct production in the first year after drought were 19–168% greater for live than dead trees. This pattern was maintained the second year after drought when live trees increased in basal area increment 8–20% and resin duct production 49–171% more than dead trees.
Temporal variation in a mean basal area increment (BAI) and b mean resin duct production from 1982 to 2007 for live and dead ponderosa pine (sample size ranged from 3 to 25 per year for both live and dead trees). Unidirectional error bars represent standard error. Only positive error bars are present for live trees and only negative error bars for dead trees
Discussion
Live and dead tree comparisons
This research represents the first known study to compare both growth rates and resin duct characteristics between live and dead trees following severe drought and bark beetle attacks. Recent growth rates did not differ significantly between live and dead trees. This finding is contrary to numerous studies that have shown differences in radial growth rates between live and dead trees (Ogle et al. 2000; van Mantgem et al. 2003; Suarez et al. 2004; Bigler et al. 2004; Das et al. 2007). Instead, we found that dead trees typically had fewer and smaller resin ducts, occupying less area of xylem radial growth compared to live trees. Our results suggest that trees containing a greater proportion of xylem composed of resin ducts were more likely to survive during a severe drought and subsequent bark beetle attack. Trees that have a larger defense infrastructure in xylem may be better at surviving herbivore attack by having a greater capacity to produce, store, and transport oleoresin (Hodges et al. 1985, Franceshi et al. 2005). Most (Schopmeyer et al. 1954; Blanche et al. 1992) but not all (Lombardero et al. 2000) empirical studies have reported a positive correlation between oleoresin flow or mass; and resin duct size or abundance. While environmental factors (e.g., soil moisture, temperature; Blanche et al. 1992; Gaylord et al. 2007) often explain considerable variation in tree resin flow, such factors were controlled by the paired tree comparisons in our study.
By applying Poiseuille’s law, with some modification (Schopmeyer et al. 1954), small increases in resin duct width should increase oleoresin flow by the power of four. For instance, our study demonstrated that living trees had 13% wider resin ducts than dead trees. This difference would equate to a 63% greater resin flow in the trees that lived than the trees that died, assuming constant resin production. Coupling this increase in resin flow with the 30% greater density of resin ducts in live trees should result in an approximate twofold increase in oleoresin delivery for trees that survived compared with trees that did not survive the drought and bark beetle attack.
Factors of tree mortality
Overall, the top models performed well in describing tree mortality and included resin duct measures, primarily resin duct production and resin duct area, but not radial growth. Percent correct classifications of live and dead trees in these models were high (>75%) and were similar or better than published values using only growth measures for other conifer species (van Mantgem et al. 2003; Bigler and Bugmann 2003; Bigler et al. 2004; Das et al. 2007). As with most models, there are limitations that should be considered in our results. For example, our analysis lacks an independent validation dataset to further test the accuracy of the model. Furthermore, our best models were slightly better at predicting dead trees than live trees (Table 2). As pointed out by Das et al. (2007), under-prediction of live trees would result in over predictions of mortality in the area of interest. Therefore, additional measurements or approaches that could better classify live trees are warranted.
The absence of growth rate variables in the top tree mortality models was unexpected and contrary to other studies (Ogle et al. 2000; van Mantgem et al. 2003; Suarez et al. 2004; Bigler et al. 2004; Das et al. 2007). While it is possible that patterns of growth, such as abrupt declines and temporal trends (e.g., Das et al. 2007), may be better parameters of tree mortality than average growth rates, our results suggest that measures and patterns of resin defense structures in xylem are more important predictors of tree mortality following severe drought and bark beetle attacks than measures of growth. The specific scenario associated with the ponderosa pine mortality event we sampled may have elevated the role of defense in tree mortality. The 2002 drought in northern Arizona was severe (precipitation 65% below normal) and coincided with a marked increase in Ips spp. populations (Negron et al. 2009). The combination of these events may have caused abrupt death to trees that had relatively “normal” growth rates but low carbon allocation to resin defense.
Patterns and relationships between radial growth and resin duct characteristics
The relationship between measures of annual xylem radial growth and resin ducts was mixed depending on the bivariate comparison. The positive correlation between temporal variation (years 1981–2007) in resin duct production and all measures of radial growth for all trees indicates that more vertical xylem resin ducts were produced annually in years of high radial growth. This result is consistent with reports of positive associations between resin flow in response to wounding and radial growth of ponderosa pine (Gaylord et al. 2007; McDowell et al. 2007). Thus, environmental conditions, such as high precipitation, or tree-intrinsic factors, such as genetic controls, that promote ponderosa pine radial growth should enhance resin duct production and tree survival in subsequent droughts because more xylem is produced to house resin ducts.
While a tradeoff between carbon allocation to growth and defense is predicted by some plant defense theories (Lorio 1986; Mattson and Haack 1987; Stamp 2003), we did not observe large tradeoffs between radial growth and resin ducts in our study of ponderosa pine. The only evidence in our study of a tradeoff in carbon allocation between growth and defense was the negative correlation between resin duct density and area with radial growth for live trees (Table 3), but two lines of evidence indicates that this tradeoff was not important to tree mortality. First, evidence for this tradeoff occurred only for live trees, not for dead trees or all trees combined. Second, in Fig. 1, both resin duct area and production were negatively related to tree mortality; an important tradeoff would produce positive or parabolic relationships. The lack of a tradeoff between growth and defense in our study can be partly explained by the physical connection between xylem growth and vertical xylem resin ducts, in that the ducts are housed within the xylem.
Our results on inconsistent relationships between tree radial growth and xylem resin duct characteristics are repeated throughout much of the literature which includes relationships that are negative (Reid and Watson 1966; Blanche et al. 1992), positive (Fahn and Zamski 1970; Rosner and Hannrup 2004), or neither (Wimmer and Grabner 1997). Throughout these studies, differences in direction of the relationship could be caused by differences in measurement methods. For example, some studies measured resin duct density per tree ring area, while others measured across multiple years and rings of growth. Another possibility is that the relationship differs by tree size or age, where seedlings and saplings may have positive relationships (Fahn and Zamski 1970) and mature trees negative relationships (Reid and Watson 1966; Blanche et al. 1992; as well as this study for live trees). Furthermore, inconsistent findings may reflect the resin duct type measured. For instance, some studies have measured resin ducts in phloem (Baier et al. 2002; Wainhouse et al. 2009) and others in xylem (Fahn and Zamski 1970, Blanche et al. 1992); additionally some studies have focused on radial resin ducts (Baier et al. 2002, Rosner and Hannrup 2004) and others on vertical resin ducts (Wimmer and Grabner 1997; Blanche et al. 1992; Lombardero et al. 2000).
Radial growth and resin duct production varied temporally. Both measures likely responded to interannual differences in climatic factors, such as precipitation, where wetter years resulted in greater radial growth and more resin ducts (Wimmer and Grabner 1997). Temporal patterns of radial growth and resin duct production after known severe drought years (1996, 2002) varied between live and dead trees. Live trees recovered in radial growth and resin duct production after drought more than dead trees. While temporal patterns in both radial growth and resin duct production varied between live and dead trees, resin duct production patterns were more pronounced than radial growth (Fig. 2). This result is consistent with our modeling results (Table 2) which determined that resin duct characteristics were more important than radial growth to tree mortality.
Conclusions
Different carbon allocation to xylem resin ducts between trees that died from severe drought and bark beetle attacks and trees of similar size in the same stand and microsite that survived suggests pronounced genetic variation within ponderosa pine stands in carbon allocation to defense. This suggestion is consistent with the large amount of genetic variation reported for other morphological and physiological traits for this species at broader spatial scales (Conkle 1973; Linhart et al. 1981; Rehfeldt 1992, 1993). Such genetic differences in resin production and composition and linkages to tree resistance to bark beetle attacks have been documented in other conifers, including pines (Strom et al. 2002; Rosner and Hannrup 2004). Our results indicate that severe droughts in the southwestern United States are selective events that favor drought- and herbivore-tolerant trees (Ogle et al. 2000) having high carbon allocation to resin defenses.
Understanding mechanisms of tree mortality (e.g., McDowell et al. 2008) is of increasing importance because of the likely role of climate change in recent pulses of tree mortality documented throughout much of the western United States (van Mantgem et al. 2009). In the southwestern United States, declines in many foundation tree species including ponderosa pine (Breshears et al. 2005; Shaw et al. 2005; Mueller et al. 2005; Gitlin et al. 2006) and increases in bark beetle populations (Raffa et al. 2008) have been documented during recent severe droughts. More frequent and severe droughts are predicted in the southwestern United States due to climate warming and greater variation in precipitation (Westerling et al. 2006; Seager et al. 2007). Given this scenario, increases in drought- and bark beetle-induced tree mortality are likely. Our results indicate that more attention needs to be given to the role of tree defense structures and processes as determinants of tree mortality.
References
Baier P, Führer E, Kirisits T, Rosner S (2002) Defence reactions of Norway spruce against bark beetles and the associated fungus Ceratocystis polonica in secondary pure and mixed species stands. For Ecol Manage 159:73–86
Bigler C, Bugmann H (2003) Growth-dependent tree mortality models based on tree rings. Can J For Res 33:210–221
Bigler C, Gricar J, Bugmann H, Cufar K (2004) Growth patterns as indicators of impending tree death in silver fir. For Ecol Manage 199:183–190
Blanche CA, Lorio PL, Sommers RA, Hodges JD, Nebeker TE (1992) Seasonal cambial growth and development of loblolly pine: xylem formation, inner bark chemistry, resin ducts, and resin flow. For Ecol Manage 49:151–165
Breece CR, Kolb TE, Dickson BG, McMillin JD, Clancy KM (2008) Prescribed fire effects on bark beetle activity and tree mortality in southwestern ponderosa pine forests. For Ecol Manage 255:119–128
Breshears DD, Cobb NS, Rich PM, Price KP, Allen CD, Balice RG, Romme WH, Kastens JH, Floyd ML, Belnap J, Anderson JJ, Myers OB, Meyer CW (2005) Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA 102:15144–15148
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer, New York
Conkle MT (1973) Growth data for 29 years from the California elevational transect study of ponderosa pine. For Sci 19:31–39
Das AJ, Battles JJ, Stephenson NL, van Mantgem PJ (2007) The relationship between tree growth patterns and likelihood of mortality: a study of two tree species in the Sierra Nevada. Can J For Res 37:580–597
Fahn A, Zamski E (1970) The influence of pressure, wind, wounding and growth substances on the rate of resin duct formation in Pinus halepensis wood. Isreal J Bot 19:429–446
Franceshi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–376
Gaylord ML, Kolb TE, Wallin KF, Wagner MR (2007) Seasonal dynamics of tree growth, physiology, and resin defenses in a northern Arizona pine forest. Can J For Res 37:1173–1183
Gitlin AR, Sthultz CM, Bowker MA, Stumpf S, Paxton KL, Kennedy K, Munoz A, Bailey JK, Whitham TG (2006) Mortality gradients within and among dominant plant populations as barometers of ecosystem change during extreme drought. Conserve Biol 20:1477–1486
Grissino-Mayer HD (2001) Evaluating cross-dating accuracy: a manual and tutorial for the computer program COFECHA. Tree Ring Res 57:205–221
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Hodges JD, Elam WW, Watson WF, Nebeker TE (1979) Oleoresin characteristics and susceptibility of four southern pines to southern pine beetle (Coleoptera:Scolytidae) attacks. Can Entomol 111:889–896
Hodges JD, Nebeker TE, DeAngelis JD, Karr BL, Blanche CA (1985) Host resistance and mortality: a hypothesis based on the southern pine beetle–microorganism–host interactions. B Entomol Soc Am 31:31–35
Hosmer DW, Lemeshow S (2000) Applied logistic regression. Wiley, New York
Leblanc DC (1990) Relationships between breast-height and whole stem growth indices for red spruce on Whiteface Mountain, New York. Can J For Res 20:1399–1407
Linhart YB, Mitton JB, Sturgeon KB, Davis ML (1981) Genetic variation in space and time in a population of ponderosa pine. Heredity 46:407–426
Lombardero MJ, Ayres MP, Lorio JL Jr, Ruel JJ (2000) Environmental effects on constitutive and inducible resin defenses of loblolly pine. Ecol Lett 3:329–339
Lorio PL Jr (1986) Growth-differentiation balance: a basis of understanding southern pine beetle-tree interactions. For Ecol Manage 14:259–273
Mattson WJ, Haack RA (1987) The role of drought stress in provoking outbreaks of phytophagous insects. In: Barbosa P, Schulz J (eds) Insect Outbreaks. Academic, New York, pp 365–407
McDowell NG, Adams HD, Bailey JD, Kolb TE (2007) The role of growth efficiency, leaf area index, and resin flow in southwestern ponderosa pine forests. Can J For Res 37:345–355
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why to some plants survive while other succumb to drought? New Phytol 178:719–739
Mueller RC, Scudder CM, Porter ME, Trotter T III, Gerhing CA, Whitham TG (2005) Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. J Ecol 93:1085–1093
Negron JF, Popp JB (2004) Probability of ponderosa pine infestation by mountain pine beetle in the Colorado Front Range. For Ecol Manage 191:17–27
Negron JF, McMillin JD, Anhold JA, Coulson D (2009) Bark beetle-caused mortality in a drought-affected ponderosa pine landscape in Arizona, USA. For Ecol Manage 257:1353–1362
Ogle K, Whitham TG, Cobb NS (2000) Tree-ring variation in pinyon predicts likelihood of death following severe drought. Ecology 81:3237–3243
Oliver WW, Ryker RA (1990) Pinus ponderosa Dougl. ex Laws. Ponderosa pine. In: Burns RM, Honkala BH (eds) Conifers, Silvics of North America, Agricultural Handbook 654, vol 1, pp 413–424. US Forest Service, Washington, DC
PASW Statistics (2009) SPSS base for windows user’s guide. SPSS, Chicago
Phipps RL, Whiton JC (1988) Decline in long-term growth trends of white oak. Can J For Res 18:28–36
Lorio Jr. PL, Sommers RA, Blanche CA, Hodges JD, Nebeker TE (1990) Modeling pine resistance to bark beetles based on growth and differentiation balance principles. In: Dixon RK, Meldahl RS, Ruark GA, Warne WG (eds) Process modeling of forest growth responses to environmental stress. Timber, Portland, pp 402–409
Raffa KF, Berryman AA (1982) Physiological differences between lodgepole pines resistant and susceptible to the mountain pine beetle and associated microorganisms. Environ Entomol 11:486–492
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517
Reeve JR, Ayres MP, Lorio PL (1995) Host suitability, predation, and bark beetle population dynamics. In: Cappuccino N, Price PW (eds) Population dynamics: new approaches and synthesis. Academic, San Diego, pp 339–357
Regent Instruments Inc (2009) WinDendro. Regent Instruments, Quebec
Rehfeldt GE (1992) Early selection in Pinus ponderosa: compromises be- tween growth potential and growth rhythm in developing breeding strategies. For Sci 38:661–677
Rehfeldt GE (1993) Genetic variation in the Ponderosae of the Southwest. Am J Bot 80:330–343
Reid RW, Watson JA (1966) Sizes, distributions, and numbers of vertical resin ducts in lodgepole pine. Can J For Res 44:519–525
Rosner S, Hannrup B (2004) Resin canal traits relevant for constitutive resistance of Norway spruce against bark beetles: environmental and genetic variability. For Ecol Manage 200:77–87
Schopmeyer CS, Mergen F, Evans TC (1954) Applicability of Poiseuilles’s law to exudation of oleoresin from wounds on slash pine. Plant Physiol 29:82–87
Seager R, Ting M, Held I, Kushnir Y, Lu J, Vecchi G, Huang H, Leetmaa A, Lau N, Li C, Velez J, Naik N (2007) Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316:1181–1184
Shaw JD, Steed BE, DeBlander LE (2005) Forest inventory and analysis (FIA) annual inventory answers the question: what is happening to pinyon-juniper woodlands? J For 103:280–285
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Stamp N (2003) Out of the quagmire of plant defense hypothesis. Q Rev Biol 78:23–55
Strom BL, Goyer RA, Ingram LL Jr, Boyd GDL, Lott LH (2002) Oleoresin characteristics of progeny of loblolly pines that escaped attack by the southern pine beetle. For Ecol Manage 258:169–178
Suarez ML, Ghermandi L, Kitzberger T (2004) Factors predisposing episodic drought-induced tree mortality in Nothofagus—site, climatic sensitivity and growth trends. J Ecol 92:954–966
van Mantgem PJ, Stephenson NL, Mutch LS, Johnson VG, Esperanza AM, Parsons DJ (2003) Growth rate predicts mortality of Abies concolor in both burned and unburned stands. Can J For Res 33:1029–1038
van Mantgem PJ, Stephenson NL, Byrne JC, Daniels LD, Franklin JF, Fulé PZ, Harmon ME, Larson AJ, Smith JM, Taylor AH, Veblen TT (2009) Widespread increase of tree mortality rates in the western United States. Science 323:521–524
Vité JP, Wood DL (1961) A study on the applicability of the measurement of oleoresin exudation pressure in determining susceptibility of second growth ponderosa pine to bark beetle infestations. Contrib Boyce Thompson Inst 21:67–78
Wainhouse D, Staley JT, Jinks R, Morgan G (2009) Growth and defence in young pine and spruce and the expression of resistance to a stem-feeding weevil. Oecologia 158:641–650
Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW (2006) Warming and earlier spring increases western US forest wildfire activity. Science 313:940–943
Wimmer R, Grabner M (1997) Effects of climate on vertical resin duct density and radial growth of Norway spruce [Picea abies (L.) Karst]. Trees 11:271–276
Acknowledgments
Funding for this research was provided in part by the Science Foundation of Arizona fellowship program. We would like to thank J. Negron and J. McMillin for providing mortality plot locations and associated data. Two anonymous reviewers, as well as, M. Gaylord, K. Waring, and T. Whitham gave helpful feedback on earlier versions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Phyllis Coley.
Rights and permissions
About this article
Cite this article
Kane, J.M., Kolb, T.E. Importance of resin ducts in reducing ponderosa pine mortality from bark beetle attack. Oecologia 164, 601–609 (2010). https://doi.org/10.1007/s00442-010-1683-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1683-4