Abstract
A large body of literature suggests that asymmetric competition, where large individuals suppress the growth of smaller individuals by intercepting a disproportionate share of incoming light, is a dominant process in tree population development. This has not been examined extensively for long-lived tree species that accumulate growth over many years under varying growing conditions. Using dendrochronological techniques, we reconstructed annual growth and mortality rates at ten stands of jack pine (Pinus banksiana Lamb.) in Western Canada. We used these data to calculate an annual index of the size asymmetry of growth for each stand for the last 50 years. Jack pine is a shade-intolerant species found in even-aged monoculture stands, so the simple hypothesis is that large trees should consistently perform relatively better than small trees. Inter-annual variation in the index of size-asymmetric growth was positively associated with interannual variation in stand productivity at eight of ten sites. The size asymmetry of growth also showed a positive trend with age at eight of ten sites, even though all sites were in a period of declining leaf area. This should have reduced the intensity of asymmetric competition for light and reduced the size asymmetry of growth over time. Alternate hypotheses for this trend are (1) that physical collisions between crowns result in asymmetric competition for growing space because they are more damaging to small trees, or (2) that a differential build up of diseases in susceptible trees suppresses their growth, even in the absence of competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large body of literature suggests that asymmetric competition, where large individuals suppress the growth of smaller individuals by intercepting a disproportionate share of incoming light, is a dominant process in tree population development (e.g. Weiner and Thomas 1986; Weiner 1990; Schwinning and Weiner 1998). However, this has not been examined extensively for long-lived tree species that accumulate growth over many years, under growing conditions that vary from year to year. Testing this assumption is important because competition indices used in most tree growth and forest-stand dynamics models assume that the growth of large and small trees in a population is consistently related to their size rank (e.g. Stadt et al. 2007). This may not be correct if the intensity of competition varies with growing conditions (e.g. Goldberg and Novoplansky 1997), or if changes in growth dominance occur over time (Binkley 2004; Binkley et al. 2006). Over time, trees are affected by long-term (multi-decadal) changes in leaf area and the density of neighbours. They are also affected by short-term (inter-annual) variation in growing conditions caused by variation in weather and periodic herbivory. These factors may affect large trees differently than small trees. Some studies suggest that increased water availability benefits large trees relatively more than small trees (Vose and Swank 1994; Orwig and Abrams 1997; Wichmann 2001). Others conclude that small trees may grow relatively better during drought (Liu and Muller 1993; Biondi 1996; Piutti and Cescatti 1997); Newton and Jolliffe (1998) reported greater mortality and slower recovery in suppressed than in dominant trees after herbivory. The growth performance of individual trees relative to their size can be described in terms of size asymmetry, which is related to the size asymmetry of competition (Weiner and Damgaard 2006). Growth is size symmetric when individuals grow in proportion to their size and size asymmetric when large trees grow disproportionately relative to their size (Schwinning and Weiner 1998). Inverse size asymmetry, where small trees grow disproportionately relative to their size, may also be observed (Biondi 1996).
Our objectives in this study were to investigate the long-term (multi-decadal) trends and short-term (inter-annual) variability in the size asymmetry of growth among individual trees. Our hypothesis was that long-term trends would be related to changes in stand characteristics, and inter-annual variability to variation in growing conditions. We used dendrochronological techniques (Metsaranta and Lieffers 2009) to obtain annual data on growth and mortality rates of individual trees over a fifty-year period at ten jack pine (Pinus banksiana Lamb.) stands in Western Canada. Jack pine is a shade-intolerant species found in even-aged monoculture stands regenerated after wildfire (Burns and Honkala 1990). The simple hypothesis is that asymmetric competition for light should dominate the long-term development of these stands. Therefore, large trees should consistently perform better than small trees relative to what would be expected based solely on differences in size, and growth should be size asymmetric over time. However, in even-aged monoculture pine forests, productivity and leaf area both tend to peak at the time that canopy closure is achieved, with declining leaf area thereafter (Long and Smith 1992; Ryan et al. 1997). Competition for light should become less important as leaf area declines in older stands, potentially reducing the size asymmetry of growth over time. In the short-term, jack pine productivity is enhanced by wet and warm springs (e.g. Larsen and MacDonald 1995; Brooks et al. 1998; Hofgaard et al. 1999). In this region, these forests are also defoliated by jack pine budworm (Choristoneura pinus pinus Freeman) on about a ten-year cycle (Volney 1988). This results in a mixture of defoliated and non-defoliated trees (Scarr 1995). We also hypothesized that short-term variability in the size asymmetry of growth will be increased by factors that increase productivity (warm spring temperatures and high precipitation), and reduced by factors that reduce productivity (herbivory and drought).
Materials and methods
Study area
Our study sites were from five regions in Western Canada. Two (Prince Albert and Candle Lake) were in the Boreal Plains ecozone and three (Flin Flon, Thompson, and Jenpeg) and three were in the Boreal Shield ecozone (Ecological Stratification Working Group 1996). In each region, we established one plot at a relatively nutrient-rich site, and another at a relatively nutrient-poor site. Further details on the study region and plots are given in the “Electronic supplementary material”.
Data collection and processing
Data collection and processing are further described in Metsaranta and Lieffers (2008) and Metsaranta et al. (2008). Briefly, we established 900 m2 (30 m × 30 m) stem mapped plots at Candle Lake and Thompson in 2005. At Prince Albert, Flin Flon, and Jenpeg, we established 100 m2 (10 m× 10 m) plots in 2006. We made a full census of the growth of live trees, snags, and downed logs. We measured breast height diameter for all trees, and height for living trees. We extracted two cores at breast height from living trees and cut cross-sectional discs from dead trees. Some trees (n = 25 at 900 m2 plots, n = 10 at 100 m2 plots) were also cored at ground level to estimate time of origin. These methods can reliably reconstruct jack pine stands for up to 50 years into the past in this region (Metsaranta et al. 2008). We prepared samples using standard dendrochronological methods, measured them with WinDendro (Regent Instruments, Quebec, Canada), and cross-dated them against master chronologies developed from the largest trees at each site. Trees without detectable radial growth were considered functionally dead (Mast and Veblen 1994). This possibly underestimated the true year of death in cases of extreme suppression (<5% of samples). The average correlation between ring widths on a dead sample and the master chronology was r 2 = 0.84 (SD 0.15, range 0.56–0.99, n = 589). For living trees, it was r 2 = 0.80 (SD 0.19, range 0.46–0.99, n = 661). Due to decomposition, ring widths could not be measured on 4.5% of n = 308 snags and 35.2% of n = 291 downed logs. We used the mean year of death of the three larger and three smaller trees nearest in diameter, of the same class (snag or downed log), and at the same plot as a decomposed tree as an estimate of its year of death, and their ring widths as an estimate of its growth. This method has a mean absolute difference of 3.3 (SD 2.4) years between true and estimated year of death (Metsaranta et al. 2008).
Quantifying size and growth rate
We used stemwood volume and volume increment to quantify tree size and growth rate (Metsaranta and Lieffers 2008). Briefly, we determined the diameter (inside bark D ib) of each tree annually from cumulative ring widths and converted this to the diameter outside bark (D ob) using (Husch et al. 2003)
We then predicted heights using the Richards function,
where H is tree height (m) and D is outside bark diameter (cm) at breast height (1.3 m). Third, we determined volume from H and D using a taper equation,
where the components of the equation are as defined in Kozak (1988). We estimated parameters for Eq. 1 and Eq. 2 from data in Halliwell and Apps (1997), supplemented with data from the present study. For Eq. 3, we obtained parameters for Saskatchewan from Gál and Bella (1994), and for Manitoba from Klos (2004). We used numerical integration of Eq. 3 to determine the total stemwood volume (m3) of each tree, and the difference in volume between subsequent years to determine stemwood volume increment (m3year−1). Volumes for a given tree i in year j (V ij ) were summed to give total standing volume in year j (Vtot j ). Volume increments for each tree i in year j (AVI ij ) were summed to give total annual volume increment in year j (AVItot j ).
Size asymmetry of growth
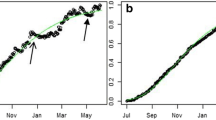
We plotted the proportion of total stand volume increment that each tree i represented in a given year j (AVI ij /AVItot j ) against the proportion of total stand volume that each tree i represented in the previous year j − 1 (V ij−1 /Vtotj−1). To quantify the size asymmetry of growth, we used the slope of a simple linear regression line fit to the proportional growth rate and size data. Due to the unit sum constraint of proportional data, we applied a centred log-ratio transformation (Aitchison 1986) prior to estimating the slope. For d trees where p i is the proportion of total size or size increment (i = 1, 2,…,d) in each tree in a year, the centered log-ratio transformation was calculated as log(p i /g), where g is the geometric mean of all the proportional tree sizes or size increments in a given year (Aitchison 1986). When the growth is proportional to size, the slope of the regression line is 1 and growth is size symmetric (Fig. 1a). When growth in large trees is disproportionately high relative to their size, the slope is greater than 1 and growth is positively size asymmetric (Fig. 1a). When growth in small trees is disproportionately high relative to their size, the slope is less than 1 and growth is inversely size asymmetric (Fig. 1a). Thus, when the slope increases from one year to the next, the growth of larger trees is relatively better than it was the previous year. When it decreases, the growth of smaller trees is relatively better than it was the previous year. An example further demonstrating how the index is interpreted and calculated is plotted in Fig. 1b. Further details on our method are given in the “Electronic supplementary material”.
Alternative relationships between proportional size in year t and growth in year t + 1 used as an index of size-asymmetric growth (a). When the slope = 1, growth is size symmetric, when it is >1, growth is size asymmetric, and when it is <1, growth is inversely size asymmetric. Data from a single year (1961) at the poor site at Thompson (b) shows size-symmetric growth (slope = 1.02). In b, proportional volume and volume increment were transformed to centred-log ratios
Data analysis: long-term trends
Our simple hypothesis was that asymmetric competition for light should dominate the development of these stands, and therefore expected size-asymmetric growth (index > 1) over time. However, in even-aged monoculture pine forests, maximum leaf area closely coincides with canopy closure and peak biomass productivity (e.g. Long and Smith 1992; Ryan et al. 1997), and declines thereafter. The timing of peak leaf area is hypothesized to be associated with a change in growth dominance (Binkley 2004; Binkley et al. 2006). To estimate the timing of peak productivity (and leaf area), we fit a Richards function to the cumulative gross stemwood volume data from each site:
where V is cumulative gross stemwood volume and A is stand age, and assumed that peak biomass productivity and maximum leaf area coincided with the peak stemwood volume growth rate. We examined the estimated timing of peak leaf area in relation to the index of size-asymmetric growth in two ways. The first was to determine if there was an overall trend in the index. We used the Mann–Kendall test for trend (τ; Hipel and McLeod 1994), which tests for a monotonic trend in a time series x(t), based on the Kendall rank correlation of x(t) and t. We assessed the robustness of the resulting significance test to autocorrelation in the time series from the 95th percentile interval of 10,000 bootstrap simulations of τ, where the input time series was re-ordered by sampling without replacement. We also tested for associations with the peak in leaf areas and a change point in the time series of the index of size-asymmetric growth. We also used a Bayesian change point detection algorithm (Barry and Hartigan 1993) to calculate the annual probability of change points in the time series of the index of size-asymmetric growth. We used the bcp package for R to perform these calculations (Erdman and Emerson 2007; R Development Core Team 2007). We compared highly probable (>90%) change points identified by this procedure to the timing of peak leaf area to determine if there was any coincidence between these events.
Data analysis: short-term variation
We obtained spatially interpolated weather data for each site from 1950 to 2000 from McKenney et al. (2006). After 2000, we obtained these data from the nearest station in the Meteorological Service of Canada’s online weather data repository. From various sources (Volney 1988; Simpson and Coy 1999 and historical annual reports of the Canadian Forest Insect and Disease Survey summarized therein), we determined that jack pine budworm defoliation most likely occurred in 1964–1966, 1976–1978, and 1984–1987 at the Prince Albert sites, 1964–1967, 1978, and 1985–1987 at the Candle Lake sites, 1965–1967 and 1984–1986 at the Flin Flon sites, 1984–1985 at the Jenpeg sites, and 1983–1985 at the Thompson sites. We hypothesized that short-term variability in the size asymmetry of growth would be increased by factors that increase productivity (warm spring temperatures and high precipitation), and reduced by factors that reduce productivity (herbivory and drought). To test this, we first used an information-theoretic approach to evaluate the ability of a series of models formalizing these hypotheses by their ability to explain inter-annual variability in the index of size-asymmetric growth at each study site. Further details on this analysis are given in the “Electronic supplementary material”. We further quantified any consistently significant relationships detected using the cross-correlation functions (ccf) of the first-differenced time series of the predictor variable and the index of size-asymmetric growth, estimating significance from the 95th percentile interval of 10,000 bootstrap simulations where the input time series were re-ordered by sampling without replacement. We also looked for associations between episodes of defoliation and change points in the time series of the index of size-asymmetric growth by comparing years with highly probable (>90%) change points with periods of historical defoliation.
Results
Long-term trends
The index of size-asymmetric growth was typically >1, indicating that larger trees were most commonly growing relatively better than expected given their size (Fig. 2). However, all sites had times where the index was near 1, indicating periods of size-symmetric growth. At some sites, the index remained fairly constant for periods of time. Other sites showed large, sudden fluctuations that occasionally resulted in periods when the index was <1, suggesting that there were some years where small trees were growing disproportionately better than expected given their size. The highly variable patterns suggest that trends in the index were site-specific, and not related to site conditions. The only consistent pattern was that the index of size-asymmetric growth had a significant positive trend over time (Fig. 2; τ = 0.31–0.83, p < 0.05) at all but the rich site at Flin Flon (Fig. 2c) and the rich site at Thompson (Fig. 2e). At all but the poor site at Thompson (Fig. 2e), leaf area peaked between 1952 and 1977 (Fig. 2). The index often remained greater than one beyond this time, and in no case was a highly probable (>90%) change point in the index associated with the timing of peak leaf area (Fig. 2).
Annual trajectories of the index of size-asymmetric growth (±SE) since 1950 at each study site. A value of 1 indicates size-symmetric growth. The dashed vertical lines indicate periods of jack pine budworm defoliation. The triangles indicate the estimated time of maximum leaf area, while stars show the occurrence of highly probable (>90%) change points in the time series. Values for rich sites are shown with filled symbols, and those for the poor sites with open symbols. Values of the Mann–Kendall trend test are provided for the poor (taup) and rich (taur) sites at each region
Short-term variation
The index of size-asymmetric growth exhibited high inter-annual variability across the ten sites (Fig. 2). We did not detect any consistently significant associations between weather variables or the occurrence of defoliation and inter-annual variability in the index of size-asymmetric growth at any of the sites. The only consistent relationship across sites was a strong association between the index and annual volume increment. Further details are provided in the “Electronic supplementary material”. The positive association between inter-annual variation in the index of size-asymmetric growth and inter-annual variation in annual volume increment was significant (p < 0.05) at all but the rich sites at Prince Albert and Flin Flon (Fig. 3). Defoliation was not a significant predictor of the index of size-asymmetric growth, but some highly probable (>90%) change points in the time series of size-asymmetric growth were associated with defoliation (Fig. 2). For example, a highly probable change point in the 1980s at the rich site at Thompson (Fig. 2e) occurs concurrently with defoliation and is associated with an abrupt decline in the index. Two probable change points occurred a few years following defoliation: in one case (Flin Flon rich; Fig. 2c) associated with a decline in the index, and in the other (Prince Albert rich; Fig. 2a) an increase. Probable change points at the rich site at Jenpeg (Fig. 2d) and the poor sites at Prince Albert (Fig. 2a) and Flin Flon (Fig. 2c) do not coincide with any known external events.
Relationships between annual growth rate and the index of size-asymmetric growth since 1950 at each study site. Both series are expressed as first differences (i.e. the change between years Y and Y − 1). Regression lines at a site are plotted when the cross-correlation function (CCF) between the two time series is significantly different from zero (p < 0.05)
Discussion
In this study, we used dendrochronological data on annual tree size, growth, and mortality to reconstruct the long-term (decadal) trends and short-term (inter-annual) variability of an index of the size asymmetry of growth at ten jack pine (Pinus banksiana) sites in Western Canada. Larger trees were typically growing better than expected relative to their size, and eight of ten sites had a positive long-term trend in the size asymmetry of growth. These observations suggested that competition was primarily size asymmetric and increasing in intensity over time. At eight of ten sites, short-term variation in the size asymmetry of growth was related to inter-annual variation in stand-level productivity. The index increased when productivity increased, suggesting that larger trees benefited most from years with good growing conditions. The index decreased when productivity decreased, suggesting that factors reducing productivity also most negatively affected large trees. These observations suggest that asymmetric competition was more important when growing conditions were good and symmetric competition when growing conditions were poor. Site quality effects would have been manifest in our analysis as differences between rich and poor sites in the direction of trends in the size asymmetry of growth, or the identity of significant predictors of its inter-annual variability. However, we did not see any strong evidence for such differences.
At present, the mechanism that would be invoked for explaining a positive trend in the index of size-asymmetric growth over time is an increase in the intensity of asymmetric competition for light. However, at the time of sampling, most of the study sites were beyond their peak leaf area (Fig. 2), and we found no evidence that a change point in the time series of the index of size-asymmetric growth was associated with the timing of peak leaf area. Logically, the intensity of asymmetric competition for light should decline as leaf area declines, reducing the size asymmetry of growth. We see two plausible alternative hypotheses for this observation. (1) Crown shyness. Crown shyness is the empty space that develops in taller stands from crowns colliding with each other during high winds (Putz et al. 1984). Crown shyness increases with height growth in even-aged monoculture pine forests (Fish et al. 2006). Studies in lodgepole pine (Pinus contorta Dougl. ex Loud.), a closely related species with similar stand development that is capable of hybridizing with P. banksiana, have shown that trees collide hundreds of times per hour in moderate winds (Rudnicki et al. 2003), that preventing these collisions increases leaf area (Meng et al. 2006), and that trees do not compensate for leaf area lost to collisions by maintaining longer crowns (Fish et al. 2006). We suggest that the impact of collisions may be asymmetric: smaller trees are disproportionately damaged when they collide with large trees because the upper branches (which are more important for intercepting light) of shorter trees collide with lower branches (which are less important for intercepting light) of the taller trees. After leaf area has peaked, physical competition for growing space could be a better explanation for continued increases in the size asymmetry of growth with age than competition for light (Newton 2006). (2) Disease load. The third hypothesis is that a disease load accumulates in the roots and stems of susceptible individuals over time. Previous studies have shown the potential for root disease to impact jack pine stand development (Mallett and Volney 1990). The proximate cause of mortality or reductions in growth rate in these susceptible individuals could be related to competition (they are likely shaded by or colliding with larger individuals), but the ultimate cause would be a high disease load that would reduce their growth even in the absence of competition for light.
Although defoliation was not a significant predictor of the index of size-asymmetric growth, some highly probable (>90%) change points in the time series of size-asymmetric growth were associated with the occurrence of defoliation (Fig. 2). The reason to expect a linkage between herbivory and the size-asymmetry of growth is as follows. Early instars of jack pine budworm feed primarily on pollen cones (McCullough 2000). Though most jack pines produce some pollen cones in most years (Nealis et al. 2003) and total cone production is correlated with growing conditions (Houle and Filion 1993), stress-induced crops of pollen cones are sometimes common on small trees or trees affected by root rot (Mallett and Volney 1990). If this was the case, then we should have observed a disproportionate impact on small trees and more size-asymmetric growth during defoliation events. To our knowledge, the size proportionality of pollen cone production in jack pine has not been quantitatively studied.
In a previous study of these stands, we found that productivity variation also influenced development of size hierarchy (Metsaranta and Lieffers 2008). Previously, we found greater inequality of tree growth rates, measured using the Gini coefficient, when productivity declined. Our current results indicate that growth became more size symmetric when productivity decreased, a seemingly paradoxical result because it suggests that growth becomes both closer to size proportional and more unequal when growth rates decline. The two results would be consistent if sizes were more unequal than growth rates, but we have shown that this was not the case (Metsaranta and Lieffers 2008). The Gini coefficient of tree growth rates and our index of size-asymmetric growth may or may not be measuring the same thing. When examined together, there are periods where they are changing in phase, and periods where they are not. This is most clearly evident at the poor site at Thompson (Fig. 4). In our previous study, the inequality in growth rates was most often due to a large number of trees with low growth rates, except during years with low productivity, when it was due to a small number of trees with high growth rates (Metsaranta and Lieffers 2008). If the few trees maintaining high growth rates were small, then the inequality of growth rates might increase at the same time that growth is becoming more size symmetric.
Relationship between the index of size-asymmetric growth and the Gini coefficient of growth from 1950 to 2004 at the Thomson poor site (see Figs. 2e, 3j), when both are expressed as first differences (i.e. the change between years Y and Y − 1). The Gini coefficient for growth rate at this site was determined as in Metsaranta and Lieffers (2008)
We found that growth was mainly size asymmetric, but also that periods of size-symmetric growth occurred at all sites. Occasional periods of inverse size asymmetry occurred at some sites, indicating that small trees sometimes regained some competitive status. Our observation of the primacy of size-asymmetric growth contrasts with the weakly expressed growth dominance of large trees noted for P. contorta (Binkley et al. 2006). The relationship between changes in productivity from one year to the next and changes in the size-asymmetry of growth suggest that a relationship exists between growing conditions and the intensity of competition (e.g. Goldberg and Novoplansky 1997), though we could not determine its specific causes. Our results have implications for the interpretation of short-term studies of competition in tree populations because they suggest that their results may be influenced by growing conditions during the study. Our results also have implications for the design of competition indices used in forest growth and stand dynamics models (e.g. Stadt et al. 2007). The possibility of variation in the size asymmetry of growth or competition is typically not accounted for by these models (Larocque 2002; Nord-Larsen et al. 2006). Our finding of a significant positive trend in the index of size asymmetry of growth over time is surprising, as the study stands were past their peak productivity and leaf area, a time when the intensity of asymmetric competition for light should be declining. However, in older stands, crown abrasion or an increase in disease load with age may be appropriate alternate hypotheses for explaining the increase in the size asymmetry of growth over time. The crown abrasion hypothesis suggests that competition continues to be intense, but it is for safe growing space rather than light. The disease hypothesis suggests that competition may not be as important as other factors in determining the population dynamics of this species. Our data do not allow us to distinguish among these alternate hypotheses.
References
Aitchison J (1986) The statistical analysis of compositional data. Chapman & Hall, London
Barry D, Hartigan JA (1993) A Bayesian analysis for change point problems. J Am Stat Assoc 88:309–319
Binkley D (2004) A hypothesis about the interaction of tree growth dominance and stand production through stand development. For Ecol Manag 236:5–16
Binkley D, Kashian DM, Boyden S, Kaye MW, Bradford JB, Arthur MA, Fornwalt PJ, Ryan MG (2006) Patterns of growth dominance in forests of the rocky mountains, USA. For Ecol Manag 236:193–201
Biondi F (1996) Decadal-scale dynamics at the Gus Pearson natural area: evidence for inverse (a)symmetric competition? Can J For Res 26:1397–1406
Brooks JR, Flanagan LB, Ehrlinger JR (1998) Responses of boreal conifers to climate fluctuations: indications from tree-ring widths and carbon isotope analyses. Can J For Res 28:524–533
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burns RM, Honkala BH (1990) Silvics of North America, vol 1: conifers. Agriculture handbook 654. United States Department of Agriculture Forest Service, Washington, DC
Ecological Stratification Working Group (1996) A national ecological framework for Canada. Agriculture and Agri-Food Canada, Research Branch, Centre for Land and Biological Resources Research and Environment Canada, State of Environment Directorate, Ottawa
Erdman C, Emerson JW (2007) bcp: an R package for performing a Bayesian analysis of change point problems. J Stat Softw 23:1–13
Fish H, Lieffers VJ, Silins U, Hall RJ (2006) Crown shyness in lodgepole pine stands of varying stand height, density, and site index in the upper foothills of Alberta. Can J For Res 36:2104–2111
Gál J, Bella IE (1994) New stem taper functions for 12 Saskatchewan timber species (Information Report NOR-X-338). Natural Resources Canada, Canadian Forest Service, Northern Forestry Centre, Edmonton, AB
Goldberg D, Novoplansky A (1997) On the relative importance of competition in unproductive environments. J Ecol 85:409–418
Halliwell DH, Apps MJ (1997) Boreal Ecosystem-Atmosphere Study (BOREAS) biometery and auxiliary sites: overstory and understory data. Natural Resources Canada, Canadian Forest Service, Northern Forestry Centre, Edmonton
Hipel KW, McLeod AI (1994) Time series modeling of water resources and environmental systems. Elsevier, Amsterdam
Hofgaard A, Tardif J, Bergeron Y (1999) Dendroclimatic response of Picea mariana and Pinus banksiana along a latitudinal gradient in the eastern Canadian boreal forest. Can J For Res 29:1333–1346
Houle G, Filion F (1993) Interannual variations in the seed production of Pinus banksiana at the limit of the species distribution in northern Quebec, Canada. Am J Bot 80:1242–1250
Husch B, Beers TW, Kershaw JA (2003) Forest mensuration, 4th edn. Wiley, Hoboken
Klos R (2004) Ecologically based taper equations for major tree species in Manitoba (MSc thesis). Lakehead University, Canada
Kozak A (1988) A variable-exponent taper equation. Can J For Res 18:1362–1368
Larocque GR (2002) Examining different concepts for the development of a distance-dependent competition model for red pine diameter growth using long-term stand data differing in initial stand density. For Sci 48:24–34
Larsen CPS, MacDonald GM (1995) Relations between tree-ring widths, climate, and annual area burned in the boreal forest of Alberta. Can J For Res 25:1746–1755
Liu Y, Muller RN (1993) Effect of drought and frost on radial growth of overstorey and understorey stems in a deciduous forest. Am Midl Nat 129:19–25
Long JN, Smith FW (1992) Volume increment in Pinus contorta var. latifolia: the influence of stand development and crown dynamics. For Ecol Manag 53:53–64
Mallett KI, Volney WJA (1990) Relationships among jack pine budworm damage, selected tree characteristics, and Armillaria root rot in jack pine. Can J For Res 20:1791–1795
Mast JN, Veblen TT (1994) A dendrochronological method of studying tree mortality patterns. Phys Geogr 15:529–542
McCullough DG (2000) A review of factors affecting the population dynamics of jack pine budworm (Choristoneura pinus pinus Freeman). Popul Ecol 42:243–256
McKenney DW, Pedlar JH, Papadol P, Hutchinson MF (2006) The development of 1901–2000 historical monthly climate models for Canada and the United States. Agric For Meteorol 138:69–81
Meng SX, Rudnicki M, Lieffers VJ, Reid DEB, Silins U (2006) Preventing crown collisions increases the crown cover and leaf area of maturing lodgepole pine. J Ecol 94:681–686
Metsaranta JM, Lieffers VJ (2008) Inequality of size and size increment in Pinus banksiana in relation to stand dynamics and annual growth rate. Ann Bot 101:561–571
Metsaranta JM, Lieffers VJ (2009) Using dendrochronology to obtain annual data for modeling stand development: a supplement to permanent sample plots. Forestry 82:163–173
Metsaranta JM, Lieffers VJ, Wein RW (2008) Dendrochronological reconstruction of jack pine snag and downed log dynamics in Saskatchewan and Manitoba, Canada. For Ecol Manag 255:1262–1270
Nealis VG, Magnussen S, Hopkin AA (2003) A lagged, density-dependent relationship between jack pine budworm Choristoneuara pinus pinus and its host Pinus banksiana. Ecol Entomol 28:183–192
Newton PF (2006) Asymptotic size-density relationships within self-thinning black spruce and jack pine stand-types: parameter estimation and model reformulations. For Ecol Manag 226:49–59
Newton PF, Jolliffe PA (1998) Temporal size-dependent growth processes within density-stressed black spruce stands: competition processes and budworm effects. For Ecol Manag 111:1–13
Nord-Larsen T, Damgaard C, Weiner J (2006) Quantifying size-asymmetric growth among individual beech trees. Can J For Res 36:418–425
Orwig DA, Abrams MA (1997) Variation in radial growth responses to drought among species, site, and canopy strata. Trees Struct Funct 11:474–484
Piutti E, Cescatti A (1997) A quantitative analysis of the interactions between climatic response and intraspecific competition in European beech. Can J For Res 27:277–284
Putz FE, Parker GG, Archibald RM (1984) Mechanical abrasion and intercrown spacing. Am Midl Nat 112:24–28
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rudnicki M, Silins U, Lieffers VJ (2003) Stand structure governs the crown collisions of lodgepole pine. Can J For Res 33:1238–1244
Ryan MG, Binkley D, Fownes JH (1997) Age-related decline in forest productivity: pattern and process. Adv Ecol Res 27:213–261
Scarr T (1995) Phenotypic variation in jack pine susceptibility to jack pine budworm. In: Volney WJA, Nealis VG, Howse GM, Westwood AR, McCullough DG, Laishley BL (eds) Jack pine budworm biology and management (Proceedings of the Jack Pine Budworm Symposium; Information Report NOR-X-342). Canadian Forest Service, Northwest Region, Edmonton, AB, pp 79–88
Schwinning S, Weiner J (1998) Mechanisms determining the degree of size symmetry in competition among plants. Oecologia 113:447–455
Simpson R, Coy D (1999) An ecological atlas of forest insect defoliation in Canada 1980–1996 (Information Report M-X-206E). Natural Resources Canada, Canadian Forest Service, Atlantic Forestry Centre, Fredericton, NB
Stadt KJ, Huston K, Coates KD, Feng Z, Dale MRT, Lieffers VJ (2007) Evaluation of competition and light estimation indices for predicting diameter growth in mature boreal mixed forests. Ann For Sci 64:477–490
Volney WJA (1988) Analysis of historic jack pine budworm outbreaks in the Prairie provinces of Canada. Can J For Res 18:1152–1158
Vose JM, Swank WT (1994) Effects of long-term drought on the hydrology and growth of a white pine plantation in the Southern Appalachians. For Ecol Manag 64:25–39
Weiner J (1990) Asymmetric competition in plant populations. Trends Ecol Evol 5:360–364
Weiner J, Damgaard C (2006) Size-asymmetric competition and size-asymmetric growth in a spatially explicit zone-of-influence model of plant competition. Ecol Res 21:707–712
Weiner J, Thomas SC (1986) Size variability and competition in plant monocultures. Oikos 47:211–222
Wichmann L (2001) Annual variations in competition symmetry in even-aged sitka spruce. Ann Bot 88:145–151
Acknowledgments
We thank Derek Bakker, Jennifer Hofman, Michael Laczek, and Theresa Mundell for assistance in the field. Field work was supported by Circumpolar-Boreal Alberta research grants and the Natural Science and Engineering Council of Canada, and JMM was supported by a Natural Science and Engineering Research Council of Canada scholarship, a Province of Alberta Graduate Fellowship, a Prairie Adaptation Research Collaborative graduate scholarship, and a Desmond Crossley Memorial Scholarship. We thank several anonymous reviewers and the handling editor, K Gross, for detailed comments on our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Katherine Gross.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Metsaranta, J.M., Lieffers, V.J. Patterns of inter-annual variation in the size asymmetry of growth in Pinus banksiana . Oecologia 163, 737–745 (2010). https://doi.org/10.1007/s00442-009-1559-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1559-7