Abstract
Forest fires remain a devastating phenomenon in the tropics that not only affect forest structure and biodiversity, but also contribute significantly to atmospheric CO2. Fire used to be extremely rare in tropical forests, leaving ample time for forests to regenerate to pre-fire conditions. In recent decades, however, tropical forest fires occur more frequently and at larger spatial scales than they used to. We studied forest structure, tree species diversity, tree species composition, and aboveground biomass during the first 7 years since fire in unburned, once burned and twice burned forest of eastern Borneo to determine the rate of recovery of these forests. We paid special attention to changes in the tree species composition during burned forest regeneration because we expect the long-term recovery of aboveground biomass and ecosystem functions in burned forests to largely depend on the successful regeneration of the pre-fire, heavy-wood, species composition. We found that forest structure (canopy openness, leaf area index, herb cover, and stem density) is strongly affected by fire but shows quick recovery. However, species composition shows no or limited recovery and aboveground biomass, which is greatly reduced by fire, continues to be low or decline up to 7 years after fire. Consequently, large amounts of the C released to the atmosphere by fire will not be recaptured by the burned forest ecosystem in the near future. We also observed that repeated fire, with an inter-fire interval of 15 years, does not necessarily lead to a huge deterioration in the regeneration potential of tropical forest. We conclude that burned forests are valuable and should be conserved and that long-term monitoring programs in secondary forests are necessary to determine their recovery rates, especially in relation to aboveground biomass accumulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest fires are a frequent and devastating phenomenon in the tropics that not only affect forest structure and diversity, but also result in the emission of large quantities of CO2 (Page et al. 2002; Cochrane 2003). The opening of old-growth forests for logging, mining and agriculture in combination with intensifying El Niño-related droughts have led to a dramatic increase in fire frequency and the spatial scale at which fires occur (Cochrane 2003). Human interference with old-growth forests, resulting in fragmentation of previously contiguous forest, has brought artificial forest—savannah fire interactions deep into once closed forests that rarely burned (Cochrane and Laurance 2002).
Although fire forms a natural phenomenon in tropical forests (Goldammer 1989; Hope et al. 2005), its occurrence used to be rare, leaving ample time for burned forests to regenerate to pre-fire conditions (Haberle and Ledru 2001). This is exemplified by what used to be one of the most diverse forests in Borneo, Kutai National Park, of which some parts are known from charcoal records to have burned as recently as 350 years ago (Goldammer 1989). Apparently, tropical rain forests can recover from fire, but little is known about the timescales involved and the pathways along which this recovery takes place. Such information is crucial for burned forest conservation and management and for determining the rate at which burned forests recapture the C lost to the atmosphere.
From previous studies in our research area, it is known that fire tree mortality rates show a negative logarithmic relationship with tree diameter and that up to 100% mortality was reported for trees with a diameter smaller than 10 cm (Slik and Eichhorn 2003; Nieuwstadt and Sheil 2005). Despite this high mortality, tree density (diameter ≥10 cm) and canopy closure are able to recover within 10 to 15 years (Slik et al. 2002). However, tree ingrowth during this initial phase of regeneration consists mostly of early successional, fast-growing tree species with low wood densities (Barlow et al. 2003; Slik and Eichhorn 2003; Hiratsuka et al. 2006) thus forest aboveground biomass remains much lower than that of old-growth forest which is characterized by heavy-wood species (Slik et al., in press). Successful recovery of forest aboveground biomass therefore depends strongly on the regeneration of the pre-fire species composition.
Fires not only occur at larger spatial scales in tropical rain forests than they used to, they also occur more frequently (Cochrane 2003). This means that the time frame between subsequent fires is shortened, which might particularly affect species characteristic of old-growth forests since these generally have regeneration times that exceed current fire intervals. Indeed, repeated fires are able to rapidly transform tropical rain forests into savannah-like grasslands (Cochrane et al. 1999; Goldammer 1999). On the other hand, some studies suggest that even repeated (twice) burned forests can maintain enough pre-fire tree species to make a successful recovery of both species composition and aboveground biomass (Slik and Eichhorn 2003; Eichhorn 2006).
This study aims to determine the recovery pathways of single and repeatedly burned forests during the 7 years of regeneration in an eastern Bornean rain forest. In addition to studying changes in forest structure, this study specifically focuses on changes in the tree species composition during burned forest regeneration. We do this because we expect the long-term recovery of aboveground biomass and ecosystem functions in burned forests to be largely dependent on the successful regeneration of the pre-fire species composition. We included both once and twice burned forests to determine the additional effect of repeat burning on forest damage and recovery in comparison to the conditions found in forests burned for the first time.
Materials and methods
Study site
The study was carried out in the Sungai Wain—Samboja area in East Kalimantan Province, Indonesian Borneo. Although this is one of the driest areas in Borneo with ca. 2,400 mm precipitation annually, the rainfall is spread equally throughout the year with no months receiving less than 100 mm of rain (Walsh 1996). Soils are relatively poor and sandy and the topography covers an elevation gradient of 10–70 m above sea level. The area includes freshwater swamps, periodically inundated river valleys and low hill ridges. Our plots were located in sites which are either covered or used to be covered by evergreen lowland mixed dipterocarp rain forest. Parts of the area were burned in 1983 and again in 1998 leaving a mosaic of unburned old-growth, once burned (1998) and twice burned (1983 and 1998) forests. The fires that occurred were low-intensity surface fires that slowly moved through the area mainly fuelled by the thin litter layer; however, they caused significant tree mortality especially in the small diameter classes (Slik and Eichhorn 2003; Nieuwstadt and Sheil 2005). The research plots were located in old-growth forest of Sungai Wain Forest Reserve and an adjacent once burned forest (~116.49°E; 1.06°S); while the plots in twice burned forest were located ca. 15 km to the north near the village of Samboja (~116.56°E; 0.59°S) neighbouring an unburned forest fragment.
Data collection
Three data sets were used for this study. Each data set has its own plot layout and plant survey methods, but all three were established in the same locations. The first data set was collected in 2000, ca. 1.5 years after the 1998 fires. It consisted of 240 plots of 10 × 20 m, of which 80 occur in old-growth, 80 in once burned and 80 in twice burned forest. The three sites had identical plot layouts consisting of 80 randomly distributed plots within a 1.5 × 3-km area. Within each plot all trees taller than 1.3 m were identified and their diameter at breast height (DBH) measured. The second data set was collected in 2001, ca. 2.5 years after the 1998 fires. It consisted of 90 plots of 10 × 10 m, of which 30 are located in old-growth, 30 in once burned, and 30 in twice burned forest. Spatial layout of the plots was identical for the three locations consisting of 30 plots spaced at 50-m intervals along a 900-m-long transect. Within each plot all trees with a DBH ≥ 10 cm were measured and identified, trees with 5 ≥ DBH < 10 cm were measured and identified in a 5 × 5-m subplot and trees taller than 1.3 m and DBH < 5 cm were measured and identified in a 2 × 2-m subplot. The third data set was collected in 2004/2005, ca. 6.5 years after the 1998 fires. It consisted of 180 plots of 10 × 10 m, of which 60 are in old-growth, 60 in once burned, and 60 in twice burned forest. Spatial layout was identical for old-growth and once burned forest, consisting of 60 plots spaced randomly within an area of 0.3 × 1.2 km, while the plots in the twice burned forest were randomly distributed over two areas of 0.3 × 0.3 km placed ca. 300 m apart (necessary to avoid a large, unburned, freshwater swamp). Within each plot all trees with a DBH ≥ 5 cm were measured and identified, while all trees taller than 1.3 m which had a DBH < 5 cm were measured and identified in a 5 × 5-m subplot. All plants were collected and vouchers are stored in the Nationaal Herbarium Nederland, Leiden University, The Netherlands.
During the 2001 and 2005 surveys each plot was classified as containing less or more than 50% herb cover by visual estimation, and hemispherical canopy photographs were taken at 2 m height in the centre of each plot to determine canopy openness and the leaf area index (LAI; surface area of leaves per ground surface area). Canopy photographs were digitalized and analysed using WINPHOT, a program especially developed to calculate canopy openness and LAI (Ter Steege 1996). Air-dry wood density values (containing ca. 15% moisture) of tree species were determined from literature (Oey 1990). However, for ca. 50% of the species in our plots no wood density values were available. For these species we used the genus average, which has been shown to explain ca. 70% of species level variance in wood density in Indonesian trees (Slik 2006a).
Data analysis
All stem-based analyses were split into three diameter classes (0–5.0 cm, 5.1–10 cm, and >10 cm) in order to differentiate regeneration stages. Since most of the data were either skewed, showed unequal variances between samples, or were not normally distributed, we decided to rank all data and use the ranked data in subsequent statistical analyses. To detect significant effects of fire history, time since fire and their interactions on the investigated variables, we applied multi-factorial ANOVA.
Biomass in the plots was calculated using the equations for moist forest given in Chave et al. (2005) for the trees with a DBH ≥ 5 cm. For smaller diameters we used the biomass equation of Hughes et al. (1999) given in Chave et al. (2003). Since both biomass equations make use of oven-dry wood density (oven-dry mass/green wood volume), our air-dry wood densities collected from literature (air-dry mass/air-dry volume) had to be converted to oven-dry wood density using Brown’s (1997) equation.
Differences in species composition between plots were calculated with cluster analysis (abundance data log transformed to reduce influence of dominant species; UPGMA cluster method; and Bray-Curtis distance index). The obtained Bray-Curtis dissimilarity matrix was converted into a similarity matrix by calculating the 1-dissimilarity value for each matrix cell. For each plot we subsequently calculated the average similarity to all unburned forest plots. Per survey year, plots were then scaled between 0 and 100% (i.e., for each plot we calculated: average similarity with all unburned forest plots/average similarity of all undisturbed forest plots) so that comparisons between survey years became possible.
Species diversity was calculated using rarefaction to reduce effects of sample size on the diversity analysis and because plot sizes differed between survey years. For each forest type and diameter class all stems were pooled. From this pool we randomly drew 30 stems and counted the number of species. This was repeated 50 times, resulting in 50 diversity values per diameter class per forest type. For each forest type the average diversity value and diameter class was tested for significant difference with other forest types and survey years by checking whether the observed average diversity value fell outside 2.5% at either tail of the calculated range of diversity values for each forest type and survey year that it was compared with (bootstrap procedure).
Results
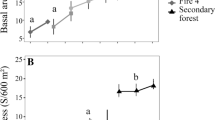
Species composition and dominance patterns changed dramatically after fire, going from a heavy- to a light-wood tree community (Table 1; Fig. 1). Only a few species that were abundant in unburned forest areas managed to maintain or re-emerge in the top ten abundant species in burned forests within 7 years after fire, i.e., Fordia splendidissima (an understorey shrub), Macaranga lowii (a small understorey tree), Crudia reticulata (a swamp shrub), Dipterocarpus confertus (a wind-dispersed upper canopy tree), and Urophyllum arboreum (an understorey shrub) (for nomenclature we refer to Slik 2006b). The burned forests also showed much stronger dominance of the ten most abundant species than the unburned forest (with 29.9–34.8%, 46.3–53.0% and 53.3–68.4% of individuals belonging to the top ten species in unburned, once burned, and twice burned forest, respectively). This difference in species composition and abundance was maintained in all three surveys, indicating no significant recovery occurred within 7 years after fire (Fig. 1).
Even though species composition and abundance in burned forests remained very different from unburned forest, the average wood density of the species community in burned forests did show strong shifts, signaling changes in these parameters. The smallest diameter class studied (0–5 cm DBH) showed strong increases in average wood density, while the large diameter classes (>5 cm DBH) showed declines (Fig. 1). This pattern was similar for species diversity, with increasing diversity in the small diameter classes (0–10.0 cm DBH), but declines in the large diameter class (DBH > 10 cm) (Table 2).
Fire also dramatically altered forest structure, greatly increasing canopy openness, while reducing LAI (Fig. 2). Both measures showed only limited recovery with time and remained significantly different from unburned forest, even 7 years after fire. Stem densities also showed strong shifts after fire, usually increasing in time after the initial strong reductions caused by the fire (Fig. 1). For the small diameter classes (DBH < 10 cm), stem densities in once burned forest were much lower than those in twice burned forest, while the opposite was observed in the largest diameter class (DBH > 10 cm). This low stem density in once burned forest coincided with a very dense herb cover in the 2001 survey, which interestingly, had returned to levels comparable with unburned and twice burned forest in the 2005 survey (Fig. 2). Aboveground biomass was reduced greatly by fire in all diameter classes and showed no, or only limited, recovery with time since fire (Fig. 1).
Discussion
Initial fire impact
Even though the fires in our research area were low-intensity surface fires that slowly moved through the forest understorey, their impact on the forest ecosystem was large. As shown in previous studies in our research area, fire significantly affected mortality rates of trees in the small diameter classes, resulting in an almost complete absence of tree seedlings, saplings and poles just after fire (Nieuwstadt and Sheil 2005). However, large diameter tree density was also significantly reduced by fire (Slik and Eichhorn 2003; Nieuwstadt and Sheil 2005). These reductions resulted in drastic declines in aboveground biomass directly after fire and significantly raised light levels in the forest understorey. This might explain the strong dominance of herbs (mainly ferns and gingers) and fast-growing, light-wood, early successional tree species present in the forest understorey just 1.5 years after fire. The recruitment of large numbers of early successional trees in the burned forest resulted in a tree species composition that deviated significantly from the adjacent unburned forest.
Fire and aboveground biomass dynamics
During the first tree survey 1.5 years after fire we observed aboveground biomass reductions between 40 and 92% in burned forests, with highest relative biomass losses in the smallest diameter classes. Our data show very limited change in the aboveground biomass during the first 7 years of burned forest regeneration, suggesting that C uptake by burned forests is limited during the early phases of forest regeneration. In the largest diameter class (DBH > 10 cm), which contains ca. 90% of forest aboveground biomass, we observed either no change (twice burned forest) or a decline (once burned forest) in biomass with time. Unburned forest also shows a trend of aboveground biomass decline with time, which can only be explained if mortality rates of large trees remain high for several years after drought or fire, something that is indeed reported in some studies (Barlow et al. 2003; Nieuwstadt and Sheil 2005). Barlow et al. (2003) suggest several mechanisms that might explain relatively high mortality rates of large trees after fire such as reduced pathogen resistance, water stress, and increased wind throw risk. An additional mechanism for the loss of aboveground biomass in the large tree diameter class might be related to drought damage in the form of cavitation of vascular tissue in large trees (Hacke et al. 2001). Nieuwstadt and Sheil (2005), who used different plots close to our study site, also observed raised mortality rates of large trees in unburned forest after the 1997/1998 El Niño-associated drought. They found that drought alone resulted in 18.5% of stem deaths within 8 months after the drought, rising to ca. 26.3% mortality after 21 months. Drought effects (i.e., independent of fire) were estimated to account for ca. 30% of large tree deaths observed in burned forests (Nieuwstadt and Sheil 2005) which roughly corresponded to an aboveground biomass reduction of ca. 57%, a value close to what we observed in our unburned forest plots.
Forest recovery after fire
Even though canopy openness and associated LAI did not return to levels observed for unburned forest during the first 7 years of succession, we did observe a rather spectacular decline in herb cover in once burned forest to levels comparable to unburned forest. This reduction is important because a dense herb cover can hinder successful recruitment of trees (Uhl et al. 1988; Kuusipalo et al. 1995; Chapman and Chapman 1999) and forms an important fuel load and ignition source for additional fires (Uhl and Kauffman 1990). At the same time stem densities showed signs of recovery in most diameter classes and the largest diameter class of once burned forest even reached similar levels as observed in unburned forest within 7 years. This corresponds to earlier research that shows quick recovery (between 10 and 20 years) of stem densities in both burned and logged forests (Slik et al. 2002). Despite this quick recovery of stem density, the species composition of burned forest remained significantly different from unburned forest in all diameter classes and did not show any sign of recovery. Apparently, the newly recruited trees belonged to species that are not, or are only rarely, found in the adjacent unburned forest. This is also reflected in the low plot average wood densities in burned forest, which indicates that the new recruits mainly consist of fast-growing early successional species with low wood densities. Plot average wood density did show a significant increase with time since fire in the smallest diameter class. This suggests that the first wave of light-wood early successional species is being replaced by heavier wood species which are more characteristic of undisturbed forest. However, because species composition remains very different from undisturbed forest, these species probably form only a subset of the pre-fire species composition and occur in different densities in the burned than they do in the unburned forest. So some recovery towards the pre-fire species composition is taking place in the burned forest but its progress is slow, and its outcome might even continue to deviate significantly from the pre-fire situation.
Why did we observe such slow recovery of the pre-fire species composition during the first 7 years of succession? First is the fact that the burned forest is quickly dominated by early successional species after fire, which will continue to result in a significantly different species similarity between unburned and burned forests for several decades. Also the recruitment of late successional forest species depends largely on seed rain from surviving adult trees in the burned forest and dispersal into the burned forest from adjacent unburned forest. As shown in this study, adult tree densities are significantly reduced in burned forest, which likely translates into fewer and (at small spatial scales) less diverse seed sources and an overall lower seed input of late successional species in burned forests. If the adult trees that survived fire form a selective subset of the pre-fire species composition due to differential species-specific fire mortality rates, as some studies suggest (e.g., Slik and Eichhorn 2003), the difference in species composition between burned and unburned forest recruits will deviate by default. Differences in dispersal abilities of late successional tree species into the burned forest from the unburned forest might further enhance differences in species composition between unburned and burned forest recruits during initial fire succession. This could occur, for example, by avoidance of burned forest by certain frugivores or general differences in animal abundances (Barlow et al. 2002; Fredericksen and Fredericksen 2002; Slik and van Balen 2006).
Another possible cause for the observed slow recovery of late successional species composition in burned forests might be related to the mast fruiting habit of most Southeast Asian tree species (Cannon et al. 2007). Mast fruiting, or the phenomenon that a large fraction of the tree species community flowers and fruits simultaneously at variable inter-annual periods, can result in long periods, usually lasting several years, in which the majority of the tree species in an area do not produce a significant amount of seeds. According to Frederiksson (personal communication) no serious mast fruiting event occurred between 1998 and 2005 in our study area, possibly explaining the low recruitment of mast-fruiting late successional species (like dipterocarps) in the burned forest.
Even if seeds reach the burned forest, seedling establishment might form another selective filter explaining the lack of late successional tree species presence. One of the problems these species might have to deal with is the thick layer of large leaves produced by the abundant early successional species Macaranga gigantea (Euphorbiaceae) in the burned forest. If seeds drop on such large leaves it is almost impossible for the roots of the germinating seeds to reach the forest floor. Since most late successional tree species have seeds that are rather vulnerable to desiccation (Daws et al. 2006), they probably do not survive for long on such exposed microsites, resulting in failed establishment. Even if they do manage to establish, differential levels of herbivory between unburned and burned forests might form another selective mechanism promoting differences in species composition between these forests (Fredericksen and Fredericksen 2002). Burned forests consist of many fresh herbs and newly recruited early successional woody plants and they might attract more, or a different set, of herbivores than the unburned forest, leading to different seed or seedling predation levels between the forest types. Eventually a combination of the above-mentioned mechanisms might inhibit recovery of the pre-fire species composition in burned forests.
Differences between once and twice burned forest
One of the more surprising outcomes of this study is the lack of strong differences in fire damage between once and twice burned forests. This is rather counterintuitive since most studies report a rapid decline of forest integrity after repeated fires (Cochrane 2003). It remains to be determined why the difference between once and twice burned forest is relatively small in our research area. It might be related to the pronounced topography of the area, in which hills are surrounded by a closed network of river valleys and swamps. Fire intensity and damage were shown to be significantly lower in these valleys and swamp areas, probably due to higher soil moisture levels, and they sometimes act as natural fire breaks (Slik and Eichhorn 2003). Many of the pre-fire species survive in these swamps and river valleys, even after repeated fires. Another more likely explanation of the small difference between once and twice burned forest in our study area might be related to the relatively long period of 15 years between the first and the second fire. As shown by Slik et al. (2002), forest structure is able to recover within 10 to 20 years in burned forests, possibly resulting in forest understorey humidity and litter conditions that resemble that of unburned forest. A second fire after 15 years of burned forest recovery might be of similar intensity and behaviour as a first fire in old-growth forest and result in damage patterns similar to those observed for once burned forest. It is likely that a shorter time interval between fires leads to considerably more damage to forest structure and species composition. The shorter the time interval post-fire, the more open the forest canopy, the more susceptible the forest understorey is to drought, and the larger the fire fuel load (Uhl and Kauffman 1990; Cochrane and Schulze 1999).
Herb cover differences were very conspicuous between once and twice burned forest. Once burned forest had low stem densities just after fire and the forest floor was almost completely covered in an impenetrable layer of ferns and gingers. Contrary to this, twice burned forests were characterized by a very dense understorey of newly recruited early successional shrub and tree species. This difference is likely related to differences in the pre-fire soil seed bank present in once and twice burned forest. The pre-fire soil seed bank of twice burned forest probably contained many seeds of early successional species simply because the area was already covered by early successional species that had successfully established and reproduced after the first fire in 1983. In contrast, the pre-fire soil seed bank of the once burned forest probably contained only a limited number of early successional species due to the fact that the area used to be covered by old-growth forest with very low densities of pioneer species. Since most old-growth species do not have dormant seeds (Garwood 1989), the understorey of once burned forest remained devoid of a dense woody understorey, which provided an opportunity for herbs to flourish during the early phase of regeneration. However, the herbs were likely outshaded by the shrubs and trees that did manage to establish in once burned forest, resulting in a loss of the initial dense herb cover within 7 years after fire. However, the small diameter stem density remained significantly lower in once burned forest compared with twice burned forest during the whole observation period.
Conclusion
Fire has a large impact on tropical forests, but this impact differs considerably depending on the variables investigated. Forest structure in the form of canopy openness, LAI, herb cover and stem densities, is strongly affected by fire but shows relatively quick recovery towards pre-fire status. In theory, a recovered forest structure creates the appropriate habitat conditions for successful regeneration of the pre-fire plant community. However, during the first 7 years of succession we did not observe significant recovery of the pre-fire species composition, indicating that regeneration of burned forests will take a considerable amount of time. Consequently, a large amount of the C released from burned forests will not be recaptured by the burned forest ecosystem in the near future. It will instead form a lasting contribution to raised atmospheric CO2 levels and associated greenhouse effect. Fortunately we also observed that repeated fire (at least in our study area and with an inter-fire interval of 15 years) does not necessarily lead to a huge deterioration of the regeneration potential of tropical forests. This means that even after two fires these forests may still have the potential to recover towards pre-fire conditions even though this might take several decades or even hundreds of years. This supports the idea that burned forests are still valuable to conserve and fire damage should not be used as an argument for commercial timber extraction, changes in conservation status, or land-use change. Instead, a long-term conservation vision and commitment is needed to protect these forests from further anthropogenic changes and fire. Our study also stresses the importance of long-term monitoring of disturbed forests because even though dynamics are high in these burned forests, it remains unclear how long it will take for burned forests to resemble their pre-fire condition.
References
Barlow J, Haugaasen T, Peres CA (2002) Effects of ground fires on understorey bird assemblages in Amazonian forests. Biol Conserv 105:157–169
Barlow J, Peres CA, Lagan BO, Haugaasen T (2003) Large tree mortality and the decline of forest biomass following Amazonian wildfires. Ecol Lett 6:6–8
Brown S (1997) Estimating biomass and biomass change of tropical forests, FAO forestry paper 134. FAO, Rome
Cannon CH, Curran LM, Marshall AJ, Leighton M (2007) Long-term reproductive behavior of woody plants across seven Bornean forest types in the Gunung Palung National Park (Indonesia): suprannual synchrony, temporal productivity and fruiting diversity. Ecol Lett 10:956–969
Chapman CA, Chapman LJ (1999) Forest restoration in abandoned agricultural land: a case study from East Africa. Conserv Biol 13:1301–1311
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi M, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Riera B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Chave J, Condit R, Lao S, Caspersen JP, Foster RB, Hubbell SP (2003) Spatial and temporal variation of biomass in a tropical forest: results from a large census plot in Panama. J Ecol 91:240–252
Cochrane MA (2003) Fire science for rainforests. Nature 421:913–919
Cochrane MA, Alencar A, Schultze MD, Souza CM Jr, Nepstad DC, Lefebvre P, Davidson EA (1999) Positive feedbacks in the fire dynamic of closed canopy tropical forest. Science 284:1832–1835
Cochrane MA, Laurance WF (2002) Fire as a large-scale edge effect in Amazonian forests. J Trop Ecol 18:311–325
Cochrane MA, Schulze MD (1999) Fire as a recurrent event in tropical forests of the eastern Amazon: effects on forest structure, biomass and species composition. Biotropica 31:2–16
Daws MI, Garwood NC, Pritchard HW (2006) Prediction of desiccation sensitivity in seeds of woody species: a probabilistic model based on two seed traits and 104 species. Ann Bot 97:667–674
Eichhorn KAO (2006) Plant diversity after rain-forest fires in Borneo. Blumea Supplement 18. Backhuys, Leiden
Fredericksen NJ, Fredericksen TS (2002) Terrestrial wildlife responses to logging and fire in a Bolivian tropical humid forest. Biodivers Conserv 11:27–38
Garwood NC (1989) Tropical soil seed banks: a review. In: Leck MA, Parker VT, Simpson RL (eds) Ecology of soil seed banks. Academic Press, San Diego, pp 149–209
Goldammer JG (1989) Natural rain forest fires in eastern Borneo during the Pleistocene and Holocene. Naturwissenschaften 76:518–520
Goldammer JG (1999) Forest on fire. Science 284:1782–1783
Haberle SG, Ledru MP (2001) Correlations among charcoal records of fires from the past 16,000 years in Indonesia, Papua New Guinea, and Central and South America. Quarternary Res 55:97–104
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Hiratsuka M, Toma T, Diana R, Hadriyantho D, Morikawa Y (2006) Biomass recovery of naturally regenerated vegetation after the 1998 forest fire in East Kalimantan, Indonesia. JARQ 40:277–282
Hope G, Chokkalingam U, Anwar S (2005) The stratigraphy and fire history of the Kutai peatlands, Kalimantan, Indonesia. Quarternary Res 64:407–417
Hughes RF, Kauffman JB, Jaramillo VJ (1999) Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of Mexico. Ecology 80:1897–1907
Kuusipalo J, Adjers G, Jafarsidik Y, Otasmo A, Tuomela K, Vuokko R (1995) Restoration of natural vegetation in degraded Imperata cylindrica grassland: understorey development in forest plantations. J Veg Sci 6:205–210
Nieuwstadt MGL, Sheil D (2005) Drought, fire and tree survival in a Borneo rain forest, East Kalimantan, Indonesia. J Ecol 93:191–201
Oey DS (1990) Specific gravity of Indonesian woods and its significance for practical use. Departemen Kehutanan Pengumuman nr. 13. Pusat Penelitian dan Pengembangan Hasil Hutan, Bogor
Page SE, Siegert F, Rieley JO, Boehm HDV, Jaya A, Limin S (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420:61–65
Slik JWF (2006a) Estimating species-specific wood density from the genus average in Indonesian trees. J Trop Ecol 22:481–482
Slik JWF (2006b) Trees of Sungai Wain. http://nationaalherbarium.nl/sungaiwain/
Slik JWF, van Balen S (2006) Bird community changes in response to single and repeated fires in a lowland tropical rain forest of eastern Borneo. Biodivers Conserv 15:4425–4451
Slik JWF, Bernard CS, Breman FC, Beek M van, Salim A, Sheil D (2008) Wood density as a conservation tool: quantification of disturbance and identification of conservation priority areas in tropical forests. Conserv Biol. doi:10.1111/j.1523-1739.2008.00986.x
Slik JWF, Eichhorn KAO (2003) Fire survival of lowland tropical rain forest trees in relation to stem diameter and topographic position. Oecologia 137:446–455
Slik JWF, Verburg RW, Kessler PJA (2002) Effects of fire and selective logging on the tree species composition of lowland dipterocarp forest in East Kalimantan, Indonesia. Biodivers Conserv 11:85–98
Ter Steege H (1996) Winphot 5: a program to analyse vegetation indices, light and light quality for hemispherical photographs. Tropenbos Guyana reports 95–2. Tropenbos Guyana Programme, Georgetown
Uhl C, Buschbacher R, Serrao EAS (1988) Abandoned pastures in eastern Amazonia. I. Patterns of plant succession. J Ecol 76:663–681
Uhl C, Kauffman JB (1990) Deforestation, fire susceptibility, and potential tree responses to fire in the eastern Amazon. Ecology 71:437–449
Walsh RPD (1996) Drought frequency changes in Sabah and adjacent parts of northern Borneo since the late nineteenth century and possible implications for tropical rain forest dynamics. J Trop Ecol 12:385–407
Acknowledgements
We would like to thank Dr Kade Sidiyasa and his herbarium crew for their excellent help in the field and for the accurate identifications of the collected plant material. We also thank the motivated and enthusiastic local field crews from Samboja; they did excellent work. Also, we are grateful to Tinus de Kam, Bas van Helvoort, and Dicky from the Tropenbos-Balikpapan office for their logistical support of our fieldwork. The Sungai Wain Management Body is thanked for granting us permission to work in the Sungai Wain area and their logistic support. Mark Cochrane is thanked for critically commenting on earlier versions of this manuscript. We thank LIPI for providing us with the necessary research permits. This research was made possible thanks to financial support from the Dutch Foundation for the Advancement of Tropical Research (NWO-WOTRO), the Alberta Mennega Stichting and the Treub Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Detlef Schulze.
Rights and permissions
About this article
Cite this article
Slik, J.W.F., Bernard, C.S., Van Beek, M. et al. Tree diversity, composition, forest structure and aboveground biomass dynamics after single and repeated fire in a Bornean rain forest. Oecologia 158, 579–588 (2008). https://doi.org/10.1007/s00442-008-1163-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1163-2