Abstract
Despite its fundamental relevance to many ecological processes in predator–prey relationships, the functional response, which relates predator intake rate to prey density, remains difficult to document in the field. Here, I document the functional response of semipalmated sandpipers (Calidris pusilla) foraging on a burrowing amphipod Corophium volutator during three field seasons at the peak of fall migration in the upper Bay of Fundy (New Brunswick, Canada). I gathered data during the ebbing tide when all sandpipers are highly motivated to feed after a lengthy hide-tide fast. As birds follow the receding tideline, foragers encounter prey at different densities and do not aggregate in the richest food patches. Results show that intake rate increased at a decreasing rate with Corophium density, yielding a type II functional response typical of many shorebird species. Intake rate decreased in the later stages of migration stopover at a time where preferred prey items have been shown to occur at lower densities due to prior depletion. At this period of lower prey availability, intake rate also decreased with sandpiper density providing evidence for interference at low prey density. The results illustrate the fact that the functional response may not be unique but instead vary as a function of the type of competitive relationship among foragers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relationship between the intake rate of a forager and the density of food is known as the functional response. Functional responses can be of many types and have been the subject of several studies (Fryxell et al. 2007; Holling 1959; Jeschke et al. 2002). In general, intake rate is reduced when food density is low since foragers spend most of their time searching for rare prey. As food density increases, intake rate increases but usually reaches a plateau since intake rate eventually becomes limited by handling time rather than searching time. Knowledge of the functional response in a predator–prey relationship is important to predict the spatial distribution of predators and more generally predator–prey population dynamics (Sutherland 1996). Such information may also be crucial to understand how changes in food availability caused by changes in the environment will affect the population of a predator (Smart et al. 2008).
Documenting the functional response is no easy task in the field to such an extent that predictive models rather than time-consuming measurements have been proposed to infer the asymptotic intake rate (Goss-Custard et al. 2006) or the functional response itself (Stillman and Simmons 2006). Documenting functional responses is problematic in part because foragers are expected to aggregate in the richer food patches making it difficult to measure intake rate in patches of poorer quality. In addition, individual phenotypic attributes, such as age or hunger level, may differ across food patches of differing quality thus creating a confounding effect when these attributes influence intake rate on their own (Sutherland and Parker 1985). Experimental manipulation of food density, which may reduce the extent of these problems, is not always feasible especially with mobile prey.
In the following, I aim to establish the functional response of an avian species, the semipalmated sandpiper (Calidris pusilla) feeding on a burrowing amphipod, Corophium volutator. The sandpiper–amphipod system is ideal to establish the functional response in a vertebrate predator. During fall migration, sandpipers gather in large numbers in the Bay of Fundy (New Brunswick, Canada) where they feed almost exclusively on large Corophium during a 2–3 week stay to accumulate fat reserves necessary to fuel the long, uninterrupted flight to their wintering grounds in coastal South America (Hamilton et al. 2006; Hicklin and Smith 1984; Peer et al. 1986).
After roosting on shore at high tide for about 3 h, sandpipers fly to exposed mudflats and follow the tideline as the tide ebbs (Boates 1980; Wilson 1990). Following the tideline is probably a response to the fact that Corophium is most active and presumably most vulnerable when first exposed by the ebbing tide (Morgan 1965). Corophium availability decreases later on during the tide cycle as individual prey may burrow deeper to avoid desiccation or to avoid birds after prior exposure to predation attempts (Minderman et al. 2006). I thus aimed to establish the functional response during first visits to food patches during the ebbing tide. This ensures that all predators are highly motivated to feed after a long fast. Since sandpipers follow the tideline, all predators are exposed to areas that vary naturally in Corophium densities and will not aggregate in the richest food patches. Finally, desiccation and interference caused by earlier sandpiper visits are minimized during these first visits.
Materials and methods
Study site
The study was conducted from late July to early August during the peak of the fall migration at Mary’s Point in 2005 and 2006 and at Daniel’s flat in 2008. The two sites are within kilometers of each other and are located in the upper Bay of Fundy, New Brunswick, Canada (45.73°N, 64.65°W). Mudflats in the area are exposed twice daily by tides averaging 11.5 m in height. Birds foraging on exposed mudflats were monitored from the shore using a 10–45× or a 15–60× spotting scope.
Setting plots and Corophium sampling
Bamboo canes were used to stake six plots in both 2005 and 2006 and five plots in 2008. Plots measuring 6 × 6 m were set at distances of 30, 60 or 90 m from the shoreline in 2005, at distances of 50, 100 or 150 m in 2006 and finally at distances of 25, 50, 100 or 125 m in 2008. I took two to three core samples from each plot during the study period and sampled the edge of each plot to avoid trampling in the delineated foraging area. Samples were collected using a circular corer (79 cm2) pressed into the sediment to the top of the anaerobic layer (approximately 5–10 cm). Contents were sieved through a 0.85-mm sieve, which is known to retain the large Corophium individuals (Crewe et al. 2001) preferentially selected by sandpipers at this time of year (Peer et al. 1986).
Sampling sandpiper behavior
Observations took place during daylight hours and started when the birds arrived at the mudflats about 3 h after high tide. High tides occurred at different times each day and therefore behavioral sampling was conducted at different times each day. Since all plots at a given location were within the field of view, it was easy to determine when each plot was first visited by birds.
Upon the first visit at a given plot, I monitored the behavior of one to several individuals selected haphazardly from those present until all birds left the plot. While the birds were not marked, repeated sampling of the same subjects is quite unlikely given the large number of birds using the site every day (1,000—15,000). A focal observation lasted until the focal bird left the plot or was lost from sight but also ceased when sandpiper density changed due to the sudden arrival or departure of other birds. The number of birds present in the plot was counted at the beginning of each focal observation and served as an estimate of sandpiper density for this focal observation. At the beginning of each focal observation, I also noted air temperature and the occurrence of wind, rain or direct sunshine.
For each focal bird, I dictated the occurrence of captures (pecks followed by visible swallowing or accompanied by handling movements) on a portable cassette recorder as events unfolded. I calculated capture rate per minute from the number of captures recorded during timed intervals. While pecks in the mud are quite unmistakable, captures are more difficult to detect as swallowing movements may be missed or if birds swallow after capturing more than one prey (Boates 1980). The fact that the same observer (G. B.) watched birds at all prey densities ensures to some extent that error in the estimation of capture rate was fairly constant from plot to plot.
Statistical analysis
Prior to analysis, sandpiper density was log transformed to normalize distributions. I calculated the average density of Corophium for each plot using all available estimates obtained during the study period. I examined the effect of Corophium density on the number of captures including the following cofactors in the analyses: year, sandpiper density, temperature, distance of the plots from shore, the occurrence of rain, wind and direct sunshine, and migration phenology (first half versus second half of fall staging). These factors have been shown to influence foraging behavior in semipalmated sandpipers (Beauchamp 2005, 2006; Hamilton et al. 2006) or are thought to play an important role and have been included in the models to control for potential confounding effects. I also considered a second-degree term for sandpiper density, Corophium density and temperature using centered data. As the distribution of captures is right skewed, I used a negative binomial regression model with the natural logarithm of focal observation duration as an offset to control for differences in focal duration. To take into account the potential lack of independence of multiple measurements with a visit at the same plot on any given day, I treated visit within a plot on a given day as a random factor. I first tested each independent variable alone and selected variables significant at the 0.15 level for a subsequent multivariable analysis. I tested the set of retained variables for multicollinearity and found little evidence for this effect using standard regression tools. I then used a backward selection process to select the final model with a level of significance set at 0.05.
Results
Using data from first visits by birds in each plot each day, I gathered a total of 139 focal observations in 2005, 101 in 2006 and 280 in 2008 for a total of 520 observations. Focal observations lasted on average between 20 and 40 s, which is the time it usually took birds to cross one plot. The range of sandpiper density was 0.03–17 m−2 in 2005, 0.03–2.2 m−2 in 2006 and 0.14–28 m−2 in 2006. The range of average Corophium density was 127–4,202 m−2 in 2005, 1,783–3,565 m−2 in 2006, and 190–1,210 m−2 in 2008. Captures varied on average from 0.5 to 4 captures min−1 across plots and up to 14 captures min−2 were recorded in the richest plots. Not all plots received visits by sandpipers with two sites in 2005 and 2006 receiving no visits at all.
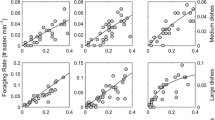
Year, the occurrence of rain or sun and temperature were not significant and were removed from the final model (P > 0.16). The fit of the final multivariable model was good as assessed with the deviance which was only 1.05 as large as the df (the higher the ratio the poorer the fit; values close to or smaller than 1 indicate a decent fit). In the final multivariable model, the number of captures increased at a decreasing rate with Corophium density (Fig. 1). The number of captures decreased later during the migration and was unrelated to sandpiper density early during the migration (Table 1). However, later in the migration, the number of captures decreased by 25% for each log unit of sandpiper density (Table 1). Capture rate increased with the distance of the plot from shore (Table 1).
Changes in capture rate (prey per minute) in fall staging semipalmated sandpipers as a function of prey density (Corophium volutator per square meter) in the upper Bay of Fundy (New Brunswick): 2005–2008. A polynomial function is shown to illustrate the non-linear trend. Bars show SD across the mean taken from each plot across the whole field season
Discussion
Using data from two field sites over 3 years, I established the functional response of a vertebrate predator when hunger levels are high and uniform and under circumstances that prevented individuals from aggregating in the richest food patches. Prey availability is expected to be high and relatively unaffected by previous encounters with predators.
Under these conditions, capture rate first increased with Corophium density in staging semipalmated sandpipers and then reached an asymptote beyond approximately 2,000 Corophium m−2. The shape of the functional response is thus a type II one which has been documented in several shorebird species (Goss-Custard et al. 2006) including the semipalmated sandpipers feeding on horseshoe crab eggs during spring staging (Gillings et al. 2007). In an earlier study of the same species, an increase in pecking rate was noted with prey density but intake rate was not measured (Wilson and Vogel 1997). The range of capture rates that I documented here is much lower than the value of 60 that would be attainable when feeding on such a small prey at high density (Boates 1980) suggesting that handling time is not the only factor that causes capture rate to plateau in this species.
The recent compilation by Goss-Custard et al. (2006) of functional responses in shorebirds indicates the presence of a wide scatter in the relationship between food intake rate and prey density making it difficult to establish what response type is actually present. The parameters of the functional response in their compilation were all from larger species outside of the Americas. The present study thus contributes rather homogeneous data from a small shorebird species from the Americas.
Intake rate in shorebirds is known to be influenced by several factors including time of year, prey type and competition (Goss-Custard et al. 2006). Competition among foragers could be assessed in this study. In a predator–prey system where disturbed prey can retreat in a burrow, the presence of competitors may be expected to decrease prey availability and thus influence prey intake rate. Indeed, in a previous study, pecking rate was found to increase with sandpiper density presumably as individuals in flocks scramble to obtain a share of the vanishing resources (Beauchamp 2007). In the present study, I documented a decrease in capture rate when sandpiper density increased but only in the second part of the study period. The immediate consequence of this finding is that the asymptote in the functional response may vary as a function of predator density and that the magnitude of competitive effects on intake rate must be established with careful measurements in the field.
That capture rate decreases with sandpiper density only later in the study period provides a clue as to the mechanism underlying competitive effects in sandpipers. Intra- and inter-species kleptoparasitism can be safely ruled out in a predator that forages on small prey in rather homogeneous flocks of the same or closely related species (Gratto-Trevor 1992). Defense of prey is not really an option in very large flocks of sandpipers and aggressive displacements are not common (Gratto-Trevor 1992). Scrambling for vanishing resources thus appears likely in sandpipers. Decreases in prey density by more than half during migratory stay have been noted in earlier studies of sandpipers (Peer et al. 1986; Schneider 1981; Sprague 2006). Therefore, the present study indicates that a negative impact of sandpiper density on intake rate probably only occurs when prey density is lower. Similar modulation of competitive effects on intake rate as a function of prey density has been predicted by models (Moody and Ruxton 1996; Nilsson et al. 2004) and documented empirically in some species (Dolman 1995; Johnson et al. 2001) but not in others (Smart et al. 2008).
Asymptotic values in functional responses have been predicted by models derived from empirical relationships in many shorebird species (Goss-Custard et al. 2006). Further research is needed before testing the predictions of these models in fall staging semipalmated sandpipers. For instance, it would be useful to document food intake rate in richer food patches to increase the range of prey densities. The present measurements have also been taken relatively close to shore where shorebirds are quite vulnerable to attacks from birds of prey (Dekker and Ydenberg 2004; Whitfield 2003). In sandpipers, capture rate has already been shown to be sensitive to predation risk as individuals allocate more time to vigilance under riskier conditions, which is not fully compatible with maintaining a high feeding rate (Beauchamp and Ruxton 2008). In the present study, capture rate was indeed lower closer to shore where one would expect predation risk from surprise attacks by falcons to be the highest. The prediction that asymptotic food intake rate will be lower when more time is dedicated to anti-predator responses, such as predator vigilance, could be tested more fully by documenting the functional response in areas with lower predation risk (Smart et al. 2008). Models also emphasize the amount of overlap between searching, handling and vigilance in animals (Smart et al. 2008). Time spent searching, handling and vigilant in semipalmated sandpipers when feeding on Corophium is not known, although given that prey size is small, handling time must be quite short and may overlap little with searching. Nevertheless, quantitative predictions about intake rate are sensitive to such foraging details and future work is thus needed.
References
Beauchamp G (2005) Low foraging success of semipalmated sandpipers at the edges of groups. Ethology 111:785–798
Beauchamp G (2006) Spatial, temporal and weather factors influencing the foraging behavior of migrating semipalmated sandpipers. Waterbirds 29:221–225
Beauchamp G (2007) Competition in foraging flocks of migrating semipalmated sandpipers. Oecologia 154:403–409
Beauchamp G, Ruxton GD (2008) Disentangling risk dilution and collective detection in the antipredator vigilance of semipalmated sandpipers in flocks. Anim Behav 75:1837–1842
Boates JS (1980) Foraging semipalmated sandpipers Calidris pusilla L. and their major prey Corophium volutator (Pallas) on the Starrs Point mudflat Minas Basin. Acadia University, Wolfville
Crewe TL, Hamilton DJ, Diamond AW (2001) Effects of mesh size on sieved samples of Corophium volutator. Estuar Coast Shelf Sci 53:151–154
Dekker D, Ydenberg R (2004) Raptor predation on wintering dunlins in relation to the tidal cycle. Condor 106:415–419
Dolman PM (1995) The intensity of interference varies with resource density: evidence from a field study with snow buntings, Plectrophenax nivalis. Oecologia 101:511–514
Fryxell JM, Mosser A, Sinclair ARE, Packer C (2007) Group formation stabilizes predator–prey dynamics. Nature 449:1041–1044
Gillings S et al (2007) Shorebird predation of horseshoe crab eggs in Delaware Bay: species contrasts and availability constraints. J Anim Ecol 76:503–514
Goss-Custard JD et al (2006) Intake rates and the functional response in shorebirds (Charadriiformes) eating macro-invertebrates. Biol Rev 81:501–529
Gratto-Trevor CL (1992) Semipalmated sandpiper. In: Poole A, Gill FB (eds) The birds of North America, vol 6. The Academy of Natural Sciences and The American Ornithologists’ Union, Philadelphia
Hamilton DJ, Diamond AW, Wells PG (2006) Shorebirds, snails, and the amphipod (Corophium volutator) in the upper Bay of Fundy: top-down vs. bottom-up factors, and the influence of compensatory interactions on mudflat ecology. Hydrobiologia 567:285–306
Hicklin PW, Smith PC (1984) Selection of foraging sites and invertebrate prey by migrant semipalmated sandpipers, Calidris pusilla (Pallas) in Minas Basin, Bay of Fundy. Can J Zool 62:2201–2210
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Jeschke JM, Koop M, Tollrian R (2002) Predator functional responses: discriminating between handling and digesting prey. Ecol Monogr 72:95–112
Johnson CA, Giraldeau LA, Grant JWA (2001) The effect of handling time on interference among house sparrows foraging at different seed densities. Behaviour 138:597–614
Minderman J, Lind J, Cresswell W (2006) Behaviourally mediated indirect effects: interference competition increases predation mortality in foraging Redshanks. J Anim Ecol 75:713–723
Moody AL, Ruxton GD (1996) The intensity of interference varies with feed density: support for behaviour-based models of interference. Oecologia 108:446–449
Morgan E (1965) The activity rhythm of the amphipod Corophium volutator (Pallas) and its possible relationship to changes in hydrostatic pressure associated with the tides. J Anim Ecol 34:731–746
Nilsson PA, Huntingford FA, Armstrong JD (2004) Using the functional response to determine the nature of unequal interference among foragers. Proc R Soc Lond B Biol Sci 271:S334–S337
Peer DL, Linkletter LE, Hicklin PW (1986) Life history and reproductive biology of Corophium volutator (Crustacea, Amphipoda) and the influence of shorebird predation on population structure in Chignecto Bay, Bay of Fundy, Canada. J Sea Res 20:359–373
Schneider DC (1981) Food supplies and the phenology of migratory shorebirds: a hypothesis. Wader Study Group Bull 33:43–45
Smart SL, Stillman RA, Norris KJ (2008) Measuring the functional responses of farmland birds: an example for a declining seed-feeding bunting. J Anim Ecol 77:687–695
Sprague AJ (2006) Factors affecting movement and habitat selection of semipalmated sandpipers (Calidris pusilla) migrating through the upper Bay of Fundy, Canada. In: Biology, M.Sc. Mount Allison University, Sackville
Stillman RA, Simmons VL (2006) Predicting the functional response of a farmland bird. Funct Ecol 20:723–730
Sutherland WJ (1996) From individual behaviour to population ecology. Oxford University Press, Oxford
Sutherland WJ, Parker GA (1985) Distribution of unequal competitors. In: Krebs JR, Davies NB (eds) Behavioural ecology. Blackwell, Oxford, pp 255–273
Whitfield DP (2003) Redshank Tringa totanus flocking behaviour, distance from cover and vulnerability to sparrowhawk Accipiter nisus predation. J Avian Biol 34:163–169
Wilson WH (1990) Relationship between prey abundance and foraging site selection by semipalmated sandpipers on a Bay of Fundy mudflat. J Field Ornithol 61:9–19
Wilson WH, Vogel ER (1997) The foraging behavior of semipalmated sandpipers in the upper Bay of Fundy: stereotyped or prey-sensitive? Condor 99:206–210
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christopher Johnson.
Rights and permissions
About this article
Cite this article
Beauchamp, G. Functional response of staging semipalmated sandpipers feeding on burrowing amphipods. Oecologia 161, 651–655 (2009). https://doi.org/10.1007/s00442-009-1398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1398-6