Abstract
Soil moisture is a key factor affecting plant abundance and distribution, both across and within species. In response to water limitation, plants have evolved numerous morphological, physiological, and phenological adaptations. In both well-watered and water-limited conditions, we identified considerable natural variation in drought-related whole-plant and leaf-level traits among closely related members of the Mimulus guttatus species complex that occupy a diversity of habitats in the field. The self-fertilizing Mimulus nasutus and serpentine-endemic Mimulus nudatus demonstrated the overall greatest tolerance to soil water limitation, exhibiting the smallest reduction in seed set relative to well-watered conditions. This may be due in part to early flowering, faster fruit development, and low stomatal density. In contrast, flowering of coastal M. guttatus was so delayed that it precluded any seed production in water-limited conditions. This range of phenotypic responses to soil water deficit in Mimulus, coupled with developing genomic resources, holds considerable promise for identifying genomic variation responsible for adaptive responses to soil water availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The local and seasonal availability of soil moisture is one of the most critical factors in determining the distribution and abundance of plant species worldwide (Cornwell and Grubb 2003). Even within species, climatic variation in conjunction with edaphic conditions drives local adaptation in natural plant populations (Turesson 1922; Clausen et al. 1940; Nagy and Rice 1997; Hall and Willis 2006). With drought being a critical selective regime influencing the evolution of plant growth, development, and physiology, understanding the mechanisms of how plants avoid or cope with water stress has been a central goal in plant biology for decades, in both natural and agricultural systems (Araus et al. 2002; Chaves et al. 2003; Maggio et al. 2006). With climate change scenarios predicting increased aridity in many regions worldwide (Canadell et al. 2007), efforts to understand the mechanistic and genetic basis of adaptive plant responses to limited soil moisture remain important, both for providing insights into potential ecological impacts of climate change on natural populations, and how plants are likely to evolve in response to such changes.
Plants have evolved numerous adaptations to cope with limited water availability, including changes in developmental, physiological, and life history traits (Geber and Dawson 1990; Ingram and Bartels 1996; Ackerly et al. 2000; Araus et al. 2002; Maggio et al. 2006). Suites of these functional traits have classically been categorized either as enabling plants to escape from drought, or to avoid the negative consequences of dehydration (Ludlow 1989). Drought-escape responses to seasonally changing patterns of soil moisture typically involve the appropriate timing of a short life cycle (e.g., spring ephemerals) or growing season (e.g., drought deciduous), so that plants reproduce before the most intense period of drought. This “live fast, die young” strategy is typically achieved through increased metabolic activity and rapid growth (Bazzaz 1979; Geber and Dawson 1990; Arntz and Delph 2001). Alternately, dehydration avoidance involves maintaining internal water status in dry environments by either regulating water loss or maximizing water uptake. For most plants, dehydration avoidance is primarily achieved through the regulation of water loss through leaves by reducing stomatal conductance (Schulze 1986; Dawson and Ehleringer 1993; Reid et al. 2003). In this case, plants typically maintain a reduced metabolic activity and growth rate to persist through the period of drought (Geber and Dawson 1997; Arntz and Delph 2001). The mechanisms responsible for each of these drought response strategies are diverse, and have been measured at multiple biological levels, ranging from the whole-plant to the molecular level (Ingram and Bartels 1996; Araus et al. 2002; Chaves et al. 2003; Hausman et al. 2005; Juenger et al. 2005; Maggio et al. 2006).
The yellow monkeyflowers of the Mimulus guttatus (Phrymaceae) species complex are well suited to studies of differentiation in drought-related traits, as this group contains populations and species that have adapted to environments that vary in the seasonal availability of water (Vickery 1978; Kiang and Hamrick 1978; Hall and Willis 2006; Martin and Willis 2007; Wu et al. 2008). In particular, the timing and speed of onset of the summer drought that characterizes their Mediterranean-type climate varies substantially with elevation, latitude, and local edaphic conditions, such that the distribution of members of the species complex across these environments may reflect varying responses to soil water availability. For example, observations that the self-fertilizing Mimulus nasutus and serpentine-endemic Mimulus nudatus are found in drier habitats in the field suggest that these species are more tolerant of low soil moisture than their presumed progenitor, Mimulus guttatus (Vickery 1978; Kiang and Hamrick 1978; Macnair and Gardner 1998; Hughes et al. 2001). Similar distribution patterns have been found with derived selfing species in other taxonomic groups (Gottlieb 2003; Culley et al. 2006; Sakai et al. 2006). At the other extreme are coastal perennial populations of M. guttatus, that unlike inland annual M. guttatus, occur in habitats where soil moisture is maintained year-round by lower temperatures and persistent fog during summer months (Hall and Willis 2006; Lowry et al. 2008). However, despite these differences in soil moisture availability among Mimulus habitats, studies to date have yet to ascertain whether these species show specific physiological adaptations to drought or tolerance to soil moisture limitation. As adaptations to water availability are identified, the emerging set of genomic tools for Mimulus offers promise for dissecting molecular mechanisms of these traits (Wu et al. 2008). A necessary first step towards this goal, then, is to identify phenotypically diverse populations or species that will facilitate future molecular analyses of naturally occurring variation in responses to soil water limitation.
To determine the extent of variation in drought-related traits in Mimulus originating from diverse habitats, and test whether trade-offs exist between drought-escape and dehydration-avoidance traits in the yellow monkeyflowers, we examined differences in several whole-plant and leaf-level traits that have been shown to influence plant response to water deficit. For example, dry habitats may exert strong selection on plants to maintain high C assimilation rates despite water limitations, which can be achieved through changes in photosynthetic physiology or vegetative morphology (Munns 2002; Culley et al. 2006). We examined the influence of water limitation on traits that can influence C uptake, including specific leaf area (SLA; the ratio of leaf area to leaf dry mass), rosette size (a proxy for photosynthetic tissue allocation), and leaf-level C isotope discrimination [C stable isotope ratio (δ13C); a time-integrated measure of water-use efficiency (WUE), Farquhar et al. 1989]. A high WUE, or ratio of C gained per unit of water lost through transpiration, is expected to confer a fitness advantage under drought stress (Dudley 1996; McKay et al. 2001), although it may come at the cost of reduced growth or lead to delayed flowering in favorable conditions (Geber and Dawson 1990, 1997). Alternately, plants may forego this high WUE, and instead maximize early growth to flower and reproduce early, thereby avoiding the negative fitness effects of drought, particularly when its onset is seasonally predictable. Indeed, several studies in annual systems suggest that trade-offs exist between dehydration-avoidance traits such as higher WUE and dehydration-escape traits such as rapid growth and early flowering (Geber and Dawson 1990, 1997; Stanton et al. 2000; McKay et al. 2003; but see Sherrard and Maherali 2006). Additionally, we used relative water content (RWC) to assess the plants’ level of water deficit and capacity for dehydration avoidance. Finally, flowering time and seed production were used as a metrics for variation in drought escape between well-watered and water-limited growth conditions.
Here, we tested for natural variation in multiple traits related to drought response within a core collection of 12 populations that encompass a range of taxa and life histories in the M. guttatus species complex, by imposing low-water and progressive water deficit conditions to examine responses to stresses that plants may experience in the field (Hall and Willis 2006; Murren et al. 2006; Lowry et al. 2008). Our goal was to quantify variation in drought-related traits in taxa from diverse habitats across the geographic range of the species complex, examine potential trade-offs among drought-related characters, and identify phenotypically extreme populations that could prove useful for future molecular analyses of drought-stress responses in this natural plant system.
Materials and methods
Plant lines
The yellow monkeyflowers of the M. guttatus species complex (sect. Simiolus, Phrymaceae) are a phenotypically diverse, yet broadly interfertile group of wildflowers with their center of diversity in western North America (Vickery 1978; Beardsley et al. 2004). Mimulus guttatus Fisch. ex DC. and its related species have evolved a variety of life history, developmental, and physiological traits that enable them to occupy a broad range of habitats, ranging from coastal sand dunes to montane meadows, serpentine barrens, and copper mine tailings (Wu et al. 2008, and references therein). This diversity of habitats has necessitated that populations within the species complex adapt to environments that vary in the seasonal availability of water.
We assembled a collection of 12 populations that span this range of edaphic conditions to explore the natural variation in drought responses found in the M. guttatus species complex. These included coastal perennial and annual inland populations of M. guttatus that have been recognized as distinct taxonomic groups (Pennell 1947; Lowry et al. 2008), the serpentine-endemic Mimulus nudatus Curran ex Greene, and two populations of the most widespread of the self-fertilizing species in the complex, Mimulus nasutus Greene (Table 1). Repeated germination difficulties prevented inclusion of the copper mine endemic Mimulus cupriphilus.
To minimize maternal effects, samples for this study originated as seeds from plants that had undergone one generation of self-fertilization in the greenhouse. Of the 12 populations included in the experiments, three [Oregon Dunes National Recreation Area, Oregon (DUN); Iron Mountain, Oregon (IM); and Sherar’s Falls, Oregon (SF)] were well-characterized inbred lines that have been used extensively in other studies of adaptation to local environmental conditions (Fishman et al. 2002; Hall and Willis 2006; Fishman and Willis 2008; Lowry et al. 2009). For the remaining populations, three to five independent maternal families per population were included to encompass as much of the genetic variation within each population as possible, while allowing for replication within treatments in our experimental growth chamber.
Growth conditions and drought-stress treatments
To compare drought responses among closely related members of the M. guttatus species complex, we used a randomized complete block design with 20 replicates of the 12 populations in each soil moisture treatment.
Plant growth conditions
Seeds were planted into 4-inch pots filled with moist Fafard 4P potting mix, and stratified in the dark at 4°C for 1 week. Pots were then moved to the Duke University greenhouses and maintained under long-day photoperiod conditions (16 h light at 24°C/8 h dark at 16°C) for 1 week to promote germination. Seedlings were transplanted into 14 × 20 × 2–3/4-inch Dyna-Flat with holes (Hummert International) heavy-duty flats filled with saturated Fafard 4P potting mix, and moved into a custom-built walk-in growth chamber (Environmental Growth Chambers, Chagrin Falls, Ohio) set at 18°C and 60% relative humidity. To allow seedlings to become established in the experimental blocks after transplanting, all flats were bottom-watered every other day for 17 days after being moved to the growth chamber, with 500 ml Peters Professional 20–15–20 fertilizer at 300 p.p.m. added after the last watering.
Experimental design
To ensure that plants from each population experienced comparable soil moisture levels in each treatment, experiments were designed so that plants were grown together in the same soil in large flats, rather than in individual pots. This design was intended to minimize variation in soil drying rates that could arise between pots from variation in overall plant size among populations—a larger plant uses more water on a daily basis than a small plant, and thus might deplete soil moisture from a similarly sized pot more rapidly. Because roots of all genotypes experienced approximately the same soil water potential in each flat, measured differences among the genotypes in their drought responses should be due to their physiological attributes, rather than to variation in the severity of stress experienced by the plants in individual pots. In each flat, the 12 focal (=experimental) plants were evenly spaced in a 3 × 4 grid that was surrounded by “edge plant” seedlings from a typical inland M. guttatus population (Road 888, Oregon; LIN). This design resulted in each focal plant being surrounded by a neighborhood of eight plants (Fig. S1a). Each flat served as an experimental block. Within each flat, the positions of one plant from each of the 12 focal populations were randomized, and families within a given population were equally represented across treatments, but haphazardly assigned to specific flats.
Soil moisture treatments
We used three watering treatments to examine physiological and whole-plant responses to variation in soil moisture conditions. Soil moisture was monitored daily using a HydroSense CS620 soil moisture system (Campbell Scientific) with 12-cm probe rods inserted into the soil at a 45° angle to a given depth marked on the probes, taking care to avoid the bottom and sides of the flat. Flats were randomly assigned to watering treatments that were initiated after the transplant establishment period, and positions within the growth chamber were rotated every 2 days throughout the duration of each study:
-
1.
High water. Flats in the high-water treatment continued to be bottom-watered every other day to keep soil at field capacity throughout the experiment, at a measured volumetric water content (VWC) of ~35–37%.
-
2.
Low water. Flats in the low-water treatment were allowed to dry to ~50% of field capacity, to a measured VWC of ~16–17%, over a 7-day period of no additional watering. During this period of drying, soil moisture dropped at a consistent rate of ~2% VWC per day across flats (Fig. S1b). The low soil moisture level was maintained by adding 500 ml water every other day to each flat with a watering can using a shower nozzle to distribute the water as evenly as possible across the flat.
-
3.
Dry down. To simulate the onset of a progressive seasonal drought, the dry-down treatment exposed plants to a sustained period of soil moisture depletion. Water was withheld from the flats to allow the soil to progressively dry from saturation (Fig. S1b). Soil moisture reached its lowest level of 5% VWC (the lower detection limit of the probe) 13 days after the last saturating watering.
Short-day measurements
Because several physiological parameters can vary when a plant transitions among life stages (Campbell et al. 2005), the first experiment was conducted under short-day photoperiod conditions (8 h light/16 h dark) to inhibit flowering and maintain plants as vegetative rosettes (Vickery 1978). Such short-day conditions are typical of the fall and early spring growing seasons throughout the range of many Mimulus species (Vickery 1978). Twenty experimental blocks for each of the three soil moisture treatments were included in the experiment, for a total 60 flats.
Rosette diameter
To examine overall growth responses to varied water treatments, we measured to the nearest millimeter the rosette diameter of plants from all three soil moisture treatments 30 days after transplanting into blocks.
Relative water content
For an estimate of plant water status that reflected cellular water deficit, we determined the RWC for plants in ten blocks from each of the three soil moisture treatments after measuring rosette diameter, following methods originally outlined in Barr and Weatherley (1962). For each plant, we collected the top-most fully expanded leaf and immediately measured fresh weight (FW) and scanned for leaf area (see “Specific leaf area” below), then placed it into a Bagette 2 × 3-inch zip-top bag to soak overnight in distilled H2O at 4°C. The following morning, the leaf was removed from the water, quickly blotted to remove surface water, and weighed immediately for turgid weight (TW), then oven-dried for 24 h at 60°C to constant weight and weighed to determine dry weight (DW). RWC (%) was calculated as [(FW − DW)/(TW − DW)] × 100.
C isotope analyses
Leaf tissue was analyzed for C stable isotope ratio (δ13C), which serves as a time-integrated measure of WUE. This provides leaf-level, or photosynthetic WUE that may differ from whole-plant WUE (biomass per water used), but is a less destructive sampling method that allows subsequent traits to be measured. As δ13C is an expensive phenotype to obtain for a large number of samples, we analyzed six replicates from seven populations (DUN, IM, LMD, MED, MEN, SF, and SWB; Table 1) in the two extreme soil moisture treatments (high water and dry down), for a total of 84 samples. Leaves were collected from each plant 30 days after transplanting, dried for ≥48 h at 60°C, and ground to a fine powder by shaking in a capped tube with two stainless steel ball-bearing pestles using a Genogrinder. Subsamples of the powder from each individual were placed in a tin capsule and sent to the Duke Environmental Stable Isotope Laboratory for δ13C determination. Data are presented as δ13C relative to the Vienna-Pee Dee belemnite (PDB) standard (R PDB), where δ13C (‰) = (R S/R PDB − 1) × 1,000.
Specific leaf area
SLA is often associated with growth rate (Lambers et al. 1998; Angert et al. 2007; Poorter et al. 2009), such that a low SLA and slower growth rate may confer a selective advantage in stressful environments. To determine SLA for plants grown in the short-day study, we used the same leaves that were collected for RWC measurements (see above). Using the leaf area scans of freshly collected leaves, along with their dry weights after drying at 60°C for ≥24 h, we calculated SLA as the ratio of leaf area to leaf dry weight.
Long-day measurements
To further explore differences in performance among differing soil moisture regimes, we conducted a second set of experiments under long-day photoperiod conditions (16 h light/8 h dark) to simulate end-of-season field light conditions that promote flowering (Vickery 1978). To focus on responses from extreme soil moisture conditions, the second experiment only included the high-water and dry-down treatments, with 20 experimental flats per treatment.
Specific leaf area
We measured SLA on all focal plants from all experimental blocks 26 days after transplanting. For each plant, the top-most fully expanded leaf was collected, scanned for leaf area on a flat-bed scanner, dried for ≥24 h at 60°C and weighed to the nearest microgram. SLA was calculated as the ratio of leaf area to leaf dry mass.
Stomatal density
Stomatal density can influence leaf conductance to H2O and CO2 (Reid et al. 2003). For plants in 11 of the high-water treatment flats, casts of the underside of fully expanded leaves were made by pressing leaves onto a microscope slide covered with polyvinylsiloxane dental impression material (Extrude medium; Kerr Manufacturing) 27 days after transplanting. Clear fingernail polish (L’Oreal Top Coat; L’Oreal, Paris) was painted onto these casts to make peels that could be examined under a light microscope. The number of stomata within each of three randomly selected fields of view per leaf was counted at 1,000× magnification, and stomatal density was averaged per plant.
Lifetime maternal fitness estimates
To estimate the effect of varied soil moisture conditions on lifetime fitness among taxa, we compared the number of days to first flower and seed set for plants in both the high-water and dry-down treatments. Flowering time was obtained by daily inspection of plants, and seed set was measured on a per fruit basis for one to two flowers per plant that were hand-pollinated with pollen donated from edge plants (LIN population) as a standardized source.
Seed maturation success
We estimated seed maturation success under dry conditions by scoring whether or not fruits had matured by day 48 (days after transplanting) of the experiment, immediately prior to the senescence of all plants in the dry treatment. A fruit was considered mature if it was dehiscent and had produced at least one brown, rather than green, seed.
Data analysis
We were interested in how traits putatively related to drought response varied across the four Mimulus taxa: coastal perennial M. guttatus, inland annual M. guttatus, M. nasutus, and M. nudatus (Table 1). For most traits we conducted ANOVAs with watering treatment and taxa as main effects. For traits that showed significant variation among taxa, we subsequently performed one-way ANOVAs within each watering treatment, followed by Tukey–Kramer honest significant difference (HSD) comparisons (JMP 7.0.1; SAS Institute, Cary, N.C.). Survival to flowering was analyzed with a generalized linear model in JMP that accounted for its binomial distribution. To compare fruit maturation 48 days after transplanting, we conducted independent χ2 analyses between M. guttatus and M. nasutus and between M. guttatus and M. nudatus, including only those plants that experienced the same amount of time to develop seed. Since no coastal M. guttatus plants survived long enough to mature seeds, we used only the inland M. guttatus for this analysis. We restricted our comparison of M. nudatus and M. guttatus to plants that initiated flowering 25–28 days after transplanting, because this was the period during which all M. nudatus plants initiated flowering. For the same reason, we also restricted our comparison of M. guttatus and M. nasutus only to plants that initiated flowering 25–31 days after transplanting. This strategy ensured that the duration for which seeds of all plants could ripen in each comparison was the same.
Results
Short-day experiment
As expected based on their association with generally moist, seepy habitats in the field, Mimulus plants overall were most robust under high-water conditions, producing larger rosettes with greater SLA and RWC than plants in the low-water or dry-down treatments (Tukey–Kramer HSD, all P < 0.05; Table 2). Plants also had more negative δ13C values, and thus lower WUE, in the high-water relative to the dry-down conditions (F 1,71 = 20.98, P < 0.001; Table S1). In particular, coastal M. guttatus had significantly lower WUE than the inland taxa (Tukey–Kramer HSD, P < 0.05).
Surprisingly, when grown in short-day conditions, the typically diminutive M. nasutus had larger rosettes than all other taxa, while M. nudatus and coastal M. guttatus plants were the smallest across all watering treatments (Table 2). Plants from the larger taxa also exhibited greater SLA, with M. nasutus on average having significantly higher SLA than coastal M. guttatus (Table 2). In contrast, RWC did not vary among the taxa (P > 0.05; Tables 2, S1).
Long-day experiment
Long-day growth conditions revealed significant flowering time differences among taxa that were consistent across watering treatments (Table 3). Overall, the self-fertilizing M. nasutus and serpentine-endemic M. nudatus flowered the earliest (26.74 ± 0.24 and 26.58 ± 0.19 days, respectively), while the coastal M. guttatus populations flowered significantly later (43.79 ± 0.65 days) than all other taxa. Survival to flowering also differed significantly among taxa (χ 2 = 44.19, P < 0.0001; Table S3), due to the particularly low survival of coastal M. guttatus populations in the dry treatment (Table 3). As a consequence of the extended time required for the coastal populations to initiate flowering, numerous coastal plants did not survive to flower, particulary in the dry treatment (Table 3).
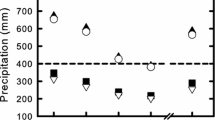
For seed production following hand pollination, we found a significant effect of both taxa (F 3,444 = 50.69, P < 0.001) and watering treatment (F 1,444 = 43.78, P < 0.001; Table S2). While M. nasutus and M. nudatus showed little change in seed set between wet and dry conditions, coastal M. guttatus set no seeds at all under dry conditions (Table 2). Successful fruit maturation in the low-water treatment also differed significantly among taxa (Fig. 1). All M. nudatus and M. nasutus plants produced mature fruit by day 48 of the experiment, but only 59% of the inland M. guttatus plants that initiated flowering during the same time period as M. nudatus had mature seeds (χ 2 = 12.31, P < 0.001). Similarly, only 46% of the M. guttatus plants that initiated flowering during the same interval as M. nasutus produced mature seeds (χ 2 = 29.53, P < 0.0001).
Variation in the ability of taxa to produce mature fruit before the onset of terminal drought on day 48 of the dry-down treatment. All Mimulus nasutus (filled circles) and Mimulus nudatus (crosses) plants were able to produce mature fruits regardless of flowering date, but fruit maturation success in inland annual Mimulus guttatus (filled triangles) depended on the day of first flower
As in the short-day experiment, leaf characters differed among Mimulus taxa when plants were grown in long days. Stomatal density differed significantly among taxa (F 3,110 = 151.27, P < 0.001), with coastal M. guttatus plants having the highest stomatal densities (Tables 3, S2). As leaf anatomical features, such as stomatal density, have been linked to variation in WUE (Masle et al. 2005) that may in turn influence flowering time through functional strategy trade-offs (e.g., Geber and Dawson 1990, 1997; McKay et al. 2003), we also examined the relationship between stomatal density and flowering time. We found a relatively strong positive correlation between the two traits (r 2 = 0.291, P < 0.0001; Fig. 2). This pattern appears to mainly be driven by coastal M. guttatus; removal of coastal individuals from the analysis led to a striking reduction in the correlation between stomatal density and flowering time (r 2 = 0.088). As in the short-day experiment, coastal M. guttatus had the smallest SLA, while M. nudatus and M. nasutus had the largest SLA (Table 3). Although SLA was significantly decreased under the dry treatment (Table 3), in either watering treatment the overall leaf morphology was much more comparable to that seen in the field under long-day growth conditions relative to short days.
Discussion
Across the 12 populations surveyed from the M. guttatus species complex, we observed a high level of phenotypic divergence in traits related to drought response in patterns consistent with the habitats from which they were derived. The coastal M. guttatus appears to be the most susceptible to soil moisture limitations, as was expected based on its distribution in areas with high year-round soil moisture levels (Hall and Willis 2006; Lowry et al. 2008). In contrast, the small-flowered M. nasutus and M. nudatus were the least affected by restricted soil moisture in our study, largely because their rapid maturation enabled the production of viable seeds before the onset of drought-induced senescence. Similar patterns have been observed in field populations, where M. nasutus is often found in drier microhabitats when it co-occurs with M. guttatus (Kiang and Hamrick 1978; Martin and Willis 2007). Likewise, as a serpentine-endemic found in rapidly draining soils, M. nudatus must cope with a faster onset of water deficit than other members of the species complex (Macnair and Gardner 1998; Gardner and Macnair 2000). In such rapidly drying sites, early flowering would be expected to maximize the time available for reproduction. In the following sections, we focus our discussion on the traits that diverged the most among the taxa in this study.
Dehydration avoidance versus escape
Many studies suggest that trade-offs may exist between traits involved in dehydration-avoidance and dehydration-escape strategies (Geber and Dawson 1990; Stanton et al. 2000; McKay et al. 2003; but see Sherrard and Maherali 2006). In particular, higher WUE is expected to be favored in water-limited environments, where minimizing water loss allows the plant to extend its growth over a longer period. In contrast, less conservative water use (a lower WUE) could enable a plant to grow as quickly as possible, completing its life cycle prior to the most severe decline in water availability. However, a majority of studies with annual plants have instead found selection for higher WUE in water-limited environments (Dudley 1996; Heschel et al. 2002, 2004; Ludwig et al. 2004; but see Heschel and Riginos 2005), suggesting that traits involved in drought escape may not always be adaptive. In this scenario, increased WUE, even if it leads to a slight reduction in development time, could be advantageous if it increases the likelihood of survival to reproduction in the shortened growing period.
Both M. nasutus and M. nudatus reproduced earlier than the other taxa and produced the highest number of seeds per fruit in both well-watered and water-limited conditions, suggestive of a “live fast, die young” annual strategy that should enable escape from seasonal drought (Geber and Dawson 1990, 1997; McKay et al. 2003; Heschel and Riginos 2005). However, M. nasutus did not show evidence for reduced WUE (based on δ13C measurements) that is expected to be associated with this drought-response strategy, at least when grown under short-day conditions. Instead, the two M. nasutus populations had δ13C values that were similar to most other populations in the dry treatment. Interestingly, when instead grown under long-day conditions, M. nasutus populations do have significantly higher WUE than M. guttatus (C. Wu, unpublished data). Additional work is needed to further explore how the photoperiod experienced by the plants affects WUE (e.g., Yu et al. 2008), as well as the relative importance of WUE for the lifetime fitness of these selfing annuals.
While several factors can influence WUE, most expectations on how WUE can vary have focused on stomatal regulation, since stomatal closure decreases transpiration more than photosynthetic C uptake, thereby increasing WUE (Ehleringer 1993). In addition to active stomatal closure, stomatal conductance can also be influenced by such factors as stomatal aperture (Reid et al. 2003) and density (Masle et al. 2005). If stomatal density affects WUE through changes in stomatal conductance, we would expect plants with lower stomatal densities to be able to maintain higher WUE, and perhaps flower later. Instead, we found a strong positive correlation between flowering time and stomatal density, such that plants with high stomatal densities flowered later (Fig. 2). This pattern appears to be mostly driven by the coastal M. guttatus, which had the highest stomatal density and flowered later than the other taxa. These characters may, in part, be explained by the year-round water availability and reduced transpiration in the coastal habitat due to persistent summer coastal fog from the Pacific Ocean (Corbin et al. 2005; Hall and Willis 2006; Lowry et al. 2008).
Trends in SLA were opposite of what was expected based on characteristics of “drought-tolerant” and “drought-sensitive” species (Lambers et al. 1998). While M. nasutus and M. nudatus are found in particularly dry areas relative to other members of the species complex, they exhibited almost identically high SLAs. This is surprising given the small, linear leaves of M. nudatus. In contrast, the coastal M. guttatus had notably large leaves but the lowest SLA, perhaps due to its thicker, larger veined leaves. However, the low SLA of the coastal M. guttatus is consistent with other studies that have also found perennial plants to have lower SLA than annual plants (Garnier et al. 1997; Li et al. 2005; Poorter et al. 2009).
Key traits for response to water limitation in Mimulus
It is well established that the interaction of many environmental and genetic factors can contribute to flowering time variation in plants (reviewed in Simpson and Dean 2002; Koornneef et al. 2004), and that the optimal timing of flowering may vary tremendously across different habitats in response to external cues, including day length (Weinig et al. 2002), light levels (Stanton et al. 2000), temperature (Eckhart et al. 2004), nutrient level (Stanton et al. 2000), and water availability (Fox 1990; Eckhart et al. 2004; Franke et al. 2006). While we are still in the early stages of identifying the specific environmental cues that influence flowering time patterns in Mimulus, recent studies suggest that local adaptive flowering-time differences are a response to the differing intensity of seasonal drought in coast and inland habitats of M. guttatus (Hall and Willis 2006; Lowry et al. 2008).
Beyond flowering time, an increase in the speed of fruit maturation may also influence variation in drought escape in Mimulus. All M. nasutus and M. nudatus plants in our study successfully matured seeds before senescence in the dry-down treatment of the long-day experiment. This fast maturation of seeds likely explains why overall seed set was not drastically reduced for M. nasutus and M. nudatus in the dry-down conditions (28 and 9% reduction in seed production, respectively). In contrast, inland and edaphic M. guttatus plants that flowered at the same time as M. nasutus and M. nudatus matured fewer seeds (Fig. 1). This suggests that annual M. guttatus generally matures seeds more slowly than M. nudatus and M. nasutus, although we did not directly measure the difference in the speed of seed maturation. The slower rate of seed maturation, coupled with an overall later flowering time, likely explains why the inland and edaphic M. guttatus plants produced so many fewer seeds in the dry-down treatment (63 and 37% reduction, respectively). Certainly, seed set in the first two fruits provides an estimate for lifetime maternal fitness, which may not fully capture potential trade-offs between number of seeds per fruit and the number of fruits per plant. Nonetheless, seed set was negatively impacted by water limitation in all of the Mimulus examined, indicating that seed set in the first two fruits may be a good proxy for estimating fitness in dry-down experiments.
Implications for the genetic dissection of water limitation response
The phenotypic variation we observed among the Mimulus in this study holds considerable promise for further genetic dissection of traits underlying responses to soil water limitation. In light of these results, efforts have been initiated to locate and characterize genomic regions that contribute to traits associated with drought escape (flowering time) and dehydration tolerance (δ13C), using existing recombinant inbred line populations between inland M. guttatus and coastal M. guttatus or M. nasutus populations. Consistent with variation in seasonal soil moisture levels in their native habitats (Macnair and Gardner 1998; Hall and Willis 2006; Lowry et al. 2008), coastal M. guttatus and the small-flowered M. nasutus and M. nudatus were often at opposite extremes for many of the traits we measured. Consequently, crosses between these extreme taxa could be especially useful for generating mapping populations with an increased likelihood of successful quantitative trait loci (QTL) identification (e.g., McKay et al. 2008). Future efforts will also focus on the simultaneous QTL mapping of multiple potentially important mechanisms of drought response, including the rate of fruit maturation, to determine if pleiotropic loci underlie various components of these strategies. Ultimately, fine-mapping and cloning of QTLs found in experimental crosses may uncover novel genes and mechanisms of drought response in these natural populations, and perhaps provide raw material for the next generation of crop improvement.
Conclusion
Our survey of morphological, physiological, and phenological traits in 12 Mimulus populations identified considerable differences within the species complex. These patterns were accentuated under reduced soil moisture conditions, indicating the evolution of mechanisms associated with inducible, as well as intrinsic, responses to water availability in Mimulus. Given the latitudinal and ecogeographic distribution of the yellow monkeyflowers (Vickery 1978; Wu et al. 2008), even greater natural variation in drought-escape- and drought-avoidance-related traits may exist across the range of the species complex, beyond that captured in this study. We are currently assembling collections from populations throughout the range of Mimulus to encompass a wide range of this environmental variability, at both large (i.e., latitudinal and elevational) and small (i.e., soil type) geographic scales. Overall, this diversity of phenotypic responses to soil water deficit in Mimulus, coupled with a suite of genomic tools under development, hold considerable promise for identifying the genomic variation responsible for adaptive responses to soil water availability.
References
Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder CR, Sandquist DR, Geber MA, Evans AS, Dawson TE, Lechowicz MJ (2000) The evolution of plant ecophysiological traits: recent advances and future directions. BioScience 50:979–995
Angert AL, Huxman TE, Barron-Gafford GA, Gerst KL, Venable DL (2007) Linking growth-strategies to long-term population dynamics in a guild of desert annuals. J Ecol 95:321–331
Araus JL, Slafer GA, Reynolds MP, Royo C (2002) Plant breeding and drought in C3 cereals: what should we breed for? Ann Bot 89:925–940
Arntz AM, Delph LF (2001) Pattern and process: evidence for the evolution of photosynthetic traits in natural populations. Oecologia 127:455–467
Barr HD, Weatherley PE (1962) A reexamination of the relative turgidity technique for estimating water deficit in leaves. Aust J Biol Sci 15:413–428
Bazzaz FA (1979) Physiological ecology of plant succession. Annu Rev Ecol Syst 10:351–371
Beardsley PM, Schoenig SE, Whittall JB, Olmstead RG (2004) Patterns of evolution in western North American Mimulus (Phrymaceae). Am J Bot 91:474–489
Campbell DR, Galen C, Wu CA (2005) Ecophysiology of first and second generation hybrids in a natural plant hybrid zone. Oecologia 144:214–225
Canadell JG, Pataki DE, Pitelka LF (eds) (2007) Terrestrial ecosystems in a changing World. Springer, Berlin
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought––from genes to the whole plant. Funct Plant Biol 30:239–264
Clausen J, Keck DD, Hiesey WM (1940) Experimental studies on the nature of species. I. Effects of varied environments on western North American plants. Carnegie Institute of Washington publication no. 520. Carnegie Institute, Washington
Corbin JD, Thomsen MA, Dawson TE, D’Antonio CM (2005) Summer water use by California coastal prairie grasses: fog, drought, and community composition. Oecologia 145:511–521
Cornwell WK, Grubb PJ (2003) Regional and local patterns in plant species richness with respect to resource availability. Oikos 100:417–428
Culley TM, Dunbar-Wallis AK, Sakai AK, Weller SG, Mishio M, Campbell DR, Herzenach M (2006) Genetic variation of ecophysiological traits in two gynodioecious species of Schiedea (Caryophyllaceae). New Phytol 169:589–601
Dawson TE, Ehleringer JR (1993) Gender-specific physiology, carbon isotope discrimination, and habitat distribution in box elder, Acer negundo. Ecology 74:798–815
Dudley SA (1996) Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution 50:92–102
Eckhart VM, Geber MA, McGuire C (2004) Experimental studies of selection and adaptation in Clarkia xantiana (Onagraceae). I. Sources of phenotypic variation across a subspecies border. Evolution 58:59–70
Ehleringer JR (1993) Carbon and water relations in desert plants: and isotopic perspective. In: Ehleringer JR, Hall AE, Farquhar GD (eds) Stable isotopes and plant carbon–water relations. Academic Press, San Diego, pp 155–172
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Fishman L, Willis JH (2008) Pollen limitation and natural selection on floral characters in the yellow monkeyflower, Mimulus guttatus. New Phytol 177:802–810
Fishman L, Kelly AJ, Willis JH (2002) Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution 56:2138–2155
Fox GA (1990) Drought and the evolution of flowering time in desert annuals. Am J Bot 77:1508–1518
Franke DM, Ellis AG, Dharjwa M, Freshwater M, Padron A, Weis AE (2006) A steep cline in flowering time for Brassica rapa in Southern California: population-level variation in the field and the greenhouse. Int J Plant Sci 167:83–92
Gardner M, Macnair M (2000) Factors affecting the co-existence of the serpentine endemic Mimulus nudatus Curran and its presumed progenitor, Mimulus guttatus Fischer ex DC. Biol J Linn Soc Lond 69:443–459
Garnier E, Cordonnier P, Guillerm JL, Sonié L (1997) Specific leaf area and leaf nitrogen concentration in annual and perennial grass species growing in Mediterranean old-fields. Oecologia 111:490–498
Geber MA, Dawson TE (1990) Genetic variation in and covariation between leaf gas exchange, morphology, and development in Polygonum arenastrum, an annual plant. Oecologia 85:153–158
Geber MA, Dawson TE (1997) Genetic variation in stomatal and biochemical limitations to photosynthesis in the annual plant, Polygonum arenastrum. Oecologia 109:535–546
Gottlieb LD (2003) Rethinking classic examples of recent speciation in plants. New Phytol 161:71–82
Hall MC, Willis JH (2006) Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 60:2466–2477
Hausman NJ, Juenger TE, Sen S, Stowe KA, Dawson TE, Simms EL (2005) Quantitative trait loci affecting δ13C and response to differential water availability in Arabidopsis thaliana. Evolution 59:81–96
Heschel MS, Riginos C (2005) Mechanisms of selection for drought stress tolerance and avoidance in Impatiens capensis. Am J Bot 92:37–44
Heschel MS, Donohue K, Hausmann N, Schmitt J (2002) Population differentiation and natural selection for water-use efficiency in Impatiens capensis (Balsaminaceae). Int J Plant Sci 163:907–912
Heschel MS, Sultan SE, Glover S, Sloan D (2004) Population differentiation and plastic responses to drought stress in the generalist annual, Impatiens capensis. Oecologia 139:487–494
Hughes R, Bachmann K, Smirnoff N, Macnair M (2001) The role of drought tolerance in serpentine tolerance in the Mimulus guttatus Fischer ex DC complex. S Afr J Sci 97:581–586
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Juenger TE, McKay J, Hausmann N, Keurentjes JJB, Sen S, Stowe KA, Dawson TE, Simms EL, Richards JH (2005) Identification and characterization of QTL underlying whole-plant physiology in Arabidopsis thaliana: δ13C, stomatal conductance and transpiration efficiency. Plant Cell Environ 28:697–708
Kiang YT, Hamrick JL (1978) Reproductive isolation in the Mimulus guttatus–M. nasutus complex. Am Midl Nat 100:269–276
Koornneef M, Alonso-Blanco C, Vreugdenhil D (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55:141–172
Lambers H, Chapin FS, Pons TL (1998b) Plant physiological ecology. Springer, New York
Li Y, Johnson DA, Su Y, Cui J, Zhang T (2005) Specific leaf area and leaf dry matter content of plants growing in sand dunes. Bot Bull Acad Sin 46:127–134
Lowry DB, Rockwood RC, Willis JH (2008) Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution 62:2196–2214
Lowry DB, Hall MC, Salt DE, Willis JH (2009) Genetic and physiological basis of adaptive salt tolerance divergence between coastal and inland Mimulus guttatus. New Phytol. doi:10.1111/j.1469-8137.209.02901.x
Ludlow MM (1989) Strategies of response to water stress. In: Kreeb KH, Richter H, Minckley TM (eds) Structural and functional responses to environmental stress. SPB Academic, Amsterdam
Ludwig F, Rosenthal DM, Johnston JA, Kane NC, Gross BL, Lexer C, Rieseberg LH, Donovan LA (2004) Selection on leaf ecophysiological traits in a desert hybrid Helianthus species and early-generation hybrids. Evolution 58:2682–2692
Macnair MR, Gardner M (1998) The evolution of edaphic endemics. In: Howard DJ, Berlocher SH (eds) Endless forms: species and speciation. Oxford University Press, New York, pp 157–171
Maggio A, Zhu J, Hasegawa PM, Bressan RA (2006) Osmogenetics: Aristotle to Arabidopsis. Plant Cell 18:1542–1557
Martin NH, Willis JH (2007) Barriers to gene flow between the monkeyflowers Mimulus guttatus, M. nasutus and their hybrids. Evolution 61:68–82
Masle J, Gilmore SR, Garquhar GD (2005) The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436:866–870
McKay JK, Bishop JG, Lin JZ, Sala A, Richards JH, Mitchell-Olds T (2001) Local adaptation across a climatic gradient despite small effective population size in the rare sapphire rockcress. Proc R Soc Lond B Biol Sci 268:1715–1721
McKay JK, Richeards JH, Mitchell-Olds T (2003) Genetics of drought adaptation in Arabidopsis thaliana. I. Pleiotropy contributes to genetic correlations among ecological traits. Mol Ecol 12:1137–1151
Mckay JK, Richards JH, Nemali KS, Sen S, Mitchel-Olds T, Boles S, Stahl EA, Wayne T, Juenger TE (2008) Genetics of drought adaptation in Arabidopsis thaliana. II. QTL analysis of a new mapping population, Kas-1 X Tsu-1. Evolution 62:3014–3026
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Murren CJ, Douglass L, Gibson A, Dudash MR (2006) Individual and combined effects of Ca/Mg ratio and water on trait expression in Mimulus guttatus. Ecology 87:2591–2606
Nagy ES, Rice KJ (1997) Local adaptation in two subspecies of an annual plant: implications for migration and gene flow. Evolution 51:1079–1089
Pennell FW (1947) Some hitherto undescribed Scrophulariaceae of the Pacific states. Proc Acad Nat Sci 99:151–171
Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Reid CD, Maherali H, Johnson HB, Smith SD (2003) On the relationship between stomatal characters and atmospheric CO2. Geophys Res Lett 30: art. no. 1983
Sakai AK, Weller SG, Wagner WL, Nepokroeff M, Culley TM (2006) Adaptive radiation and evolution of breeding systems in Schiedea (Caryophyllaceae), and endemic Hawaiian genus. Ann Mo Bot Gard 93:49–63
Schulze ED (1986) Carbon dioxide and water vapor exchange in response to drought in the atmosphere and in the soil. Annu Rev Plant Physiol Plant Mol Biol 37:247–274
Sherrard ME, Maherali H (2006) The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution 60:2478–2489
Simpson CG, Dean C (2002) Arabidopsis, the Rosetta stone of flowering time? Science 296:285–289
Stanton ML, Roy BA, Thiede DA (2000) Evolution in stressful environments. I. Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution 54:93–111
Turesson G (1922) The genotypic response of the plant species to habitat. Hereditas 3:211–350
Vickery RK (1978) Case studies in the evolution of species complexes in Mimulus. Evol Biol 11:405–507
Weinig C, Ungerer MC, Dorn LA, Kane NC, Toyonaga Y, Halldorsdottir SS, Mackay TFC, Purugganan MD, Schmitt J (2002) Novel loci control variation in reproductive timing in Arabidopsis thaliana in natural environments. Genetics 162:1875–1884
Wu CA, Lowry DB, Cooley AM, Wright KM, Lee YW, Willis JH (2008) Mimulus is an emerging model system for the integration of ecological and genomic studies. Heredity 100:220–230
Yu H, Chen X, Hong Y-Y, Wang Y, Xu P, Ke S-D, Liu H-Y, Zhu J-K, Oliver DJ, Xiang C-B (2008) Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. Plant Cell 20:1134–1151
Acknowledgments
We thank Meg Peterson, Calvin Sheng, Eugene Wu, and Mike Yan for assistance at various stages of the experiments, Chantal Reid for discussions of plant physiology, Diane Campbell for statistical advice, and two anonymous reviewers for comments that improved this manuscript. This work was funded by the National Science Foundation though FIBR Grant EF-0328636, Doctoral Dissertation Improvement Grant DEB-0710094, and Environmental Genomics Grant EF-0723814.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Todd Dawson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, C.A., Lowry, D.B., Nutter, L.I. et al. Natural variation for drought-response traits in the Mimulus guttatus species complex. Oecologia 162, 23–33 (2010). https://doi.org/10.1007/s00442-009-1448-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1448-0