Abstract
In most egg-laying vertebrates, maternal responses to stressful conditions are translated into the release of glucocorticoid hormones such as cortisol, which are then transmitted to their developing embryos. Although such maternally transmitted hormonal resources have been shown to influence or even interfere with the optimal developmental trajectories of offspring in many taxa, their influence on the dynamics of wild fish populations remains largely unexplored. Here, we examined the extent to which simulated hormonally mediated maternal effects influence the development and early survival of the coral reef damselfish, Pomacentrus amboinensis. Concentrations of cortisol in the eggs were manipulated within naturally occurring limits by immersion. We found that the proportion of embryos that delayed hatching when exposed to high levels of cortisol was considerably lower than in the other two treatments (low cortisol dose and control). High cortisol levels in P. amboinensis eggs resulted in increased egg mortality and greater asymmetry in hatchlings. For embryos that successfully hatched, individuals from the elevated cortisol treatments (especially low dose) survived longer after hatching. Although individuals that originated from eggs with elevated cortisol levels survived longer after hatching, they may not gain an overall survival advantage. Our results suggest that subtle increases in the allocation of maternally derived hormones, such as cortisol, to offspring are a direct way for stressed mothers to endow their young with an immediate survival advantage. We propose that this immediate benefit outweighs the developmental costs which may be expressed as reduced fitness at later life stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of wild populations to survive environmental challenges and ecological perturbations depends on the rapidity and severity of these stressors. Although organisms have evolved a wide range of behavioural responses to deal with stressful conditions, the effectiveness of their physiological response determines their potential to survive and adapt to further environmental changes (Dillon and Lynch 1981). Differences in tolerance or adaptability to stressful conditions among individuals often arise from a response to the stressful conditions experienced by their parents, and are increasingly recognized as the outcome of environmentally induced parental effects (Lacey 1998). When parents are exposed to highly variable and stressful conditions, the quality of parental investment in the future generation may be significantly challenged with major repercussions for the number and quality of individuals surviving to recruit into the reproductive population. By altering the frequencies of phenotypes within a population, parental effects play a key role in shaping population responses to environmental changes occurring over ecologically relevant evolutionary scales (see review by Räsänen and Kruuk 2007).
Despite substantial naturally occurring and human-induced environmental variability, developmental systems appear to show a high level of stability (Gibson and Wagner 2000). The mechanisms that regulate the phenotypic stability of any single trait are mostly maintained by the ability of individuals to express different phenotypes under different environmental conditions (plasticity) and buffer slight genetic abnormalities or environmental disturbance (canalization) or any random error arising spontaneously with development (developmental stability) (Debat and David 2001). In particular, developmental stability buffers against stochastic perturbations of cellular processes, which may affect the expression and development of morphological structures (Santos et al. 2005).
Developmental errors in morphological traits that occur early during ontogeny may be directly linked to the physiological condition of the mother at the time of vitellogenesis (Eriksen et al. 2006). In egg-laying vertebrates, stressful conditions are translated into a physiological response primarily involving the neuroendocrine system and culminating in the release of glucocorticoid hormones, which are then transmitted from mother to offspring (Braastad 1998). Maternally transmitted hormonal resources have been shown to influence or even interfere with the optimal developmental trajectories of offspring in a wide range of taxa, particularly in mammalian and avian species (e.g. Eriksen et al. 2003; Hayward and Wingfield 2004; Crespi and Denver 2005; Groothuis et al. 2005). In mammals, for example, hormonally mediated maternal responses to stressful conditions have been shown to have a permanent effect on the hypothalamic–pituitary–adrenal axis of the progeny, markedly affecting the ability of juvenile and adult offspring to cope with unfavourable environments (reviewed by Braastad 1998; Dufty et al. 2002). The physiological stress response of fishes is similar to that found in mammals, and there is thus the potential for environmental challenges to affect parental condition in fishes and modify the developmental program and viability of their offspring. Experimental manipulations of maternal cortisol within naturally occurring limits were able to produce larvae that spanned the complete size range that naturally occurred in the wild, emphasizing the importance of this mechanism in wild fish populations (McCormick 1999, 2006). Nonetheless, the extent to which hormonally mediated maternal responses to stress influence the dynamics of these populations remains largely unexplored.
The present study explored the extent to which simulated hormonally mediated maternal effects affect the development and early survival of a common coral reef damselfish, Pomacentrus amboinensis. It also explores the implications of these findings for future fitness. Cortisol levels experienced by embryos were experimentally manipulated to mimic stress conditions experienced by mothers on coral reefs and the development and fate of the offspring were recorded. Because of the logistical difficulty of following the fate of a cohort of pelagic larval offspring under field conditions, we quantified fluctuating asymmetry (FA) as an index of developmental perturbations to embryogenesis and related this to events that occur in natural fish populations.
Materials and methods
Study species
The model species, P. amboinensis, is typical of many reef fishes, having a site-attached adult phase that breeds to produce dispersive larvae, which have a pelagic duration of 15–23 days (Kerrigan 1996) prior to settlement back into a reef-based fish assemblage. The species is loosely haremic. Males guard a benthic nest (often an upturned clam shell), and attract females to spawn on the nest just prior to dawn (McCormick and Smith 2004). Fertilized eggs develop for 4.5–5.5 days (depending on temperature) and hatch synchronously ~30 min after sunset at which time they have a small yolk sac, well-developed eyes and are capable of feeding directly on rotifers (McCormick and Nechaev 2002).
Previous studies have shown that females differ widely in their levels of ovarian cortisol and this is related to factors that correlate with habitat (McCormick 1998, 2006; McCormick, in press). Breeding pairs on isolated patch reefs have the lowest levels of ovarian cortisol compared to fish from other habitats (McCormick 1998) and adults naturally occur on such spatially isolated patch reefs. This low level of maternally derived cortisol is in part due to the low interaction regime of females on patch reefs (McCormick 2006) and low number of egg predators that cause breeding females stress (McCormick 1998; McCormick, in press). Monitoring studies and field experiments have shown that there is a good correspondence between the levels of cortisol in newly spawned eggs and the concentrations in maternal ovarian tissue (McCormick 1998), suggesting that maternal cortisol is being transferred to the eggs during vitellogenesis. Field sampling has shown that concentrations of cortisol within eggs can vary widely during the spawning season (0.2–0.4 pg/egg modal concentration, with maximum 12.8 pg/egg; McCormick 1999). Moreover, McCormick (1999) experimentally demonstrated that levels of cortisol within newly fertilized eggs could be elevated, within naturally occurring limits, by emersion in an aerated solution of cortisol and seawater. The present study is only made possible through this foundation of field and laboratory methodology.
Preliminary examination of egg cortisol concentrations through development
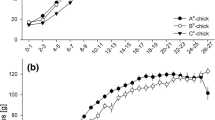
Studies of temperate and cold water fish, which have a protracted embryonic period compared to tropical fish, have found that embryos use cortisol during embryogenesis and do not develop the capacity to produce their own cortisol until after hatching (e.g. De Jesus and Hirano 1992; Barry et al. 1995; see also studies in birds, e.g. Elf and Fivizzani 2002; Eising et al. 2003 and reptiles, e.g. Bowden et al. 2002; Elf et al. 2002). Unfortunately, the usage trajectories of cortisol within the developing eggs of tropical fishes are unknown. As the first part of the present study, it was necessary to determine the trajectory of cortisol usage by P. amboinensis embryos in order to mimic these trajectories in the laboratory. Spawning was monitored on mapped male nest sites at the back reef of Lizard Island on the northern Great Barrier Reef (14°40′S, 145°28′E) during the austral summer. Samples of embryos were collected every ~24 h until just prior to hatching. Eggs were collected in situ from the monolayer clutch with a new scalpel, floated into a 1.5-ml Eppendorf tube, and preserved in liquid nitrogen for radioimmunoassay (RIA) analysis of cortisol levels (below). Between 500 and 1,000 eggs were removed. Unfortunately, clutches could seldom be serially sampled since embryo removal often lead to the consumption of the clutch by the parents. The relationship between egg cortisol concentrations and time post-fertilization was examined with a linear regression and differences among means at different time points were tested by Mann–Whitney U-test.
Egg collection and experimental protocol
Eggs used in the laboratory experiment were obtained in November 2005 from P. amboinensis breeding pairs on patch reefs constructed within the Lizard Island lagoon as described in Gagliano and McCormick (2007). Breeding females in isolated patch reef habitats have been shown to produce eggs with low levels of cortisol compared to females in other reef habitats (McCormick 1999).
In the morning following a pre-dawn spawning, clutches of newly fertilized eggs spawned on artificial nesting substrata (McCormick 1999) were collected from five breeding pairs (one clutch from each pair) and transferred into well-aerated flow-through seawater aquaria in the laboratory. Individual eggs were removed from each clutch using a scalpel and transferred with a fine brush to 12 replicate six-well tissue culture plates (n = 72 eggs per clutch). Each plate was then housed in a 1-l plastic container and submerged in a seawater bath (~4 cm deep). A total of 360 embryos (from the five clutches) were randomly assigned to three stress treatments (n = 120 per treatment): control (no cortisol added to the seawater bath); a low dose of cortisol (LD; 102 ng/ml added to the seawater bath); and a high dose of cortisol (HD; 104 ng/ml added to the seawater bath). A pilot study found concentrations of cortisol within these seawater solutions were stable for greater than 36 h. All embryos were allowed to develop in isolation at the same seawater temperature recorded on the patch reefs where the clutches were collected (28°C). Each plastic container was flushed daily and replaced with a clean one where cortisol concentrations were immediately re-established.
The levels of cortisol that embryos were exposed to during this study were chosen to span the naturally occurring range determined from previous field observations (see McCormick 1999). McCormick (1999) found that concentrations of cortisol in newly spawned P. amboinensis eggs from field collections were 0.2–0.4 pg/egg, with the highest concentration being 12.8 pg/egg. McCormick (1999) also used RIAs on egg samples removed from control and cortisol treatments after 3 days of immersion and showed that the highest dosage level used in the present study (104 ng/ml) induced an egg concentration of 1.2 pg/egg; well below the maximum concentrations found in the wild (see McCormick 1999). McCormick (1999) found that 22.7% of eggs clutches had cortisol concentrations equal or greater than 1.2 pg/egg soon after spawning in natural conditions. Few studies have described cortisol usage trajectories during embryogenesis in perciformes, but those that have show only minor reductions in maternally endowed cortisol within the egg prior to hatching (e.g. Szisch et al. 2005). The preliminary data on embryonic cortisol usage in the present study provide no evidence of a change in cortisol levels during embryogenesis in P. amboinensis (Fig. 1). Accordingly, embryos were maintained in their respective cortisol treatments until hatching to ensure a constant developmental environment and were all transferred to a cortisol-free seawater bath only following hatching. This experimental set up allowed us to investigate the effects of cortisol on early life history characteristics while controlling for the potentially confounding effects of other hormones and substances transferred from mother to offspring.
To define the extent to which pre-natal exposure to cortisol influences development prior to hatching and influences subsequent survival of offspring, half the embryos from each treatment were sacrificed at hatching to quantify the degree of bilateral asymmetry at the end of the embryonic phase, while the other half was monitored to gauge the post-hatching longevity of newly hatched larvae.
Measurements of development and fitness
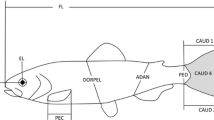
A total 360 embryos from all treatments were monitored and photographed under a compound microscope (at 10× magnification) just prior to the formation of main organs and systems [i.e. 36 h post-fertilization (hpf); McCormick and Nechaev 2002)] and 2–4 h prior to hatching (i.e. 84 hpf). To quantify the early life history characteristics of the embryos, egg size (volume in cubic millimetre), yolk-sac size (yolk-sac area in millimetre square; YK) and oil globule size (oil globule area in millimetre square; OG) were measured from these calibrated digital images using the image analysis programme, OPTIMAS 6.5. Heart rate (heart beats/min) was used as a proxy for embryonic metabolism and measured at 84 hpf only by three replicated 1-min counts of heart beats in embryos submerged in their experimental water treatment. The sequence in which embryo characteristics were recorded were randomized across treatments to reduce the potential effect of unavoidable delays caused by the time required to photograph each individual embryo (maximum 45 min to measure all embryos). Pre-hatching mortality and time of hatching were recorded. Hatching in this species generally occurs on the evening of the 3rd day post-fertilization (i.e. 84 hpf). However, embryos were also observed to hatch on the evening of the 4th day post-fertilization (i.e. 108 hpf) and this is referred to as “delayed hatching” in this paper. Upon hatching, larval body size (total length, millimetre) was measured under a compound microscope (at 10× magnification) and the recently formed circular sagittal otoliths of individual hatchlings were then located under a compound microscope (at ×40 magnification). To measure otolith dimensions, individual larvae were placed on a slide and covered with a cover slip, the weight of which was sufficient to dorsally flatten the head region, allowing for easy identification and measurement of the otoliths. Area (square micrometre) of the left and right otolith was recorded as a measure of otolith size-at-hatching and used to quantify the degree of FA (see Palmer and Strobeck 2003). For each individual fish, we calculated the signed asymmetry value (R–L) of the otolith pair. We then examined the distribution of these values to ensure that otolith area exhibited true FA (distribution with a mean equal to 0 and normal variation), as opposed to different forms of asymmetry, such as directional asymmetry and antisymmetry. We used a one-sample t-test to test for signs of directional asymmetry (i.e. kurtosis values smaller than 0) and a Shapiro–Wilks’s statistic for antisymmetry (i.e bimodality) as described by Palmer and Strobeck (1986). To eliminate the possibility of inflated asymmetry estimates due to measurement error (ME), otolith area was independently re-measured for each hatchling without knowledge of previous measurements. Adopting an approach similar to Gagliano et al. (2008), we then classified as “asymmetrical” all individuals for which FA was greater than the ME for this trait. Following hatching, unfed larvae were inspected every 6 h until death as a measure of post-hatching longevity. This condition was chosen to represent the realistic pelagic environment in tropical waters, where food availability is notoriously limited and patchy at both temporal and spatial scales, and starvation is implicated as one of the major sources of mortality in fish larvae (Leis and McCormick 2002).
Cortisol content of eggs
Concentrations of cortisol in the eggs were determined by standard RIA techniques as described for the study species by McCormick (1999). To enable detection of cortisol levels within the eggs, assays were run on homogenates from 200 to 511 eggs from individual clutches and concentrations converted to picogram per egg. Extraction efficiencies, determined by the recovery of 3H-steroid added to triplicated egg homogenates, ranged between 84.1 and 93.1%.
Data analyses
Data were checked for all relevant assumptions of normality and homogeneity of variance before performing statistical analyses (Sokal and Rohlf 2001). The effects of treatment and clutch identity within treatments on hatching success, hatching time, degree of otolith asymmetry and post-hatching survival were analysed using mixed model ANOVAs with clutch identity as the hierarchically nested random factor. The effect of hatching time on the otolith asymmetry observed in hatchlings, as well as the extent to which both hatching time and level of asymmetry affected embryonic traits among treatments, was analysed using factorial ANOVAs. All differences among treatments were identified using post-hoc Tukey’s [honest significant difference (HSD)] tests.
Results
Egg cortisol concentrations through development
There was no relationship between the levels of cortisol within the developing eggs and time since fertilization (r 2 = 0.04, P = 0.22, n = 42; Fig. 1) and no differences among means at different time points were observed (Mann–Whitney U-test, P > 0.05 for all comparisons).
Hatching success and cortisol levels
Hatching success was significantly affected by the level of cortisol embryos experienced during development (F 2,229 = 7.15, P < 0.05), resulting in an increased hatching failure as cortisol levels increased (Tukey’s HSD, P < 0.001 for all comparisons; 83, 60 and 44% hatching success rates for C, LD and HD treatment, respectively). While hatching success varied among clutches (F 9,229 = 2.91, P < 0.05), this component accounted for only 8.7% of the overall variation in mortality observed.
Hatching time and cortisol levels
Increased cortisol levels affected hatching time of embryos (F 2,169 = 6.06, P < 0.05), independently of clutch of origin (F 9,169 = 1.17, P = 0.32). The proportion of embryos that delayed hatching (i.e. hatched at 108 instead of 84 hpf) in the control and LD treatment was similar (Tukey’s HSD, P = 0.99) and represented about 38% of all embryos in each treatment. However, only 10% of embryos in the HD treatment hatched later.
Asymmetry and cortisol levels
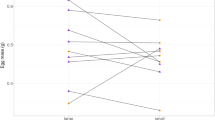
No matter which clutch the hatchlings originated from (F 9,77 = 1.24, P = 0.28), exposure to different levels of cortisol resulted in significantly different level of otolith asymmetry at hatching (F 2,77 = 3.43, P < 0.05). Otolith asymmetry increased as the concentration of cortisol experienced during embryogenesis increased (Fig. 2a) and the proportion of asymmetrical individuals was on average 2.11 and 1.71 times greater in the HD and LD treatment, respectively, than the control (control 28%, LD 48%, HD 59%).
Mean ± SE level of a otolith asymmetry observed at hatching and b heart rates immediately prior to hatching at 84 hpf in P. amboinensis embryos developing from control and cortisol-treated [low dose (LD), high dose (HD)] eggs. Different letters indicate statistical differences between means based on Tukey’s honest significant difference (HSD) at P < 0.05
Asymmetry, hatching time and phenotypic traits
Heart rates increased significantly with increasing cortisol levels, mirroring the pattern observed for otolith asymmetry (Fig. 2b). Across treatments, both heart rates prior to hatching and overall level of otolith asymmetry observed at hatching appeared to be independent of hatching time (treatments × hatching time, F 2,35 = 0.84, P = 0.44 for heart rates and F 2,84 = 0.62, P = 0.54 for asymmetry). Independently of the treatments they originated from, embryos hatched at 108 hpf had significantly different heart rates from embryos hatched at 84 hpf (F 1,35 = 4.56, P < 0.05). Interestingly, embryos that delayed hatching in both the control and LD treatment exhibited consistently lower heart rates compared to those hatching at 84 hpf (Fig. 3). In contrast, no difference in heart rates was observed between embryos that hatched at 84 hpf and those that delayed hatching while exposed to higher cortisol levels (HD treatment; Fig. 3). In embryos from the HD treatment, higher heart rates were strongly associated with lower energy reserves left just prior to hatching (for YK, r = −0.65, P < 0.05; for OG, r = −0.35, P < 0.05; see Electronic supplementary material). Moreover, among HD embryos, asymmetrical individuals had significantly lower energy reserves available to hatch (for YK, F 2,81 = 3.84, P < 0.05; for OG, F 2,81 = 6.35, P < 0.05; Fig. 4). In embryos exposed to higher cortisol levels, heart rates were also positively correlated with mean otolith size at hatching (r = 0.52, P < 0.05). Nevertheless, mean otolith size at hatching of HD embryos indicated that these embryos, contrary to individuals in the control and LD treatment, failed to grow during the extra day spent in the egg envelop (treatments × hatching time, F 2,84 = 8.91, P < 0.001; Fig. 5) and ultimately, the body size of newly hatched larvae was similar among treatments (treatments × hatching time, F 2,87 = 2.04, P = 0.14).
Mean ± SE heart rates (heart beats/min) recorded in P. amboinensis embryos that hatched at 84 (white) and 108 hpf (grey) from control and cortisol-treated (LD and HD) eggs. Asterisks indicate significant differences between hatching times for each treatment [**P < 0.05; *α = 0.01, P = 0.07; no significant difference (n.s.), P = 0.91]. Numbers above each histogram bar indicate sample sizes. For abbreviations, see Fig. 2
Mean ± SE a yolk sac area and b oil globule area measured just prior to hatching in P. amboinensis embryos that hatched with symmetrical (white) and asymmetrical (grey) otoliths from control and cortisol-treated (LD and HD) eggs. Asterisks indicate a significant difference (*P < 0.05) and n.s. between hatching times for each treatment. Numbers above each histogram bar indicate sample sizes. For abbreviations, see Figs. 2 and 3
Mean ± SE otolith size-at-hatching in P. amboinensis embryos that hatched at 84 (white) and 108 hpf (grey) from control and cortisol-treated (LD and HD) eggs. Asterisks indicate a significant difference (**P < 0.05) and n.s. (P = 0.39) between hatching times for each treatment. Numbers above each histogram bar indicate sample sizes. For abbreviations, see Figs. 2 and 3
Post-hatching survival and cortisol levels
Even after accounting for substantial variability observed among clutches within treatments (F 9,63 = 2.16, P < 0.05), post-hatching survival was generally higher for embryos in the LD treatment than in both HD and control treatments (F 2,63 = 4.25, P < 0.05). In the control and HD treatments, 50% of all hatchlings died within 18 and 36 h post-hatching respectively, while the same proportion was still alive at 48 h following hatching in the LD treatment (Fig. 6). By 24 h post-hatching, only 15% of all hatchlings in the control treatment remained alive, while individuals in the HD and LD treatments reached 15% survival at 60 and 72 h, respectively.
Post-hatching longevity of P. amboinensis larvae hatched from control and cortisol-treated (LD and HD) eggs, expressed as percentage of surviving individuals. For abbreviations, see Fig. 2
Discussion
Many environmental challenges and ecological circumstances may induce a stress response, which in vertebrates is in part manifested by increased concentrations of circulating glucocorticoids, such as cortisol. Early exposure to high levels of this stress-associated hormone is often assumed to be deleterious to embryos, being an unavoidable consequence of maternal stress (Braastad 1998). In this study, the experimental elevation of cortisol levels in newly fertilized eggs of P. amboinensis induced significant physiological changes in developing embryos, resulting in altered metabolic rhythms, hatchability and hatching timing. Specifically, high cortisol levels in P. amboinensis eggs resulted in increased egg mortality and greater asymmetry in hatchlings, indicating that the normal or homeostatic state of these individuals was adversely affected by stress. Nevertheless, we also found that embryos that successfully hatched from either cortisol-enhanced treatments had greater survival on endogenous yolk reserves compared to control individuals. These findings on the effects of different concentrations of cortisol available during egg development are interesting as they suggest an underlying tradeoff between normal development during the embryonic stage and the proper functioning after hatching. We have previously proposed that eggs with low hormonal endowment may be less efficient in transforming yolk into body tissue than those with a different (higher) cortisol levels, thereby retarding growth and reducing survivorship (Gagliano and McCormick 2007). Hence, we conclude that high concentrations of cortisol, and possibly other maternal glucocorticoids experienced during embryonic development, may also have beneficial effects on offspring phenotype and survival, as proposed for other vertebrate taxa (e.g. birds, Kitaysky et al. 2003; lizards, de Fraipont et al. 2000; rodents, Welberg and Seckl 2001).
The elevation of cortisol levels in our experiment induced changes in the timing of hatching, hence revealing a degree of phenotypic plasticity of this trait in response to real or perceived stressful conditions. In fact, we found that the proportion of embryos that delayed hatching when exposed to high levels of cortisol was considerably lower than in the other two treatments. This may be because cortisol speeds up developmental rhythms (McCormick and Nechaev 2002) and the consequence of this phenotypic response to higher concentrations of the stress-associated hormone may be greater chances of survival if delaying hatching increases egg-specific risks. Two previous field studies of P. amboinensis, showed that the presence of egg predators around the nest site is one of the major factors triggering an increase in the level of cortisol in breeding females and their eggs (McCormick 1998; McCormick, in press). Moreover, field studies have shown that egg mortality through predation is variable (0–100%) and at times high while the male is guarding the benthic egg clutch (Emslie and Jones 2001). Accordingly, the observed cortisol-induced effects may represent an adaptive mechanism by which mothers communicate information about the prevailing environment to their offspring to ensure their immediate survival. Similar hormonally mediated maternal effects that induce a transition between life stages to avoid deadly conditions or deteriorated environments have been demonstrated in mammals, amphibians and reptiles (Denver 1997; Crespi and Denver 2005; Weiss et al. 2007), illustrating the beneficial effects of maternal glucocorticoids in shaping the neuroendocrine responses of offspring, albeit at the potential cost of reduced fitness later in life.
Consistent with the fact that stress is an energy-demanding process, we found that embryos exposed to higher concentrations of cortisol exhibited higher heart rates, which were strongly associated with lower energy reserves left just prior to hatching (see also McCormick and Nechaev 2002; McCormick and Gagliano, in press). Supposedly, the amount of energy mothers allocate to an individual egg for embryonic development sufficiently meets basic energetic costs of the growth of new tissue and the maintenance of developed tissue. While fish embryos generally allocate most of their energy resources to fuel high growth rates (Rombough 1994), the way energy is partitioned may change significantly under stressful conditions. Our experiment suggests that individuals from the high cortisol treatment who delayed hatching stopped growing over the extra day spent in the egg envelop. This suggests that cortisol-induced stress initiated faster depletion of energy resources, probably resulting from elevated metabolic expenditure for maintenance of higher levels of movement rather than growth (McCormick and Nechaev 2002).
Although cortisol clearly affected embryonic developmental rhythms, exposure to the hormone did not appear to have immediate consequences for the survival of newly hatched larvae. Nonetheless by initiating elevated metabolic expenditure and increasing the depletion of a limited endogenous nutritional supply, stress may induce subtle perturbations to the stable development of individuals (Møller and Swaddle 1997). In this study, we found that embryos developing in cortisol-enhanced conditions displayed reduced developmental stability as indicated by increased bilateral asymmetry (FA) in otolith morphology at hatching. In fishes, the degree of otolith asymmetry can be established at the initial development of the paired otoliths (i.e. at the embryonic stage) and, since the aragonite-protein matrix is not subsequently reworked, the degree of asymmetry may be retained during a fish’s lifetime and accentuated through further accretion (Lychakov et al. 2006). By comparing the levels of otolith FA in newly hatched larvae of P. amboinensis, to the otolith FA of fish at the end of the larval phase, Gagliano et al. (2008) suggested that larvae with asymmetrical otoliths suffer significantly higher rates of mortality during the larval phase. A similar link between otolith asymmetry and larval persistence has been found for a tropical lizardfish (Lemberget and McCormick 2009). If asymmetry persists during crucial ontogenetic periods, such as the larval phase, and phenotypic selection penalizes asymmetrical individuals, the data presented herein suggest that hormonally mediated maternal effects are likely to have important repercussions on annual recruitment variability and play a key role in shaping the dynamics of future fish populations.
References
Barry TP, Malison JA, Held JA, Parrish JJ (1995) Ontogeny of the cortisol stress response in larval rainbow trout. Gen Comp Endocrinol 97:57–65
Braastad BO (1998) Effects of prenatal stress on behaviour of offspring of laboratory and farmed mammals. Appl Anim Behav Sci 61:159–180
Bowden RM, Ewert MA, Nelson CE (2002) Hormone levels in yolk decline throughout development in the red-eared slider turtle (Trachemys scripta elegans). Gen Comp Endocrinol 129:171–177
Crespi EJ, Denver RJ (2005) Ancient origins of human developmental plasticity. Am J Hum Biol 17:44–54
de Fraipont M, Clobert J, John-Alder H, Meylan S (2000) Increased prenatal maternal corticosterone promotes philanthropy of offspring in common lizard (Lacerta vivipara). J Anim Ecol 69:404–413
De Jesus EG, Hirano T (1992) Changes in whole body concentrations of cortisol, thyroid hormones and sex steroids during early development of the chum salmon, Oncorhynchus keta. Gen Comp Endocrinol 85:55–61
Debat V, David P (2001) Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol Evol 16:555–561
Denver RJ (1997) Environmental stress as a developmental cue: corticotrophin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm Behav 31:169–179
Dillon TM, Lynch MP (1981) Physiological responses as determinants of stress in marine and estuarine organisms. In: Barrett GW, Rosenberg R (eds) Stress effects on natural ecosystems. Wiley, New York, pp 227–241
Dufty AM Jr, Clobert J, Møller AP (2002) Hormones, developmental plasticity and adaptation. Trends Ecol Evol 17:190–196
Eising CM, Muller W, Dijkstra C, Groothuis TGG (2003) Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. Gen Comp Endocrinol 132:241–247
Elf PK, Fivizzani AJ (2002) Changes in sex steroid levels in yolks of the leghorn chicken, Gallus domesticus, during embryonic development. J Exp Zool 293:594–600
Elf PK, Lang JW, Fivizzani AJ (2002) Dynamics of yolk steroid hormones during development in a reptile with temperature-dependent sex determination. Gen Comp Endocrinol 127:34–39
Emslie MJ, Jones GP (2001) Patterns of embryo mortality in a demersally spawning coral reef fish and the role of predatory fishes. Environ Biol Fish 60:363–373
Eriksen MS, Haug A, Torjesen PA, Bakken M (2003) Prenatal exposure to corticosterone impairs embryonic development and increases fluctuating asymmetry in chickens (Gallus domesticus). Br Poult Sci 44:690–697
Eriksen MS, Bakken M, Espmark Å, Braadstad BO, Salte R (2006) Prespawning stress in farmed Atlantic salmon Salmo salar: maternal cortisol exposure and hyperthermia during embryonic development affect offspring survival, growth and incidence of malformations. J Fish Biol 69:114–129
Gagliano M, McCormick MI (2007) Maternal condition influences phenotypic selection on offspring. J Anim Ecol 76:174–182
Gagliano M, Depczynski M, Simpson SD, Moore JAY (2008) Dispersal without errors: symmetrical ears tune into the right frequency for survival. Proc R Soc B 275:527–534
Gibson G, Wagner G (2000) Canalization in evolutionary genetics: a stabilizing theory? Bioessays 22:372–380
Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C (2005) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev 29:329–352
Hayward LS, Wingfield JC (2004) Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol 135:365–371
Kerrigan BA (1996) Temporal patterns in the size and condition of settlement in two tropical reef fishes (Pomacentridae: Pomacentrus amboinensis and P. nagasakiensis). Mar Ecol Prog Ser 135:27–41
Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC (2003) Benefits and costs of increased levels of corticosterone in seabird chicks. Horm Behav 43:140–149
Lacey EP (1998) What is an adaptive environmentally induced parental effect? In: Mousseau TA, Fox CW (eds) Maternal effects as adaptations. Oxford University Press, New York, pp 54–66
Leis JM, McCormick MI (2002) The biology, behavior and ecology of the pelagic, larval stage of coral reef fishes. In Sale PF (ed) Coral reef fishes—dynamics and diversity in a complex ecosystem. Academic Press, London, pp 171—199
Lemberget T, McCormick MI (2009) Replenishment success linked to fluctuating asymmetry in larval fish. Oecologia 159:8–93
Lychakov DV, Rebane YT, Lombarte A, Fuiman LA, Takabayashi A (2006) Fish otolith asymmetry: morphometry and modeling. Hear Res 219:1–11
McCormick MI (1998) Behaviourally induced maternal stress in a fish influences progeny quality by a hormonal mechanism. Ecology 79:1873–1883
McCormick MI (1999) Experimental test of the effect of maternal hormones on larval quality of a coral reef fish. Oecologia 118:412–422
McCormick MI (2006) Mothers matter: crowding leads to stressed mothers and smaller offspring in marine fish. Ecology 87:1104–1109
McCormick MI (in press) Indirect effects of heterospecific interactions on progeny quality through maternal stress. Oecologia doi:10.1111/j.1600-0706.2008.17410.x
McCormick Mi, Gagliano M (in press) Carry-over effects: the importance of a good start. Eleventh International Coral Reef Symposium
McCormick MI, Nechaev I (2002) Influence of cortisol on developmental rhythms during embryogenesis in a tropical damselfish. J Exp Zool 293:456–466
McCormick MI, Smith SA (2004) Efficacy of passive integrated transponder tags to determine spawning site visitations by a tropical fish. Coral Reefs 23:570–577
Møller AP, Swaddle JP (1997) Asymmetry, developmental stability and evolution. Oxford University Press, Oxford
Palmer AR, Strobeck C (1986) Fluctuating asymmetry: measurement, analysis, patterns. Annu Rev Ecol Syst 17:391–421
Palmer AR, Strobeck C (2003) Fluctuating asymmetry analyses revisited. In: Polak M (ed) Developmental instability (DI): causes and consequences. Oxford University Press, Oxford, pp 279–319
Räsänen K, Kruuk EB (2007) Maternal effects and evolution at ecological time-scales. Funct Ecol 21:408–421
Rombough PJ (1994) Energy partitioning during fish development: additive or compensatory allocation of energy to support growth? Funct Ecol 8:178–186
Santos M, Iriarte PF, Cespedes W (2005) Genetics and geometry of canalization and developmental stability in Drosophila subobscura. Evol Biol 5:7–7
Sokal RR, Rohlf FJ (2001) Biometry. Freeman, New York
Szisch V, Papandroulakis N, Fanouraki E, Pavlidis M (2005) Ontogeny of the thyroid hormones and cortisol in the gilthead sea bream, Sparus aurata. Gen Comp Endocrinol 142:186–192
Weiss SL, Johnston G, Moore MC (2007) Corticosterone stimulates hatching of late-term tree lizard embryos. Comp Biochem Physiol A Mol Integr Physiol 146:360–365
Welberg LAM, Seckl JR (2001) Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 13:113–128
Acknowledgments
We thank U. Siebeck for assistance in the field, M. Depczynski and J. A. Y. Moore for comments on an earlier version of the manuscript. This research was supported by the ARC Centre of Excellence for Coral Reef Studies (to M. I. McCormick), a JCU Doctoral Scholarship and a Nancy Vernon Rankine grant (to M. Gagliano), and is also an output of the AIMS@JCU joint venture. The experiments in this study comply with the current laws of Australia and were conducted under permits from the Great Barrier Reef Marine Park Authority and the JCU Animal Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Joel Trexler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gagliano, M., McCormick, M.I. Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia 160, 657–665 (2009). https://doi.org/10.1007/s00442-009-1335-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1335-8