Abstract
The impact of alien species on native organisms is a cause for concern worldwide, with biological invasions commonplace today. Suppression efforts targeting many invasive species have included introductions of biological control agents. The numerous releases of biological control agents in the Hawaiian archipelago have resulted in considerable concern for non-target impacts, due to high levels of non-target parasitism observed to occur in some cases. This study investigated the impact of introduced Hymenoptera parasitoids on a Hawaiian moth. The endemic Hawaiian moth Udea stellata (Butler) has seven alien parasitoids associated with it, two purposely introduced, three adventive, and two of uncertain origin. The objective of this study was to determine the relative contribution of the seven parasitoid species to the population dynamics of U. stellata by constructing partial life tables. Marginal attack rates and associated k-values were calculated to allow comparison of mortality factors between experimental sites. Sentinel larvae were deployed on potted host plants and left in the field for 3-day intervals in open and exclusion treatments. The factors that contributed to total mortality in the open treatment were: disappearance (42.1%), death due to unknown reasons during rearing (16.5%) and parasitism (4.9%). The open treatment incurred significantly higher larval disappearance compared to the exclusion treatment (7.8%), which suggests that in large part disappearance is the result of predation. Adventive parasitoids inflicted greater total larval mortality attributable to parasitism (97.0%) than purposely introduced species (3.0%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
International and interregional commerce continues to break down biogeographical boundaries (Loope and Howarth 2003), and this is accelerating the rate of biological invasions to a degree without precedent. The practice of classic biological control for pest management has been commonly recognized as an effective suppression method for invasive species, and its use was encouraged to reduce dependence on insecticides for the management of invasive insect pests. Biological control is an important component of any integrated pest management program as it is not only an option to control invasive species in agricultural settings but is also a tool for conservation, when it targets invasive species that threaten native species and natural habitats (Hoddle 2004; Messing and Wright 2006).
Practitioners of classic biological control have traditionally regarded their method as environmentally safe (van den Bosch and Messenger 1973; De Bach 1976; Simmonds and Bennett 1977; Caltagirone and Huffaker 1980) and even though concerns about the potential negative effects of purposely introduced species on endemic fauna were expressed more than a century ago in Hawaii by Perkins (1897), it is only since the 1980s that there has been an increase in concerns about the environmental impact of introduced biocontrol agents on native species in the USA (Howarth 1983, 1991; Gagne and Howarth 1985; Simberloff 1992; Simberloff and Stiling 1996, Henneman and Memmott 2001).
Retrospective studies on biological control introductions provide an important tool in the evaluation of potential non-target effects of future biological control programs. They help build case histories that can provide patterns to aid the identification of key biological and ecological factors that need to be investigated to provide a robust estimate of the candidate’s non-target potential (Louda et al. 2003). Adventive species (species that have been accidentally introduced to a new area) provide a further set of species for developing a greater understanding of non-target impacts, because they also offer opportunities to study impacts of new introductions, albeit accidental, upon indigenous species.
Hawaii provides an excellent set of circumstances to study non-target effects from biological control as well as accidental introductions. The Hawaii archipelago is home to a greater proportion of endemic species than any other place of similar size on earth (Kaneshiro 1995), has a steady rate of arrival of non-indigenous species, and has a long history of biological control introductions; more than 700 species released in the past 100 years (Funasaki et al. 1988). Hawaii has also been the center of controversy regarding non-target impacts on native and desirable species, and in some cases it has been suggested that extinctions have resulted from mortality caused by purposefully introduced species (Howarth 1983, 1991; Gagné and Howarth 1985). However, the actual impacts of introduced biological control agents on indigenous species have seldom been quantified.
The species used in our investigation, Udea stellata Butler (Lepidoptera: Crambidae), is a multivoltine species endemic to Hawaii and is widely distributed throughout the state. The larval stages of this moth feed on the endemic host plants Pipturus spp. (Urticaceae) (common name, mamaki) which occur in mesic forests, under variable conditions in terms of canopy cover, disturbance level, presence of invasive plant species and elevation. A separate study (Kaufman 2008 ) assessed the parasitoid guild associated with U. stellata and quantified field parasitism rates at different sites. Results from these studies showed that the parasitoid guild associated with U. stellata larvae includes seven koinobiont solitary endoparasitoids, all of them polyphagous species. Three of the parasitoids are of adventive origin: Casinaria infesta (Cresson), Trathala flavoorbitalis (Cameron) and Triclistus nr. aitkeni; two species were purposely introduced for biological control purposes: Meteorus laphygmae (Viereck) and Cotesia marginiventris (Cresson); and two are of uncertain origin. Diadegma blackburni (Cameron), listed as adventive (Nishida 2002) is possibly endemic to Hawaii (Oboyski et al. 2004), and Pristomerus hawaiiensis (Perkins) listed as endemic (Nishida 2002) may be adventive to the islands (Fullaway and Kraus 1945; Stein 1983). C. marginiventris and M. laphygmae were introduced to Hawaii in 1942 to control the sugar cane pest Spodoptera exempta (Walker) (Nishida 2002). All adventive species in this study were first observed in Hawaii before 1942 except for T. nr. aitkeni, which was only detected in the last decade.
In the 2 years of surveys of field parasitism (apparent mortality) reported above, 27.55% of the total larvae collected (or 42.92% of the larvae that reached adulthood) yielded parasitoids, and parasitism rates varied significantly across sites (Kaufman 2008). Adventive parasitoids, notably T. flavoorbitalis, rather than purposely introduced species inflicted the majority of the field parasitism observed in samples. It was also noted that in low and low to medium elevations (below 850 m) the parasitoid assemblage was dominated by adventive species, whereas purposely introduced species where detected only from sites above 850 m.
Such faunistic surveys provide good background information on parasitoid guild composition, levels of parasitism of samples taken, and seasonal trends in different locations. However, caution should be exercised when interpreting results from field parasitism since these data do not often provide an effective measure of parasitoid impact at the host population level, such as information on density of the host and the ecological role of the parasitoids in the population dynamics of the target or non-target hosts (Van Driesche et al. 1991; Duan and Messing 2000). Field surveys also do not provide information on the role of parasitism relative to other mortality factors influencing fluctuations in population densities. To date, there are some detailed studies quantifying to what extent non-target attacks are impacting the populations of non-target species (Duan et al. 1998; Boettner et al. (2000); Duan and Messing 2000; Benson et al. 2003a, b; Barron et al. 2003; Van Driesche et al. 2004; Johnson et al. 2005). This study aims to determine the relative contribution of parasitoid species with respect to other mortality factors, to the population dynamics of U. stellata by constructing partial life tables, estimating marginal attack rates and their associated k-values, using artificial cohorts (Carey 2001) in the field. Life table studies provide important information on the contribution of different mortality factors for life stages over a generation and among generations, and therefore for an understanding of the ecological impact of the mortality factors in insect populations. The calculation of marginal attack rates and associated k-values allows comparison of mortality factors between experimental sites.
Materials and methods
Study sites
Field experiments were conducted at six locations on three of the Hawaiian Islands from August 2005 to October 2006. The Kokee site (22°7′54″N, 159°37′54″W, elevation 981 m), is located at the Ditch Trail at the Kokee State Park, on the island of Kauai. Three sites were located on the island of Oahu: Kunia (21°27′46″N, 158°5′44″W, elevation 550 m) and Palikea (21°24′46″N, 158°5′58″W, elevation 781 m) are located in the Waianae Mountains and are managed by the Nature Conservancy of Hawaii, and Tantalus (21°19′48″N, 157°49′21″W, elevation 460 m) is located near Honolulu. The last two sites were located on the island of Hawaii, Kipuka Ki (19°26′36″ N, 155°18′59″W, elevation 1315 m) at the Hawaii Volcanoes National Park, and Olaa (19°28′25″N, 155°15′40″W, elevation 1245 m) which is located inside the University of Hawaii Volcano Experimental Station adjacent to the Olaa forest. Sites varied in many environmental features such as elevation, level of disturbance by alien species, type of overstory, percentage of overstory and type of understory. All study sites had naturally occurring mamaki plants. We made an effort to deploy our sentinel plants in the vicinity of these plants.
Plant material
Potted Pipturus spp., locally known as mamaki, grown from seed were used as substrate for sentinel larvae exposed in the field. Plants for each experiment were grown from seeds that were collected from the respective island in 2004 and 2005. Plants used at all sites were planted in 3.8-l pots and were approximately 50-cm tall when they were deployed at study sites.
We chose to use potted plants instead of wild plants in the field to avoid unobserved “recruitment” of wild larvae that could bias the data collected. Plants were grown at Kauai Research experimental station on Kauai, Gilmore Hall greenhouse facility at the University of Hawaii at Manoa on Oahu, and at the USDA Forest Service facility at Hawaii Volcanoes National Park on Hawaii.
Insect material
To ensure they were not parasitized before field exposure, larvae of U. stellata were reared in the laboratory [22°C (±2) and ~62% (±10) relative humidity (RH)] on mamaki plants, until exposed to create artificial “cohorts” in the field. Since the experiments were conducted on three different islands, we maintained three colonies of U. stellata, each initiated from field-collected larvae from the respective island; this was to ensure parasitism or other mortality factors measured in each study site were not influenced by the origin of the colonies. To produce larvae of specific instars, we caged host plants with moths that emerged from field-collected larvae and replaced oviposition plants every 2–3 days. Larvae emerging from eggs laid were used to initiate colonies. From quantification of larval morphometrics in the laboratory, it is known that U. stellata undergoes six larval stages (Kaufman and Wright, in press). All six larval instars of U. stellata were used for field experiments. Stages were differentiated by head capsule diameter (Kaufman and Wright, in press).
Field experiments
We deployed sentinel larvae on potted host plants in the field, where they were exposed to parasitoids and other sources of mortality for a 3-day interval. This interval was based on an estimate of the time that larvae take to molt to the next instar under laboratory conditions (Kaufman and Wright, in press). Potted plants with larvae were randomly placed into one of two treatments: exposed to natural enemies (open treatment) and caged to exclude natural enemies (exclusion treatment). Exclusion of predators and larval parasitoids was accomplished by placing a fine mesh bag over the plants (covering foliage and soil). The exclusion treatment served to estimate predation levels and as a control for mortality due to transportation and stress during infestation of the plants. A total of nine deployments were conducted in a total of six sites, and each of them considered to constitute a “generation” (Table 3).
To quantify mortality at each larval instar, we exposed groups of larvae of all instars at the same time, at a density of four larvae of similar instar per plant (comparable to the density in the Palikea site, which was the one with the highest density of larvae per plant). Instars were kept separate on different plants. Twenty to 40 larvae of each instar were exposed at deployment time (deployment time refers to a particular time × site combination) in the open treatment, and four to eight larvae in the exclusion treatment. The differences in number of larvae exposed at a particular time were dictated by the availability of larvae. Plants in the open and enclosed treatments were randomly placed within natural stands of mamaki at all sites. The total area where sentinel plants were placed in the field was between 30 and 40 m2. Since the fifth and sixth instar larvae are very mobile, plastic basins painted with fluon at the rim and provisioned with small drainage holes, were placed under each pot to facilitate recovery of larvae, in case they attempted to migrate to pupation sites. After 3 days sentinel plants were inspected in the field, and larvae found were retrieved and returned to the laboratory. Larvae that were not found during the retrieval were classed as “disappeared”. Once returned to the laboratory (22°C and ~62% RH), each larva was placed individually into a labeled plastic container. Feces and old plant material were removed and new plant material was added to the containers every day or every other day depending on the rate of feeding. All larvae were reared to the adult stage, or until they died or parasitoids emerged. Emerging parasitoids were pinned for identification. Specimens were identified by using unpublished keys to the Hawaiian Ichneumonidae (Beardsley, unpublished) and also by comparing adult voucher specimens with specimens at the Hawaii Department of Agriculture insect collection, University of Hawaii Insect Museum and Bishop Museum. Identifications were confirmed by Dr David Wahl at the American Entomological Institute. Voucher specimens are deposited at the American Entomological Institute, Gainesville, Florida.
Partial life table construction
The number of larvae deployed at each stage and mortality data for each stage for both treatments were used to construct partial life tables. Partial life tables were constructed using the method described by Morris and Miller (1954) and Morris (1963), where l x denotes the number of larvae that enter each stage (in our case numbers deployed at each stage), d x F is the mortality factor acting during each stage, and d x the numbers dying during each stage. Proportion dying at each larval stage or q x (also known as apparent mortality) was obtained by diving d x by the corresponding l x .
Calculation of marginal attack rates and associated k-values
We used life table data to calculate marginal attack rates, k-values and to conduct key factor analysis.
Marginal attack rates
Since assessing the strength of individual mortality factors which act contemporaneously is not usually possible from simple analysis of numbers observed dying, we calculated marginal attack rates, defined as the levels of mortality that would have occurred if the agent had acted alone (Royama 1981; Bellows et al. 1992; Elkinton et al. 1992). Marginal attack rates were calculated as:
where m i is marginal probability of attack from the ith cause, q i is apparent mortality from the ith cause and q is mortality rate from all causes combined (Elkinton et al. 1992).
k-values
“Killing powers” or k-values were estimated as the negative logarithm of the estimated proportion surviving in each stage:
where k i is the k-value for the ith cause. The sum of all successive mortality factors (k 1 − k i ) equals the total generational mortality (K). The major advantage of k-values as compared to percentages of organisms dying is that k-values are additive: the k-value of a combination of independent mortality processes is equal to the sum of k-values for individual processes.
Proportion of larvae “disappearing” or dying from unknown causes was compared between exposed and exclusion treatments using two-way contingency table analysis, pooling data from all exposures. The null hypothesis that disappearance and mortality from unknown causes were not associated with exclusion or exposure was tested.
Results
The mortality factors that contributed most to the total generation mortality were: disappearance, unknown cause of mortality during rearing, and parasitism. From the 1,975 larvae deployed across all sites in the open treatments, we were able to retrieve 1,144 (57.9%) larvae, while 831 (42.1%) disappeared. Of the larvae retrieved, 28.5% (326/1,144) died due to unknown causes during rearing, 8.5% (97/1,144) were parasitized and 63.0% (721/1,144) completed their life cycle and emerged as moths. Six parasitoid species were reared from the larvae recovered. Of the larvae parasitized, 1.0% (1/97) were P. hawaiiensis, 1.0% (1/97) Cotesia marginiventris, 2.1% (2/97) M. laphygmae, 7.2% (7/97) Casinaria infesta, 40.2% (39/97) Trathala flavoorbitalis and 48.5% (47/97) Triclistus nr. aitkeni.
In the exclusion treatment we deployed a total of 295 larvae across all sites. Of these, 23 (7.8%) disappeared and 272 (92.2%) were recovered. Since this treatment excluded predators, and Udea larvae are not cannibalistic, it is reasonable to say that our search efficiency exceeded 90%. Of those recovered, 16.2% (44/272) died for indeterminate reasons during rearing and 83.8% (228/272) emerged as moths. The open treatments incurred significantly higher “disappearance” compared to the exclusion treatment (χ2 = 127.06; df = 1; P < 0.0001). Death due to unknown causes was not significantly different in the exclusion treatment compared to the open treatment (χ2 = 0.367; df = 1; P = 0.545). In the exclusion treatment, total mortality, as well as the impact of individual mortality factors decreased with increasing larval stage. The major mortality factor for all larval instars in exclusion cages was death by unknown causes during rearing. This unknown death can be attributed to natural mortality (which was comparable to natural mortality in our laboratory colony) but was possibly also due to unknown bacterial and fungal infections.
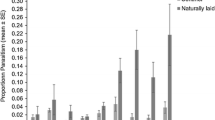
Life table data, marginal attack rates and k-values across all sites by larval stage for the open and exclusion treatments are presented in Table 1. In the open treatment, the major mortality factor across all sites in all larval stages was disappearance, which accounted for >57% of the mortality. The highest observed k-values due to disappearance were in the first and sixth instar (0.366–0.317, respectively). The highest attack rates and k-values due to parasitism were incurred in the fourth and fifth larval instar (0.045–0.078, respectively) in which T. nr. aitkeni had the highest individual k-values (0.031 and 0.064, respectively). Triclistus nr. aitkeni and Trathala flavoorbitalis were the species with the greatest k-values summed over all larval instars. Individual k-values for T. flavoorbitalis were the highest in second and third larval instar. The killing power due to parasitism by the purposely introduced species M. laphygmae and C. marginiventris summed across all six larval stages were 0.007 and 0.002, respectively, and together with P. hawaiiensis (k = 0.002), these were the species that contributed the least to the total mortality (K = 2.651).
Table 2 shows k-values attributable to parasitism for U. stellata per larval instar, by site for the open treatment. Not all k-values followed the same trend in different sites and not all parasitoid species were reared from all sites. In the Kauai study site, mortality due to T. nr. aitkeni had the greatest k-value attributable to parasitism (0.171). Palikea had the highest total mortality summed over all larval stages (3.4014) and also the highest killing power due to parasitism when summed over all parasitoid species (0.373); Olaa had the lowest total mortality (1.835) and Tantalus the lowest killing power due to parasitism (0.031). The purposely introduced parasitoid species were reared from the sites with highest elevation. M. laphygmae was reared from Kauai and Olaa and C. marginiventris from Kipuka Ki.
Partial life tables were also constructed for each of the nine deployment times (nine artificial generations). The k-values by mortality factor for each generation are presented in Table 3. Mortality due to disappearance had the greatest k-value by generation and also the greatest average k-value (k a = 1.8383) and therefore contributed the most to total generational mortality of larvae (K = 2.7086), followed by “dead by unknown causes” (k b = 0.6775). Although T. nr. aitkeni had the highest average k-value (k e = 0.0938) among the various parasitoids, its contribution was only the highest for generations 3, 8 and 9 (Kauai 2005, Kipuka Ki and Olaa, respectively), which did not correlate with the highest observed total mortality (K). T flavoorbitalis had the second highest average k-value (k c = 0.0716), and had the highest killing power in generations where K was also the highest (generations 1 and 2). Casinaria infesta, P. hawaiiensis, M. laphygmae and Cotesia marginiventris had the lowest contributions to total mortality in all generations.
Discussion
Larval disappearance (most probably due to predation) was consistently the most important mortality factor. This is consistent with other studies on insects that report high rates of disappearance attributable to predation and/or migration (Midega et al. 2005; Barron et al. 2003; Kellogg et al. 2003; Furlong et al (2004); Johnson et al. 2005). The significantly larger number of larvae recovered from the exclusion treatment (search efficiency was >90%) when compared to the open treatment, may be attributed to reduced predation and possibly reduced larval migration. The high rate of disappearance in the first larval instar was possibly mainly due to predation, whereas the high rate of disappearance in the last instar might have been also due to larval migration. This is supported by our field observations; at the time of larval exposure we tagged leaves where we placed the larvae and mostly recovered the first three instars on the same leaf, whereas fourth, fifth and sixth instar larvae were more frequently recovered from leaves other than the tagged ones, and occasionally we recovered these older larvae from the plastic basins below the plants. Active predation by Coccinellidae and spiders was observed.
This study attempted to evaluate the relative contribution of parasitism with respect to other sources of mortality as well as the impact of different parasitoids on U. stellata larvae. A previous study on parasitism rates of field-collected larvae (Kaufman 2008) reported apparently high parasitism rates (27.6% of all larvae collected or 42.9% of the larvae that reached adulthood). The present study, which employed rigorous demographic procedures, shows a low impact of parasitoid species on U. stellata larvae (percentage parasitism across all sites and all instars was 4.9%). Even though larvae that died during rearing were not dissected, comparison of mortality due to unknown causes between open and exclusion treatments showed no significant differences, suggesting that other causes such as natural mortality or stress during rearing, rather than parasitism, were the causal factors.
The number of species of adventive parasitoids outnumbers species that have been introduced intentionally worldwide, often by a factor of 10 or more (van Lenteren et al. 2006). In this study, mortality resulting from parasitism by adventive species played a more important role as a mortality factor than purposely introduced species in the study system. This is consistent with previous studies of non-target impacts in insect biological control (Duan and Messing 1996; Barron et al. 2003; Johnson et al. 2005) that similarly showed that adventive species had far more serious impacts than purposefully introduced species, and is consistent with our observations of parasitism of wild larvae where adventive species were dominant. C. marginiventris and M. laphygmae have been recorded from low to high altitude sites in Hawaii in malaise traps (Peck et al. 2008). Nevertheless, as in our survey data, these species were only reared from U. stellata at our least-disturbed high altitude sites (above 950 m), far from their original release areas and target habitat, perhaps due to the lack of their preferred target hosts at these sites, forcing them to exploit indigenous species.
Results from the present study contrasted with results from surveys of wild larvae (Kaufman 2008) in that Trathala flavoorbitalis was not the species that contributed the most to mortality due to parasitism, but Triclistus nr. aitkeni (48.5% of all parasitoid reared in this study). This was possibly due to over- and under-representation of certain larval stages during field surveys. This emphasizes the importance of interpreting results of field surveys with caution since they can potentially overestimate or underestimate the actual level of mortality in situations where susceptible stages are over- or under-sampled. Parasitism by Triclistus nr. aitkeni, was restricted to sites at higher elevations (above 980 m), whereas Trathala flavoorbitalis had the greatest contribution in low- to medium-elevation sites (below 800 m). These results are congruent with the previous study, where T. flavoorbitalis was also found to contribute the highest parasitism rates in low- to medium-altitude sites. From this and results from the study on field parasitism (Kaufman and Wright, in press), it is known that T. nr. aitkeni can parasitize second to sixth instar larvae and the observed pattern in parasitism is consistent with accumulative parasitism over larval stages; however, only larvae deployed from the fourth to sixth instar were parasitized in the present study. Results from both studies suggest that Triclistus nr. aitkeni prefers parasitizing later larval instars, perhaps to avoid competition with other parasitoid species that parasitize earlier instars (such as Trathala flavoorbitalis).
It is common to construct life tables for economically important pests in agricultural and forest settings, providing an important component in the understanding of the population dynamics of a species (Southwood 1978). The practical application of life table studies in agroecosystems is to help identify key mortality factors that can be manipulated to reduce pest population densities. In the case of non-target studies in the field of insect biological control, life table data have been used to assess the impact at the population level of species suspected to have experienced population declines due to attacks by purposely introduced species (Barron et al. 2003; Johnson et al. 2005). The application of such studies has been to the benefit of the practice of biological control itself, by providing quantitative data/evidence that non-target impacts are typically relatively small and build case histories that can help reduce negative impacts of current and future programs. Detailed life table studies that do not only focus on the role of a specific biological control agent but take into account the full complement of mortality factors that act on a population could also be used in the field of insect conservation, since they can help identify key mortality factors, susceptible stages in the life cycle, and susceptible sites in order to develop efficient conservation strategies (e.g., if exotic predators are identified as important mortality factors then measures for control of these predators could be part of the agenda for conservation of that specific species of concern). Studies of this nature will likely be difficult to conduct, as it will often be difficult to acquire adequate sample sizes from the field to trace individuals through a generation or to start colonies to create artificial cohorts of rare species.
Asquith and Miramontes (2001) examined the composition of the braconid and ichneumonid fauna collected in malaise traps over a 2-year period in a mesic forest in Kokee State Park on the island of Kauai, in an area adjacent to one of the study sites used in the current study. The majority of wasps collected in their traps were exotic rather than native species. The authors expressed concern since this site harbors a rich community of endemic species, and called for cessation of biological control introductions. The presence of purposefully introduced species in native areas is highly undesirable, but their presence alone is not evidence of attack on indigenous species, or measure of the severity of any such attack. Many non-native Lepidoptera species are also present and readily attracted to light traps (L. Kaufman, personal observation). Henneman and Memmott (2001) in their study in a remote site in the island of Kauai spanning a 2-year period, found that most of the parasitoid species associated with the immature stages of many native Lepidoptera species were purposely introduced, and suggested that the introduced species significantly altered food web structure. From their data it is evident that these purposely introduced species were using native species as hosts; however, it is difficult to translate this to the level of impact on the non-target species involved.
Most studies on non-target impact in the field of biological control have been done on species observed to have experienced serious population declines, and with clear evidence that these declines have been influenced by attacks of purposely introduced species. Attention has been concentrated on beneficial organisms, organisms of commercial, cultural, or aesthetic significance and some with conservation concern (Boettner et al. 2000; Kellogg et al. 2003; Babendreier et al. 2003; Babendreier and Bigler 2005). The field of biological control (and conservation) would benefit greatly if in addition to selecting a particular non-target species with obvious high rates of field parasitism, researchers would select non-target species with no such obvious evidence of non-target attacks. This study is one of the few addressing non-target impacts on a species that is not of special concern, with no previous report on severe attacks by introduced species, but on a species that is distributed across a wide range of ecological conditions. This kind of study offers the opportunity to make a more general case, and improve our understanding of the impact that introduced species can have on an “average” non-target host in a range of circumstances.
Similarly, a way to gain insights on what mediates the extent of impact on certain non-target species can be achieved by selecting particular purposely introduced species such as M. laphygmae, known to attack many non-target species in Hawaii (Funasaki et al. 1988; Henneman and Memmott 2001) with different rates of field parasitism by individual species, and address a broader question such as: how has M. laphygmae impacted non-target native species in Hawaii in a more general sense. To answer this, not only a particular species known to have high rate of field parasitism should be included in the study but also other non-target species without such evidence of high parasitism rates. This might give a more realistic picture of the role that purposely introduced species have played on non-target species, and build stronger case histories. One obstacle for this kind of investigation will be the lack of historical data, which makes it difficult to assess the current status of non-target species (Stiling and Simberloff 2000; Follett et al. 2000; Kellogg et al. 2003; Barron et al. 2003, 2004). Current parasitism rates in the field may not reflect accurately the parasitoid’s earlier parasitism rates when initially introduced, and original potential to destabilize non-target populations (Follett et al. 2000), as it is also possible that some non-target species might be absent from part of their original range or have gone totally extinct (Henneman and Memmott 2001).
Results of our study have shown that k-values for the different parasitoid species vary among sites that differ in disturbance and/or altitude. This study system provides the opportunity to investigate environmental factors associated with differences in level of impact of introduced parasitoids (Kaufman 2008). The marginal attack rates estimated in this study will provide quantitative data for the validation of risk assessment procedures (Wright et al. 2005).
References
Asquith A, Miramontes E (2001) Alien parasitoids in native forests: the Ichneumonidae wasp community in a Hawaiian rainforest. In: Lockwood J, Howarth F, Purcell M (eds) Balancing nature: assessing the impact of importing non-native biological control agents (an international perspective). Say, Entomologocal Society of America, Lanham, MD
Babendreier D, Bigler F (2005) How to assess non-target effects of polyphagous biological control agents: Trichogramma brassicae as a case study. Proceedings of the 2nd International Conference on Athropod Biological Control, Davos, Switzerland, September 2005, pp 603–610
Barron MC, Wratten SD, Barlow ND (2003) Non-target parasitism of the endemic New Zealand red admiral butterfly (Bassaris gonerilla) by the introduced biological control agent Pteromalus puparium. Biol Control 27:329–335
Babendreier D, Kuske S, Bigler F (2003) Non-target host acceptance and parasitism by Trichogramma brassicae (Hymenoptera: Trichogrammatidae) under laboratory conditions. Biol Control 26:128–138
Barron MC, Wratten SD, Barlow ND (2004) Phenology and parasitism of the red adminal butterfly Bassaris gonerilla (Lepidoptera: Nymphalidae). New Zeal J Ecol 28:105–111
Benson J, Pasquale A, Van Driesche R, Elkinton J (2003a) Assessment of risk posed by introduced braconid wasps to Pieris virginiventris, a native woodland butterfly in New England. Biol Control 26:83–93
Benson J, Van Driesche RG, Pasquale A, Elkinton J (2003b) Introduced braconid parasitoids and range reduction of a native butterfly in New England. Biol Control 28:197–213
Bellows TS Jr, Van Driesche RG, Elkinton JS (1992) Life-table construction and analysis in the evaluation of natural enemies. Annu Rev Entomol 37:587–614
Boettner GH, Elkinton JS, Boettner CJ (2000) Effects of a biological control introduction on three non-target native species of Saturniid moths. Consev Biol 14:1798–1806
Butler AG (1883) On a small series of Lepidoptera from the Hawaiian Islands. Entomol Mon Mag 19:179
Caltagirone LE, Huffaker CB (1980) Benefits and risks of using predators and parasites for controlling pests. Ecol Bull 31:103–109
Carey JR (2001) Insect biodemography. Annu Rev Entomol 46:79–110
DeBach P, Huffaker CB, MacPhee AW (1976) Evaluation of the impact of natural enemies. In: Huffacker CB, Messenger PS (eds) Theory and practice of biological control. Academic press, New York, p 778
Duan JJ, Messing RH (1996) Response of two opine fruit fly parasitoids (Hymenoptera: Braconidae) to the lantana gall fly (Diptera: Tephritidae). Environ Entomol 25:1428–1437
Duan JJ, Messing RH (2000) Evaluating non-target effects of classical biological control: fruit fly parasitoids in Hawaii as a case study. In: Follet PA, Duan JJ (eds) Non-target effects of biological control. Kluwer, Norwell, pp 95–109
Duan JJ, Purcell MF, Messing RH (1998) Association of the opiine parasitoid Diachasmimorpha tryoni (Hymenoptera: Braconidae with the lantana gall fly (Diptera: Tephritidae) on Kauai. Environ Entomol 27:419–426
Elkinton JS, Buonaccorsi JP, Bellows TS, Van Driesche RG (1992) Marginal attack rate, k-values and density dependence in the analysis of contemporaneous mortality factors. Res Popul Ecol 34:29–44
Follett PA, Duan J, Messing RH, Jones VP (2000) Parasitoid drift after biocontrol introductions: re-examining Pandora’s box. Am Entomol 46:82–94
Fullaway DT, Krauss NLH (1945) Common insects of Hawaii. Tongg, Honolulu, p 228
Funasaki GY, Lai P, Nakahara LM, Beardsley JW, Ota A (1988) A review of biological control introductions in Hawaii: 1890 to 1988. Proc Hawaiian Entomol Soc 28:105–160
Furlong MJ, Shi Z, Liu S, Zalucki MP (2004) Evaluation of the impact of natural enemies on Plutella xyllostella L. (Lepidoptera: Yponomeutidae) populations on commercial brassica farms. Agric For Entomol 6:311–322
Gagne WC, Howarth FG (1985) Conservation status of the endemic Hawaiian lepidoptera. In: Proceedings of the 3rd Congress of European Lepidopterologists, Cambridge (1982) Societus Europaea Lepidopterologica, Karluhe, pp 74–84
Henneman ML, Memmott J (2001) Infiltration of a Hawaiian community by introduced biological control agents. Science 293:1314–1316
Hoddle MS (2004) Restoring balance: using exotic natural enemies to control invasive exotic species. Conserv Biol 18:38–49
Howarth FG (1983) Classical biocontrol: panacea or Pandora’s box? Proc Hawaiian Entomol Soc 24:239–244
Howarth FG (1991) Environmental impacts of classical biological control. Annu Rev Entomol 36:485–509
Johnson MT, Follett PA, Taylor AD, Jones VP (2005) Impacts of biological control and invasive species on a non-target native Hawaiian insect. Oecologia 142:529–540
Kaneshiro KY (1995) Evolution, speciation, and the genetic structure of island populations. In: Vitousek P, Andsersen H, Loope L (eds) Islands: biological diversity and ecosystem function. Springer, New York, pp 23–34
Kaufman LV (2008) Non-target impacts of introduced parasitoids and validation of probabilistic risk assessment for biological control introductions. Doctoral dissertation, Entomology, University of Hawaii at Manoa
Kaufman LV, Wright MG (in press) Life history, seasonal phenology and parasitoids of the Hawaiian endemic moth Udea stellata (Lepidoptera: Crambidae). Ann Entomol Soc Am 120
Kellogg SK, Fink LS, Brower LP (2003) Parasitism of native luna moths, Actia luna (L.) (Lepidoptera: Saturniidae) by the introduced Compsilura concinnata (Meigen) (Diptera: Tachinidae) in central Virginia, and their hyperparasitism by Trigonalid wasps (Hymenoptera: Trigonalidae). Environ Entomol 32:1019–1027
Loope LL, Howarth FG (2003) Globalization and pest invasion: where will we be in five years? In: Proceedings of the international symposium on biological control of arthropods, Honolulu, Hawaii, January 2002, United States. Department of Agriculture, Forest Service, Morgantown, West Virginia, FHTET-2003–05, pp 34–39
Louda SM, Pemberton RW, Johnson MT, Follett PA (2003) Non-target effects––the Achilles heel of biological control? retrospective analysis to reduce risk associated with biological control introductions. Annu Rev Entomol 48:365–396
Messing RH, Wright MG (2006) Biological control of invasive species: solution or pollution. Front Ecol Environ 4:132–140
Midega CAO, Ogol CKPO, Overholt WA (2005) Life table, key factor analysis and density relations of natural populations of the spotted maize stemborer, Chilo partellus (Lepidoptera: Crambidae), under different cropping systems at the Kenyan coast. Int J Trop Ins Sci 25:86–95
Morris RF (eds) (1963) The dynamics of epidemic spruce budworm populations. Mem Ent Soc Can 31:223–228
Morris RF, Miller CA (1954) A development of life-tables for the spruce budworm. Can J Zool 32:283–301
Nishida GM (2002) Hawaiian terrestrial arthropod checklist, 4th edn. Technical report 22 BP. Bishop Museum, Honolulu, Hawaii, p 263
Oboyski PT, Slotterback JW, Banko PC (2004) Differential parasitism of seed-feeding Cydia (Lepidoptera: Tortricidae) by native and alien wasp species relative to elevation in subalpine sophora (Fabaceae) forests on Mauna Kea, Hawaii. J Insect Conserv 8:229–240
Peck RW, Banko PC, Schwarzfeld M, Euaparadorn M, Brink KW (2008) Alien dominance of the parasitoid wasp community along an elevation gradient on Hawai’i Island. Biol Invas, online first version
Perkins RCL (1897) The introduction of beneficial insects into the Hawaiian Islands. Nature 55:499–500
Royama T (1981) Evaluation of mortality factors in life-table analysis. Ecol Monogr 5:495–505
Simberloff D (1992) Conservation of pristine habitats and unintended effects of biological control. In: Kauffman WC, Nichols JE (eds) Selection criteria and ecological consequences of importing natural enemies. Entomological Society of America, Say, Lanham, pp 103–117
Simberloff D, Stiling P (1996) How risky is biological control? Ecology 77:1965–1974
Simmonds FJ, Bennett FD (1977) Biological control of agricultural pests. Proceedings of the XV International Congress in Entomology, pp 464–472
Stein JD (1983) The biology, host range, parasites, and hyperparasites of koa seed insects in Hawaii: a review. Proc Hawaiian Entomol Soc 24(2/3):317–326
Stiling P, Simberloff D (2000) The frequency and strength of non-target effects of invertebrate biological control agents on plant pests and weeds. In: Follett PA, Duan JJ (eds) Non-target effects of biological control. Kluwer, Boston, pp 31–43
Wright MG, Hoffmann MP, Kuhar TP, Gardner J, Pitcher SA (2005) Evaluating risks of biological control introductions: a probabilistic risk-assessment approach. Biol Control 35:338–347
Van den Bosch R, Messenger PS (1973) Biological control. Intext, New York, p 180
Van Driesche RG, Bellows TS, Elkinton JS, Gould JR, Ferro DN (1991) The meaning of percentage parasitism revisited: solutions to the problem of accurately estimating total losses from parasitism. Environ Entomol 20:1–7
Van Driesche RG, Nunn C, Pasquale A (2004) Life history pattern, host plants and habitat as determinants of population survival of Pieris napi oleracea interacting with an introduced braconid parasitoid. Biol Control 29:278–287
Van Lenteren JC, Bale J, Bigler F, Hokkanen HMT, Loomans AJM (2006) Assessing risks of releasing exotic biological control agents of arthropod pests. Annu Rev Entomol 51:609–634
Acknowledgments
The authors would like to acknowledge the USDA-TSTAR grant program for funding. The Hawaii Department of Land and Natural Resources and the Nature Conservancy of Hawaii are thanked for facilitating collection permits and access to field sites. Peter Follett and Tracy Johnson are thanked for reviewing an earlier version of the manuscript. Roy Van Driesche is thanked for advice on life table analysis. Numerous field assistants are thanked for their help in field collections, as well as Clesson Higashi and Sasha Grant for helping to rear the larvae and growing the plants used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Hoffmeister.
Rights and permissions
About this article
Cite this article
Kaufman, L.V., Wright, M.G. The impact of exotic parasitoids on populations of a native Hawaiian moth assessed using life table studies. Oecologia 159, 295–304 (2009). https://doi.org/10.1007/s00442-008-1226-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1226-4