Abstract
Predator–prey studies often assume a three trophic level system where predators forage free from any risk of predation. Since meso-predators themselves are also prospective prey, they too need to trade-off between food and safety. We applied foraging theory to study patch use and habitat selection by a meso-predator, the red fox. We present evidence that foxes use a quitting harvest rate rule when deciding whether or not to abandon a foraging patch, and experience diminishing returns when foraging from a depletable food patch. Furthermore, our data suggest that patch use decisions of red foxes are influenced not just by the availability of food, but also by their perceived risk of predation. Fox behavior was affected by moonlight, with foxes depleting food resources more thoroughly (lower giving-up density) on darker nights compared to moonlit nights. Foxes reduced risk from hyenas by being more active where and when hyena activity was low. While hyenas were least active during moon, and most active during full moon nights, the reverse was true for foxes. Foxes showed twice as much activity during new moon compared to full moon nights, suggesting different costs of predation. Interestingly, resources in patches with cues of another predator (scat of wolf) were depleted to significantly lower levels compared to patches without. Our results emphasize the need for considering risk of predation for intermediate predators, and also shows how patch use theory and experimental food patches can be used for a predator. Taken together, these results may help us better understand trophic interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most theoretical and almost all field studies have addressed predator–prey interactions as a three trophic level phenomenon (carnivore ↔ herbivore ↔ plant). They assume that predators forage free from predation risk, and that their choice of habitat is determined primarily by the distribution of their prey (Rosenzweig 1973; Sih et al.1998; Rosenheim 2004; Luttbeg and Sih 2004). However, in several aquatic and terrestrial communities, intraguild predation (Polis et al. 1989) is a widespread phenomenon (Polis and Holt 1992; Holt and Polis 1997; Rosenheim 1998). In many systems, it is likely that smaller predators forage under risk from larger top predators, and this is certain to influence their behavior and ecology. Top predators impose constraints on the foraging decisions of the meso-predators, which in turn could also affect their herbivore prey (Rosenheim 2004). Thus, predator–prey interactions are often four trophic level systems (top predator ↔ meso-predator ↔ herbivore ↔ plants,) in which meso-predators also have to trade-off between obtaining food and being safe.
Small mammalian carnivore studies have almost always focused only on their roles as predators. In many ecosystems, several species of predators that differ in body size may coexist (Rosenzweig 1966), and in such systems small carnivores themselves may become prospective prey to larger predators (see Ralls and White 1995; Helldin et al. 2006), or might suffer harassment and injury (e.g., interactions between hyenas and lions, Kruuk 1972) that may affect their hunting abilities. For example, interference competition from wolves limits the distribution and abundance of coyotes (Berger and Gese 2007). Even though such interactions between different sized carnivores have often been documented (Donadio and Buskirk 2006, and references within), rarely has any study addressed interactions between predation risk and foraging behavior of a meso-predator. Most studies (e.g., Ralls and White 1995) have instead focused on quantifying the actual predation events (i.e., intraguild predation), although these events may be difficult to document in many systems. Top predators do not only kill or injure, but may also induce fear in meso-predators. Since this has the potential to affect both their behavior and ecology (e.g., increased vigilance, reduced activity, avoiding certain areas, etc.), a focus on understanding risk-driven foraging behavior can at times be much more important than quantifying actual predation events (Brown et al. 1999). Also, since relative body size, trophic relationships, and taxonomic relatedness are important factors governing the frequency and intensity of interspecific predation, there is a need to focus on the role that predation risk plays in determining the foraging behavior of meso-predators (Case and Gilpin 1974; Polis et al. 1989).
We used patch use theory and experimental food patches to study the foraging behavior of a free-living meso-predator (the red fox, Vulpes vulpes) exposed to changing risk of predation. In particular, we used the giving-up density approach (Brown 1988, 1992) based on an extension of Charnov’s (1976) marginal value theorem. Charnov’s theory states that an animal feeding from a resource patch, in which harvest rate declines with time, should stay in the patch until its harvest rate equals the environmental average. When the forager also has to contend with risk of predation and has alternative activities, it should quit a depletable food patch when the benefit derived from its present harvest rate [i.e., patch quitting harvest rate (QHR)] no longer exceeds the sum of metabolic, predation, and missed opportunity costs of foraging (Brown 1988). The resource density at which the forager leaves the patch is its giving-up density (GUD).
In the Negev Desert, Israel, red foxes co-occur with two larger predators and hence may face substantial risk. Using patch use behavior and activity data, we tested the following predictions regarding trade-offs between food and safety for foxes. Since moonlight affects the foraging behavior of various desert organisms (Kotler et al. 1991, 2002), we predicted higher GUDs (less amount of food harvested) for foxes on full moon nights for two reasons: (1) foxes may be exposed to higher risk of predation (or harassment) from larger predators (e.g., the porcupines described by Brown and Alkon 1990), or (2) hunting is more profitable on moonlit nights due to better visibility, which makes it easier to locate active prey, thus increasing missed opportunity cost of foraging in assay patches. Providing GUDs are higher on moonlit nights, reduced activity on moonlit nights would support hypothesis 1, and higher activity would support hypothesis 2. Here, activity and GUD data complement each other.
Microhabitat may also affect risk of predation and GUDs. “Bush” and “open” are the two main microhabitats in desert systems. If having clear, unobstructed sightlines is important for foxes for predator detection, then foraging patches placed in the open should have the lower GUD. If concealment from a larger predator is more important, then patches in the bush microhabitat should be of greater value (lower GUD, Brown and Alkon 1990).
Finally, odors may provide clues for a forager regarding the whereabouts and identity of its predators (Dickman and Doncaster 1984). If so, then we expected higher GUDs in patches with predator cue.
Before applying the GUD approach to wild foxes, we first standardized our assay patches, and tested the two main assumptions (a forager experiences diminishing returns, and uses QHR rule while exploiting resource patches) of optimal patch use theory with captive foxes. We then applied this technique to wild foxes. We show that foxes respond to food patches in much the same way as do small mammals, hence showing the applicability of concepts from foraging theory to carnivores. Though the use of optimal patch use techniques have been more prevalent in the study of small mammals (especially rodents), we show how such an approach should also prove valuable for studying predators, especially as they are often rare, secretive, and at times difficult to study directly.
Methods
Experiments with the captive foxes were conducted at the Beer Sheva Zoo (Beer Sheva, Israel), and the field study took place at the Holot Mashabim Nature Reserve (31.01° N and 34.45° E) in the north-central Negev Desert, Israel, between 1999 and 2000. The Holot Mashabim area receives an average rainfall of 108 mm. It has two distinct habitats, the sandy areas containing patches of semi-stabilized and stabilized sand dunes with Artemisia monosperma and Retama raetam as the dominant plant species, and the loess plateau containing high amounts of loessal soil with a dense, firm soil crust. The dominant plants in this habitat are Noaea mucronata and Thymelaea hirsuta.
The red fox (Vulpes vulpes) is the most common small carnivore (3–4.5 kg) in the study area as well as in the entire Middle East (Mendelssohn and Yom-Tov 1999). It is a solitary, mainly nocturnal, and territorial animal with an omnivorous diet consisting mainly of rodents, insects, arachnids, birds, reptiles, fruits, and human refuse (Mendelssohn and Yom-Tov 1999). C.R. Dickman and B.P.K. (unpublished data) found that desert gerbils constituted 26% of its diet at our study site. Larger predators in our study area are the striped hyena (Hyaena hyaena, 25–55 kg) and the wolf (Canis lupus pallipes, 18–22 kg).
Testing diminishing returns and equalization of GUDs
We presented six captive adult foxes with artificial food patches. Each patch consisted of an 18 l bucket (29 cm diameter and 36 cm height) with ten pieces of chick (each wet piece weighing approximately 8 g, feathers included) mixed into 16 l sand. The buckets were placed into a hole cut into a wooden platform, so that its lip was flush with the platform. Each fox foraged individually from its own food patch. Its foraging was interrupted after different amounts of feeding time (cumulative time), and the remaining pieces of chick counted (its GUD). We obtained a total of 39 data points from the six foxes (mean ± SD data points per individual; 6.5 ± 0.84), and this data was used to quantify the relationship between the amounts of food harvested versus the cumulative time spent in the resource patch (i.e., the harvest rate curve). As a forager depletes a patch, its harvest rate declines through time, i.e., a forager experiences diminishing returns. As it continues to forage, in time, its harvest rate in that patch becomes so low that it is better for the forager to stop foraging from it (Brown 1988; Kotler and Brown 1990). Thus a harvest rate curve that rises steeply first and then flattens off would signify diminishing returns.
A forager that uses a QHR rule for patch exploitation should equalize GUDs between resource patches differing in initial resource abundance. We tested this assumption with wild red foxes in the field by offering them resource patches with different initial amounts of food. Ten stations of assay patches (same as above) were set in sandy habitats. Each station had two patches (buckets) with unequal initial resource abundance, one with 10 and the other with 20 pieces of chick. The distance between the patches at a station was small enough (2–3 m apart) to allow negligible travel time between them, and conditions at each patch were as similar as possible to ensure similar metabolic, predation, and missed opportunity cost of foraging. Chick pieces were placed in these patches just before sunset, and GUDs collected by sieving the sand and counting the remaining pieces of chicks at dawn. The tendency to equalize GUDs at a station was tested using the ratio of GUDs between the rich (high density) and poor (low density) patch. The experiment was repeated for 11 nights, and an average of the ratios of all the stations was calculated for each night (one data point each night), yielding a total of 11 data points. The initial ratio between patches in a given station was 2:1, and a ratio of GUDs significantly less than this was deemed as evidence in support of equalization of GUDs. To a forager, time is “money”; hence the time it saves by equalizing GUDs can be used in other fitness-enhancing activities or to harvest other patches (Valone and Brown 1989).
Foraging behavior of foxes and their predator/competitor
Fox GUDs were collected across two microhabitats (bush and open) and four moon phases (full, wane, new, and waxing) in loess and sandy habitats. The field site was first divided into different sections of loess and sandy habitats. In the 1st year (1999), a sandy and a loess area (600 m apart) were selected as study areas. Within each area, ten GUD stations spaced 60 m apart were established. In the following year, two more areas were added (one in each habitat type), but the numbers of GUD stations in the two locations were reduced to five in each (from ten). Thus, in total we had two replicates each of sandy habitat and loess habitat, with five GUD stations within each replicate. The distance between two habitat types and stations within a habitat was kept the same, at 600 and 60 m, respectively.
In the 2nd year, we also tested the effect of predator cue on the patch use behavior of foxes in the wild. We placed a wolf scat at each of the five stations in one of the two areas in each habitat type, creating a treated and control section within each habitat. GUDs were setup before dusk in all 20 stations (with wolf scats next to the ten stations), and these were collected at dawn. We used fresh wolf feces obtained from a captive wolf at the beginning of each moon phase and kept frozen until use. There are three reasons why we used wolf scat instead of hyena scats: (1) prior to these experiments we did not know that wolves were rare at our study site; (2) since wolves were available in a nearby zoo, we could obtain sufficient quantities of fresh wolf scats for our experiment; and (3) several studies (e.g., Thurber et al. 1992; Berger and Gese 2007) have shown wolves to pose a significant threat to smaller carnivores.
Each GUD station had two patches, one near a bush (a 1 m perennial shrub, representing the bush microhabitat) and the other 2–3 m away in the open (representing the open microhabitat) with each patch containing ten pieces of chick mixed in 16 l sand. Patches were provisioned before dusk, and the GUDs collected at dawn. The foragers were identified up to specific level from tracks around a patch. These experiments were carried out for three consecutive nights per moon phase, during the first summer month, and for two consecutive nights per moon phase in the following month.
Fox GUD data from the wild were first checked for normality using a one sample KS-test, Lilliefors option, and also checked for homogeneity of variance by viewing residuals against estimated figures. Post-Hoc tests were carried out using Tukey's HSD test. Activity data were analyzed using SYSTAT 7.0 (Wilkinson et al. 1992).
Foxes and their predator activity were quantified in the two habitats using sand tracking. Before dusk, the substrate of a 1 km long and 80 cm wide tracking transect was smoothed in each habitat. For making the transects in sand we used a squeegee, while in the loess we used a 1.5-m long metal pole wrapped with barbed wire (which helped to break the hard crust and to create a loose, relatively smooth surface) dragged behind a vehicle. Transects were smoothed each evening and activity (numbers of track crossings) recorded the following dawn. In these transects we also recorded the shortest distance between a line of fox tracks and the nearest bush (a perennial shrub, at least 1 m high and 0.5 m wide). Any fox tracks more than 3 m away from a bush were considered to be in the open. These transect data were collected on the same nights as the patch use experiment. Transects were located at least 500 m from the GUD patches to avoid affecting the experiment.
Results
Diminishing returns and equalization of GUDs
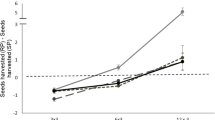
Fox harvest rates, i.e., the amounts of food harvested versus the cumulative time spent in the resource patch, showed a significant positive linear relationship (r 2 = 0.496, P < 0.001, F = 36.405, N = 39), but showed a stronger quadratic fit (r 2 = 0.902, P < 0.0001, F = 161.6, N = 39; Fig. 1). The F of improvement from a linear to a quadratic fit is highly significant (P < 0.001). The slopes of the harvest rate curves decreased as the time spent foraging increased (Fig. 1), indicating that the foxes experience diminishing returns from their harvest as they increased their foraging time (Charnov 1976).
The foxes did not perfectly equalize GUDs among pairs of patches. They only tended to equalize GUDs. The average ratios of GUDs between rich and poor patches was 1.7, and differed significantly (t 10,0.05 = 2.861, P = 0.017) from the initial ratio of 2. This significant difference suggests that foxes did devote more time/effort to exploiting the richer patch, leaving it with a lower GUD, and resulting in GUD ratios lower than 2. Under-use of rich patches suggests that foxes did not have perfect information about patch quality. Perfect information would have resulted in GUD ratios not differing from 1 (Valone and Brown 1989). Nonetheless, they can assess patch quality and exploit time accordingly.
Foraging behavior of foxes and their predators/competitors
Moon phase affected patch use by foxes. Fox GUDs were significantly affected by moon phase only in the 2nd year, but in both years similar trends were recorded (ANOVA, repeated measures, 1999: MS = 19.96, F 3,39 = 2.38, P = 0.21; 2000: MS = 21.112, F 3,84 = 6.34, P = 0.05). GUDs on new and waning nights were significantly lower than those during the waxing phase (Tukey HSD post-hoc test, P = 0.02 and P = 0.016, respectively).
Moon phase similarly affected activity. Moon phase had a significant effect on fox activity as measured by tracking plots in both years (Multi-way contingency table, Log-Linear Model, 1999: χ2 = 16.36, P = 0.001; 2000: χ2 = 8.36, P = 0.015). Fox activity during both years was significantly higher during new moon compared to full moon [Mann–Whitney tests (Bonferroni adjusted), 1999: u = 46, P = 0.04; 2000: u = 150.5, P = 0.05, Fig. 2a]. In 2000, a significant difference was also found between the new and waning half moon (u = 52, P = 0.04).
Hyena activity was also affected by moon phase but, in contrast to the foxes, hyenas were least active during new moon (Fig. 2b). In 1999, although moon phase had a significant effect on hyena activity (Multi-way contingency table, Log-Linear Model, G = 13.76, P = 0.001), no differences were found between moon phases using t-tests. In 2000, however, levels of activity during new moon were significantly lower than during a full moon (Mann–Whitney, u = 48, P = 0.03; Fig. 2b). Wolf activity data were scarce and hence are not presented in our results.
Foxes responded less strongly to habitat. Fox GUDs did not differ significantly between the sandy and loess habitats in either year (ANOVA, repeated measures, 1999: MS = 0.287, F 1,13 = 0.05, P = 0.82; 2000: MS = 9.472, F 1,28 = 4.56, P = 0.1). None of the interactions between habitat and other factors were found to be significant. However, fox activity did differ between habitats. In 1999, foxes had nearly double the activity in the sandy habitat compared to that in the loess (Multi-way contingency table, Log-Linear Model, G = 9.96, P < 0.001, mean number of crossings per night ± SE. Sand: 2.54 ± 0.49, Loess: 1.29 ± 0.31; n = 24). This trend did not exist in 2000, when foxes did not show any habitat preference (Multi-way contingency table, Log-Linear Model, χ2 = 0.02, P = 0.9). With regards to predators, there was no interaction of habitat with other factors in either year. Hyena activity was significantly different between the two habitats only in 2000 [Log-Linear Model, 1999: G = 0.6, P = 0.43; 2000: G = 7.1, P = 0.007; mean number of crossings per night ± SE (in 2000). Sand: 0.35 ± 0.11; n = 26, Loess: 0.80 ± 0.33; n = 30], with the hyenas being more active in the loess. No interaction was found with other factors.
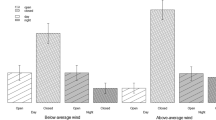
Microhabitat had an effect on the fox GUD only in 1999, with lower GUDs in the bush (ANOVA, repeated measures, 1999: MS = 0.149, F 1,13 = 23.96, P = 0.008; 2000: MS = 0.034, F 1,28 = 0.09, P = 0.78). A significant interaction between moon phase and microhabitat was also found in 1999 (ANOVA, repeated measures, MS = 0.1, F 3,39 = 16.24, P = 0.01). The most significant difference in GUDs between the two microhabitats was observed during waning phase, when foxes had higher GUDs in the open. Foxes and hyenas were both significantly more active in the open compared to in the bush in both years (Multi-way contingency table, Log-Linear Model, (1999 Fox: G = 6.33, P = 0.05; Hyena: G = 9.01, P = 0.002), (2000 Fox: G = 17.56, P < 0.001; Hyena: G = 16.31, P < 0.001). No interaction was found between microhabitat and other factors.
Surprisingly, the presence of the predator cue of wolf scats led foxes to leave patches at lower GUDs (ANOVA, repeated measures, MS = 9.355, F 1,28 = 8.80, P = 0.04). There was a significant interaction between predator cue and illumination on the fox GUDs (ANOVA, repeated measures, MS = 9.394, F 3,84 = 8.84, P = 0.03; Fig. 3). When wolf scats were absent, GUDs were relatively high across different moon phases, but when present there was a significant difference in GUDs across the different moon phases, with lower GUDs on dark nights (new and waning half moon).
To summarize, the patch use behavior of the red fox is consistent with the assumptions of the optimal patch use model (Brown 1988, 1992), in that they experienced diminishing returns (as evidenced by a quadratic fit) while foraging in assay patches. Foxes were unable to determine patch quality accurately and hence could not entirely equalize GUDs between a pair of rich and poor patches, but they did put more effort in foraging from the richer patch and tended to equalize GUDs. This supports the notion that foxes use a QHR rule for patch exploitation. These results allowed us to apply the method to foxes in the field. Patch use behavior of wild foxes did not differ between habitats, but foxes were more active in the sandy habitat (during 1999) compared to the loess habitat. They were also more active in open microhabitats, but had lower GUDs in the bush. Patch use behavior of foxes was significantly affected by moonlight, with the lowest GUDs (maximum harvest) during the waning and new phase. Foxes were also most active during new moon nights compared to full moon nights. Hyenas on the other hand were more active in the loess, and they too preferred open areas. Hyena activity was also significantly affected by moonlight levels, with lowest activity during the new moon. These data suggest that foxes avoid hyenas, and perceive a foraging cost arising from risk from hyenas. Finally, the presence of predator cue had a significant effect on the patch use decisions of foxes, with lower GUDs in patches where the cue was present.
Discussion
Here we showed that predators, too, conform to the assumptions of optimal patch use theory. First, foxes tended to equalize GUDs between patches of different initial densities, supporting a QHR patch departure strategy as opposed to a fixed number or fixed time strategy (Valone and Brown 1989). Although foxes were unable to assess patch quality with complete accuracy, they still spent more time/effort in richer patches. Their under-exploitation of richer patches (an average GUD ratio of 1.7 between rich and poor patch), suggests the use of Bayesian sampling to estimate QHR (Iwasa et al. 1981; Valone and Brown 1989). This implies that a fox perceives its environment to be heterogeneous, has an a priori expectation of patch quality, and updates its estimate of patch quality while foraging from it. The information that the red fox acquires about patch quality may not be complete, but it is enough to enable it to make the optimal foraging decisions regarding the amount of effort and time it should devote to a patch.
Second, the significant F of improvement obtained by adding the quadratic term to a linear model for harvest rates demonstrates that foxes experienced diminishing returns while exploiting a depletable food patch. Since foxes followed a QHR rule and experienced diminishing returns in our assay patches, we applied Brown’s (1988) patch use model for understanding their foraging behavior in a risky world.
Perceived risk of predation gives rise to a foraging cost that can be measured both qualitatively and quantitatively. A forager’s GUD, its QHR, and its activity patterns across time and space can all provide us with estimates that help us better understand the costs and consequences of predation risk (Brown and Kotler 2004). Foxes responded most strongly to changing risk factors during our study. Low GUDs along with the higher activity of foxes on darker nights reveal that the foxes perceived moonless nights to be safer. Increased activity on darker nights when hyenas were least active allowed foxes to avoid these predators and reduce their risk. Higher fox activity (along with high GUDs) during full moon nights would have suggested higher missed opportunity costs (MOC) of foraging (Brown 1988), i.e., foxes devoted more time to alternative activities, such as hunting, maintaining territories, etc., than to foraging in their assay patches. That did not happen. Instead, we found reduced fox activity and high GUDs during full moon nights, indicating higher costs of predation on these nights.
These results are not consistent with foxes responding to changing resource availability. Since several studies (e.g., Kotler et al. 2002, 2004) have shown reduced rodent activity during moonlit nights (and higher activity on dark nights), one might guess that the high GUDs and reduced fox activity during moonlit nights is a result of foxes adjusting their behavior to changing prey availability. However, this alternative hypothesis would actually predict lower GUDs on bright nights because the lower hunting success of foxes should increase their marginal value of energy and thereby decease their cost of predation [the cost of predation is given by μ · F/(∂F/∂e), where μ is the risk of predation, F is survivor’s fitness, and (∂F/∂e) is the marginal value of energy, Brown 1988]. Also, since desert rodents constitute only 26% of the diet of red foxes in our field site (Chris Dickman and B.P.K., unpublished data), it is less likely that the foxes will synchronize their activity completely with that of the rodents, i.e., being least active during the full moon. Thus, taken together, our GUD and activity results suggest that foxes reduce predation risk by shifting activity to darker nights, a result similar to that found in other taxa such as porcupines (Brown and Alkon 1990) and scorpions (Skutelsky 1996).
Intraguild predation (Sih et al. 1985) can give rise to temporal and/or spatial segregation that helps reduce risk of predation and promote coexistence among sympatric carnivores. In our system, we find some evidence for this. Foxes reduced their chances of interaction with hyenas by being less active in the loess habitat where hyenas are most active. This should help reduce the encounter rate with predators and hence the risk. Similar active avoidance of the habitats frequented by larger predators has been recorded in other systems, e.g., foxes avoid areas extensively used by lynxes (Fedriani et al. 1999) and coyotes (Voigt and Earle 1983), and coyotes avoid areas used by wolves (Berger and Gese 2007).
Interestingly, foxes showed similar GUDs in sand and loess. Equal GUDs might signify similar costs of foraging in the two habitats. The lower activity of foxes in the loess suggests that only some foxes are willing to take more risk and forage there. This indicates some form of density dependence, where foxes adjust their density between the two habitats in order to equalize fitness and GUDs.
Foxes perceived less risk from predators when foraging in the bush microhabitat where they had lower GUDs than in the open. Foraging close to bushes may help reduce the chances of being detected by their predators, thus reducing predation risk. This anti-predator response is similar to that observed in many desert rodents (see Kotler et al. 2002). Interestingly, even though foxes preferred foraging near bushes, they were more active in the open. Open terrain may have allowed them clearer sightlines, thus increasing their predator detection ability. Detecting a predator early may be crucial for a prey, as it can give it enough time to escape and thus survive. Alternatively, the higher activity logged in the open in tracking plots may reflect the use of the open to travel between patches.
Prey often evaluate predation risk using direct cues (e.g., feces and urine) and respond by either dispersing or aggregating (Dickman 1992). We used wolf scats, a cue that is easily manipulated. The highly significant interaction between predator cue and moon phase (Fig. 3) yet again reiterates the importance of moonlight on the foraging decisions of the red foxes. However, contrary to our predictions, foxes had lower GUDs in stations with cue. The low GUDs could be because the feces provided information to the foxes regarding its predator’s whereabouts, i.e., an older scat may indicate that a predator had visited, but has likely moved on. Another possibility is that, in the presence of hyenas, foxes prefer to be closer to wolves. Since hyenas are more common in the area, they are more of a threat to the foxes. In this situation, the foxes may possibly benefit by staying closer to the lesser threat, i.e., the wolves, because wolves may help deter hyenas.
Under natural conditions foxes may not often forage in patches that resemble those used in our experiments. However, the relevance of the GUD results does not depend on how closely these patches resemble a natural patch. Rather, it depends on whether GUDs can reveal the costs and benefits of foraging. For this, foxes need to experience diminishing returns while exploiting the artificial patches, assess patch quality, and use a QHR patch departure. The foxes do all of these things.
Though our patches more closely resemble scavenging behavior than hunting rodent prey, our results may still be relevant to these situations. While hunting for rodent prey in a natural resource patch (necessarily much larger than our artificial patches), foxes capture prey infrequently. As such, they cannot depend on harvest rates per se when deciding when to depart the patch. If instead they can estimate capture probabilities, they can then base their patch departure on the expected patch value. This is also a QHR rule of sorts. Consequently, foraging behavior as revealed by GUDs should also reflect the costs and benefits faced by foxes hunting rodents.
Regardless of the nature of the resource patch exploited by foxes, the salient points of this study are that: (1) predators often forage in a context of diminishing returns, be it in regards to actual harvest rates (as in our experiments) or expectations of prey capture; and (2) foraging decisions of meso-predators are influenced by risk from their own predators, regardless of the food they are exploiting. Our simplification of the system, by offering non-responsive prey in food patches, allowed us to apply an optimal patch use model that has been successfully tested with several organisms.
Studying the behavior of small carnivores based on foraging theory is easy compared to techniques such as radio telemetry, measuring predation rates, etc., but, more than that, it allows the foragers to tell us how they perceive their own environment. Measures of patch use and activity are in fact behavioral indicators that yield information not only on behavior, but also on population well-being (Kotler et al. 2007). Behavioral responses are, by their nature, rapid, and so reflect the current situation better than responses reflected in population dynamics. Thus they can be more effective in helping us understand trophic interactions in any given system. Evidence from different systems, showing that risk from larger predators affects the movement and foraging decisions of meso-predators, suggests a need for similar experiments to help understand ecological and behavioral interactions at a range of trophic levels.
References
Berger KM, Gese EM (2007) Does interference competition with wolves limit the distribution and abundance of coyotes? J Anim Ecol 76:1075–1085
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Brown JS (1992) Patch use under predation risk: I. Models and predictions. Ann Zool Fenn 29:301–309
Brown JS, Alkon PU (1990) Testing values of crested porcupine habitats by experimental food patches. Oecologia 83:512–518
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014
Brown JS, Laundre JW, Gurung M (1999) The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal 80:385–399
Case TJ, Gilpin ME (1974) Interference competition and niche theory. Proc Natl Acad Sci USA 71:3073–3077
Charnov EL (1976) Optimal foraging, marginal value theorem. Theor Popul Biol 9:129–136
Dickman CR (1992) Predation and habitat shift in the house mouse, Mus domesticus. Ecology 73:313–322
Dickman CR, Doncaster CP (1984) Responses of small mammals to red fox (Vulpes vulpes) odour. J Zool 204:251–253
Donadio E, Buskirk SW (2006) Diet, morphology and interspecific killing in carnivore. Am Nat 167:524–536
Fedriani JM, Palomares F, Delibes M (1999) Niche relations among three sympatric Mediterranean carnivores. Oecologia 121:138–148
Helldin JO, Liberg O, Glöersen G (2006) Lynx (Lynx lynx) killing red foxes (Vulpes vulpes) in boreal Sweden—frequency and population effects. J Zool 270:657–663
Holt RD, Polis GA (1997) A theoretical framework for intraguild predation. Am Nat 149:745–764
Iwasa Y, Higashi M, Yamamura N (1981) Prey distribution as a factor determining the choice of optimal foraging strategy. Am Nat 117:710–723
Kotler BP, Brown JS (1990) Harvest rates of two gerbilline rodents. J Mammal 71:591–596
Kotler BP, Brown JS, Hasson O (1991) Factors affecting gerbil foraging behavior and rates of owl predation. Ecology 72:2249–2260
Kotler BP, Brown JS, Dall SRX, Gresser S, Ganey D, Bouskila A (2002) Foraging games between owls and gerbils: temporal dynamics of resource depletion and apprehension in gerbils. Evol Ecol Res 4:495–518
Kotler BP, Brown JS, Bouskila A, Mukherjee S, Goldberg T (2004) Foraging games between gerbils and their predators: seasonal changes in schedules of activity and apprehension. Isr J Zool 50:255–271
Kotler BP, Brown JS, Morris DW (2007) Behavioral indicators and conservation: wielding “the biologist’s tricorder”. Isr J Ecol Evol 53:237–244
Kruuk H (1972) The spotted hyena. University of Chicago Press, Chicago
Luttbeg B, Sih A (2004) Predator and prey habitat selection games: the effects of how prey balance foraging and predation risk. Isr J Zool 50:233–254
Mendelssohn H, Yom-Tov Y (1999) Fauna plaestina, mammalia of Israel. The Israel Academy of Science and Humanities, Jerusalem
Polis GA, Holt RD (1992) Intraguild predation—the dynamics of complex trophic interactions. Trends Ecol Evol 7:151–154
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Ralls K, White PJ (1995) Predation on San Joaquin kit foxes by larger canids. J Mammal 76:723–729
Rosenheim JA (1998) Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol 43:421–447
Rosenheim JA (2004) Top predators constrain the habitat selection game played by intermediate predators and their prey. Isr J Zool 50:129–138
Rosenzweig ML (1966) Community structure in sympatric carnivore. J Mammal 47:602–612
Rosenzweig ML (1973) Exploitation in 3 trophic levels. Am Nat 107:275–294
Sih A, Crowley P, McPeek M, Petranka J, Strohmeier K (1985) Predation, competition, and prey communities: a review of field experiments. Annu Rev Ecol Syst 16:269–311
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Skutelsky O (1996) Predation risk and state-dependent foraging in scorpions: effects of moonlight on foraging in the scorpion Buthus occitanus. Anim Behav 52:49–57
Thurber JM, Peterson RO, Woolington JD, Vucetich JA (1992) Coyote coexistence with wolves on the Kenai Peninsula, Alaska. Can J Zool 70:2494–2498
Valone TJ, Brown JS (1989) Measuring patch assessment abilities of Desert granivores. Ecology 70:1800–1810
Voigt DR, Earle BD (1983) Avoidance of coyotes by red fox families. J Wildl Manage 47:852–857
Wilkinson L, Hill M, Welna JP, Birkenbeuel GK (1992) SYSTAT for windows: statistics, version 5. SYSTAT, Evanston, IL
Acknowledgments
Thanks to Beer Sheva Zoo, Aviv (Ranger, Holot Mashabim Nature Reserve) and the Israel Nature and National Parks Protection Authority. We would like to thank Amos Bouskila for his valuable advice at all stages of this study. We also thank Yaron Ziv and Aziz Subach for their ideas and suggestions. We thank Toby Goldberg for her assistance during fieldwork. M.Z. would like to thank Yaffa and Itay for their support and immense technical help. We would also like to thank Joel Brown, Barney Luttbeg and an anonymous reviewer for their valuable comments. The experiments comply with the current laws of Israel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Rights and permissions
About this article
Cite this article
Mukherjee, S., Zelcer, M. & Kotler, B.P. Patch use in time and space for a meso-predator in a risky world. Oecologia 159, 661–668 (2009). https://doi.org/10.1007/s00442-008-1243-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1243-3