Abstract
Species’ functional traits may help determine rates of carbon gain, with physiological and morphological trade-offs relating to shade tolerance affecting photosynthetic capacity and carbon allocation strategies. However, few studies have examined these trade-offs from the perspective of whole-plant biomass gain of adult trees. We compared tree-level annual diameter increments and annual above-ground biomass (AGB) increments in eight long-term plots in hyper-diverse northwest Amazonia to wood density (ρ; a proxy for shade tolerance), whilst also controlling for resource supply (light and soil fertility). ρ and annual diameter increment were negatively related, confirming expected differences in allocation associated with shade tolerance, such that light-demanding species allocate a greater proportion of carbon to diameter gain at the expense of woody tissue density. However, contrary to expectations, we found a positive relationship between ρ and annual AGB increment in more fertile sites, although AGB gain did not differ significantly with ρ class on low-fertility sites. Whole-plant carbon gain may be greater in shade-tolerant species due to higher total leaf area, despite lower leaf-level carbon assimilation rates. Alternatively, rates of carbon loss may be higher in more light-demanding species: higher rates of litterfall, respiration or allocation to roots, are all plausible mechanisms. However, the relationships between ρ and AGB and diameter increments were weak; resource availability always exerted a stronger influence on tree growth rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the functional differences between the many co-existing tree species in tropical forests is an important task for both theoretical and applied ecology. Species can be grouped according to shared taxonomic, physiological and morphological traits, which indicate carbon allocation strategies and ecosystem functions (Woodward and Cramer 1996; Cornelissen et al. 2003; Wright et al. 2006). These differences between functional groups can help us to understand how species composition may influence ecosystem dynamics. Improving our understanding of these relationships is particularly important in equatorial forests where species diversity is very high.
Plant responses to light environments are fundamental to their life strategies and are therefore frequently used to classify functional groups (Boardman 1977; Givnish 1988; Swaine and Whitmore 1988). A continuum of light requirements exists, from light-demanding “pioneer” species, which require high light levels for germination and survival, to shade-tolerant species which can persist under the canopy for sustained lengths of time (Denslow 1987; Whitmore 1989). Although the majority of species fall between these two extremes, there are apparent trade-offs between both physiological and morphological traits that enable rapid growth in high light and those that promote persistence in the shade (Pacala et al. 1994; Walters and Reich 1996; Wright et al. 1998).
Shade-tolerant species are adapted to survive under the forest understorey at the expense of photosynthetic capacity (Boardman 1977). Low whole-plant light compensation points maximise light capture in the shade (Givnish et al. 2004) and slow metabolism (Poorter and Bongers 2006), slow leaf turnover (Lusk 2002; Poorter and Bongers 2006) and greater investment in defence and storage (Coley and Barone 1996; Kobe 1997; Westoby et al. 2002) allow species to maintain a positive carbon balance in the shade and enhance survival. However, these allocation strategies limit overall growth potential, with low light-saturated photosynthetic rates restricting carbon assimilation in high-light environments (Boardman 1977; Givnish et al. 2004).
Light-demanding species, on the other hand, typically have high photosynthetic capacity and are adapted to outgrow competitors in canopy gaps (Boardman 1977; Denslow 1987; Kitajima 1994). Rates of photosynthesis are maximised, with high leaf nitrogen contents (Poorter and Bongers 2006) and short leaf life spans which allow easy replacement of over-shaded and unproductive leaves (King 1994). Also, less dense woody tissue permits higher hydraulic conductivity, which supports greater xylem activity (Roderick and Berry 2001) and consequently greater photosynthetic capacity (Santiago et al. 2004). However, these strategies to maintain high leaf-level carbon gain, along with greater allocation to rapid diameter and height growth, are at the expense of defence and storage (Coley and Barone 1996; Westoby et al. 2002). This increases vulnerability to breakage and herbivory, especially in the shade where biomass losses cannot be offset by rapid growth (King et al. 2006). Also, short leaf life spans lead to high whole-plant light compensation points, with higher production rates necessary to maintain leaf area (King 1994).
Despite these well-known differences between shade-tolerant and light-demanding species, few empirical studies have compared rates of biomass gain of adult trees across functional types. Tropical seedling studies have demonstrated a negative relationship between shade tolerance and relative biomass growth rate in both shade and sun conditions, with inter-specific differences attributed to varying seed reserve utilisation and allocation strategies (Kitajima 1994; Veenendaal et al. 1996; Poorter 1999). However, while it is relatively straightforward to manipulate the environmental conditions in experimental seedling studies, assessment of adult trees in natural surroundings is more complex. Also, seedling behaviour may not represent patterns observed for adult trees. For example, in the Solomon Islands, larger trees of species with shade-tolerant seedlings had greater radial growth rates than those with light-demanding seedlings (Burslem and Whitmore 1996).
Theoretical allometric scaling laws suggest that, under constant resource supply, carbon gain should remain invariant with species type (Enquist et al. 1999). Rather, the differential diameter and height growth rates observed between functional groups represent variation in allocation strategies, with a trade-off between investment in rapid diameter growth (light-demanding species) and allocation to higher wood density (ρ) (shade-tolerant species) (Enquist et al. 1999; Enquist and Niklas 2001). Several tropical studies have demonstrated negative relationships between diameter growth rate and ρ (Enquist et al. 1999; Muller-Landau 2004; King et al. 2005), but only one study has directly tested the prediction that rates of biomass gain should be constant across species types. Consistent with the predictions of Enquist et al. (1999), Chambers et al. (2004) found no significant correlation between relative biomass growth rates and ρ for 60 species in a Brazilian forest. However to date, there has been no detailed study of adult tree above-ground biomass (AGB) growth which also controls for environmental constraints on carbon assimilation rates such as light and edaphic conditions.
Improved knowledge of tree-level processes is important for gaining an overall understanding of the processes that drive stand-level ecosystem dynamics. For example, changes in species composition may have an important impact on carbon storage in tropical forests (Bunker et al. 2005), although the significance of species composition in determining rates of carbon gain across stands is still poorly understood. Very high species diversity, and consequently poor replication of species within study sites, limits species-level studies in tropical forests. However, the assessment of patterns associated with tree functional groups can aid our understanding of stand-level processes. As compositional shifts may already be occurring in mature tropical forests (Phillips et al. 2002; Laurance et al. 2004), identifying the role of functional traits in determining productivity is essential.
Here, we use long-term growth records in north-western Amazonia, the most tree-species-rich region on the planet (Gentry 1988; ter Steege et al. 2003), to explore these ideas further. We first determine how tree-level diameter growth rates vary with shade tolerance and whether this is dependent on resource availability. We then test whether rates of tree-level AGB gain vary with species’ shade tolerance. The sampling design (i.e. controlling for resource availability) allows us to compare the influence of functional traits over differing soil fertility levels.
We propose the following alternative hypotheses to describe how functional traits may influence tree biomass-gain rates, with regards to light availability:
-
1.
There is no difference in rates of biomass gain with shade tolerance (H1). Enquist et al. (1999) predicted that biomass gain is invariant across species’ types, therefore both shade-tolerant and light-demanding species should respond equally to variation in the light environment.
-
2.
Shade-tolerant taxa gain biomass faster in low light, and light-demanding taxa gain biomass faster in high light (H2). Physiological and morphological trade-offs imply that shade-tolerant species maximise biomass gain in the shade due to lower whole-plant light compensation points; in high light, light-demanding species have faster biomass gain due to greater photosynthetic capacity (e.g. Pacala et al. 1994; Walters and Reich 2000).
-
3.
Light-demanding taxa have faster rates of biomass gain in all light environments (H3). The light available to adult trees throughout the observed light gradient is generally sufficient for light-demanders to maintain greater rates of photosynthesis and biomass gain than shade-tolerators.
Materials and methods
Study area
The study was carried out in mature, lowland humid tropical forest in north Peru (north-western Amazonia) at three sites: Allpahuayo (3°57′S, 73°26′W, 114 m), Yanamono (3°26′S, 72°51′W, 104 m) and Sucusari (3°16′S, 72°54′W, 107 m). These regions average > 250 tree species per hectare and include the most speciose landscapes in the world (Gentry 1988; Tuomisto et al. 1995; Vasquez and Phillips 2000). The regional climate is hot and humid, with mean annual precipitation of ca. 2,800 mm, average precipitation in each month > 100 mm and mean annual temperature ca. 26°C (WORLDCLIM data, Hijmans et al. 2005). Edaphic conditions range from sandy soils derived from fluvial sediments, which characterise the small hills, to more fertile, clay-rich soils which dominate the lowest locations (Vormisto et al. 2000). Two, 1-ha plots were studied in both Allpahuayo [ALP-20 and ALP-30, with ALP-20 divided into ALP-21 (0.48 ha) and ALP-22 (0.44 ha) to represent distinct soil types], and Yanamono (YAN-01 and YAN-02) and four 1-ha plots were studied in Sucusari (SUC-01, SUC-02, SUC-03 and SUC-04).
The study plots are highly species rich, in particular those located on more fertile, clay-rich soils (YAN-01, 02, SUC-01, 02, 04) with 214-263 species per plot (from 445–521 study trees per plot) and 122 different species represented by 177 stems in ALP-22 (0.44 ha). The most common species in Yanamono are Otoba glycarpa and Otoba parvifolia and at Sucusari include Eschweilera coriacea, Iryanthera juruensis, Virola calophylla and Anaueria brasiliensis. Basal area of these plots ranges from 26.8 m2 ha−1 (ALP-22) to 32.4 m2 ha−1 (YAN-01) (Baker et al. 2004).
The plots located on less fertile soils have lower species richness (ALP-30, SUC-03, 71–89 species per plot, from 465 to 493 study trees; ALP-21, 113 species from 273 stems). Relatively common species found at Allpahuayo include Micrandra elata, Macrolobium microcalyx, Tachigali tessmannii and Virola pavonis, and at SUC-03 include Campsiandra chrysadenius, Cynometra spruceana, Eschweilera albiflora, Eschweilera parvifolia, Pouteria gomphiifolia, Caraipa densiflora and Tachigali paniculata. Basal area is lower in these plots, ranging from 23.1 m2 ha−1 (ALP-30) to 27.2 m2 ha−1 (ALP-21) (Baker et al. 2004).
Overall, 792 species are represented in this study.
Growth data
Inventory data for an approximate 4-year census period (2001–2005) are available for each plot, with diameter at 1.30 m, or above buttresses, measured for all trees with diameter ≥ 10 cm, collected as part of the RAINFOR project (Malhi et al. 2002). Diameter and AGB increments were calculated for 2001 trees which survived to 2005. Palms were excluded from this study because their biomass growth is largely determined by apical extension rates and we lack accurate measures of tree height. Trees which had fallen, had snapped stems or were otherwise substantially damaged were also excluded.
AGB gain represents the coarse wood productivity, defined as the rate of biomass increment of tree boles, limbs and branches (Malhi et al. 2004). Tree AGB was calculated using two different allometric equations, derived from independent datasets that relate tree diameter (d; cm) and ρ (g cm−3) to AGB (kg dry weight). Chave et al. (2005) developed an allometric model (Eq. 1) for wet forests (high rainfall, aseasonal), based on data from America, Asia and Oceania (n = 725)
Chambers et al. (2001) provide an allometric model (Eq. 2) developed in Central Amazonia (n = 315) that, compared to Eq. (1), produces more conservative estimates for very large trees [>60 cm diameter at breast height (DBH)] but higher biomass estimates for smaller trees. The model was corrected for tree ρ to account for differences in allocation between species (Baker et al. 2004).
Annual increment was calculated by dividing absolute increment in diameter and AGB by the exact census period.
Light availability
The light environment of each tree was assessed using a crown illumination index (CII; Clark and Clark 1992), which is based on visual assessments of vertical and lateral light availability [no direct light (1); low lateral light (1.5); medium lateral light (2); high lateral light (2.5); 10–90% vertical light (3); crown completely exposed to vertical light, some or all lateral light blocked (4); and crown completely exposed to vertical and lateral light (5)]. The index has been calibrated for our sites using hemispherical photography to derive the visible sky fraction and global site factor (the proportion of total light available, compared to an open location) associated with each CII class (a detailed methodology is provided in Keeling and Phillips 2007). Where poor visibility prevented confident assessment using the CII, these trees were excluded from the analysis (1.4% of trees assessed).
An assessment of the between- and within-observer error associated with the CII has been presented elsewhere (Keeling and Phillips 2007). Briefly, a good degree of consistency was found both between the classifications made by two independent observers (H. K. and one other) (n = 54, Kendall’s τ of concordance 0.934, P = 0.0001) and also by a single observer at the beginning and end of a 2-week period (H. K.) (n = 111, Kendall’s τ of concordance 0.866, P < 0.0001). Additionally, the ability to accurately assess a tree’s canopy light environment from the forest floor was tested by recording the CII of 50 trees both at ground level and also from a canopy walkway (at heights from 7 to 17 m) where visibility was much improved (H. K.). The same class or one class different was given on 90% of occasions (n = 50, Kendall’s τ of concordance 0.885, P = 0.0007).
High- and low-ρ species are common in all light environments, within these study plots. In low light (CII = 1, 1.5 & 2), both low-density species (e.g. O. glycycarpa and V. calophylla) and high-density species (e.g. Cynometra spruceana, E. coriacea and E. albiflora) are relatively common, and again, in high light (CII = 4 & 5), both high-density species (e.g. E. coriacea, P. gomphhiifolia, M. elata) and lower density species (e.g. Tachigali paniculata, O. glycycarpa, Apeiba aspera, V. calophylla) are found.
Species trait data
We used ρ as a surrogate for shade tolerance. ρ provides an indicative trait of plant life strategy (cf. Introduction; ter Steege and Hammond 2001; Wright et al. 2003; Chave et al. 2006) and is significantly correlated with independent measures of shade tolerance (e.g. r = 0.73, P < 0.001, n = 17; Augspurger 1984) and pioneer status of Amazon taxa (r = −0.38, P < 0.0001, n = 777; O. L. Phillips et al. unpublished data). As the study sites are permanent inventory plots for the long-term study of natural forest dynamics, it is not desirable to sample each tree to measure individual ρ, because of the risk of introducing pathogens. Therefore, an extensive species’ ρ database, collated from various sources, was supplied by Chave (cf. Chave et al. 2006 for data description and methods). This database provides, where available, species-level averages based on multiple individuals from the Neotropics, presenting the best possible species’ estimates (Chave et al. 2006). Species-level ρ data were used, where available, to represent the light requirements of each tree (54.3% trees used in study). Otherwise, as trait values are generally conserved within genera and families (Baker et al. 2004; Chave et al. 2006), generic- or family-level means were used (39.9 and 5.8% of study trees, respectively). Where no family-level data were available, trees were excluded from the analysis (0.4% of trees with known CII). The ρ data used in this analysis therefore represent the best available measure for the taxon, not necessarily the actual density of the subject trees. Taxon averages present the best possible way to study tree-level processes across these very diverse forest stands, where tree-specific data, and often species-specific data, are unavailable (e.g. ter Steege et al. 2006; Chao et al. 2007).
Mean plot ρ ranged from 0.59 g cm−3 (YAN-01) to 0.71 g cm−3 (SUC-03) with all other plots between 0.62 and 0.66 g cm−3. Across all plots, mean tree ρ is very similar between light environments (low light, CII = 1, 1.5 and 2, 0.65 g cm−3; high light, CII = 4 and 5, 0.63 g cm−3).
Data analysis
Environmental factors other than light availability are also likely to influence growth rates. Climatic variation was controlled for by locating the study in three climatically similar sites. However, edaphic conditions do vary between study sites. We tested for first-order edaphically mediated differences in growth rates by grouping plots into the clay-enriched, well-drained “more fertile” soils with pH > 3.6 (1 M KCl) and estimated cation exchange capacity (ECEC) > 3 cmol kg−1 (ALP-22, SUC-01, -02, -04, YAN-01, -02), and “less fertile” soils, with pH < 3.6 and anoxic and seasonally flooded conditions (SUC-03) or nutrient-deficient white sands with ECEC ≤ 1 cmol kg−1 (ALP-21 and -30). These broad groupings provided adequate group sizes to preserve statistical power. To test for the possibility of spatial autocorrelation confounding the results, we plotted the similarity in median plot annual diameter and AGB increment against the distance between plots, for all plot combinations. For this test we only included trees less likely to be limited by competition (d ≥ 20 cm) or light (CII = 4,5), to provide a more homogeneous dataset for comparison across plots.
We used non-parametric statistical techniques, to enable us to incorporate the (ordinal) CII and (positively skewed) diameter and AGB increment datasets.
Specifically, two separate approaches were used to analyse the influence of species’ functional traits (mean taxon ρ) on annual diameter and AGB increments, calculated using both allometric models.
-
1.
Firstly, we used multivariate non-parametric rank regression to assess the significance of ρ, whilst also controlling for environmental variation (light availability, soil fertility) as well as tree size.
Rank regression is an asymptotically distribution-free rank-based method for multiple linear regression. Minitab 14.0 (Minitab 2004) was used to generate the multivariate regression equation, as well as the data necessary to calculate the Jaeckel-Hettmansperger-McKean (HM) test statistic:
where D * J represents the reduction in dispersion from the full model to the reduced model, and \( \hat{\tau } \) represents the Hodges–Lehmann estimate of τ (Hollander and Wolfe 1999). The HM statistic has an asymptotic χ2 distribution and therefore at the appropriate α-level of significance, is statistically significant if HM ≥ χ 2q,α , where χ 2q,α is the upper α percentile point of a χ2 distribution with q df (Hollander and Wolfe 1999). The HM statistic was calculated and the statistical significance tested for the full model as well as for each independent predictor variable.
Independent variables in the rank regression models were mean taxon ρ, light availability (CII), initial tree size (diameter at beginning of census period; d t0), and the potential interaction variables between light and ρ (CII × ρ) and between light and initial tree size (CII × d t0), which were orthogonalised to minimise multicollinearity. The multivariate models were tested firstly using the whole data, then separately for each soil fertility group, and finally individually for each plot.
-
2.
Secondly, we categorised the data to allow separate analyses for differing light levels and soil fertility groups. For ρ, categories were “low” ≤ 0.49 g cm−3, “intermediate” 0.5–0.69 g cm−3 and “high” ≥ 0.7 g cm−3. Light availability classes were “low” CII = 1, 1.5 & 2 [little or medium lateral light; ca. ≤ 15% full light, from Keeling and Phillips (2007)], “intermediate” CII = 2.5 & 3 (high lateral or medium vertical light; ca. 15–45% full light) and “high” CII = 4 & 5 (high vertical light; ca. 45–100% full light). Correlation between growth rates and ρ, and descriptive statistics (i.e. mean, median, maximum annual increment) for each ρ group, were calculated for each light environment, within both soil groups, to allow comparisons. Maximum annual increment was computed as the 95th percentile in order to minimise the influence of outliers.
We undertook these analyses both for the dataset as a whole, and also for three diameter size classes: 10 ≥ d < 20 cm, 20 ≥ d < 40 cm and d ≥ 40 cm. Controlling for size is important since carbon allocation to growth and reproduction varies throughout ontogeny; stem diameter growth rates typically peak at intermediate sizes (Wright et al. 2003) and larger trees invest more carbon in ρ for structural support (Chambers et al. 1998). Many of the same species, covering a range of wood densities, are found in all size classes, including: P. gomphiifolia, M. elata, V. pavonis, Anaueria brasiliensis and O. glycarpa. The mean ρ of trees 10 ≥ d < 20 cm and 20 ≥ d < 40 cm is 0.64 g cm−3, although slightly lower for trees d ≥ 40 cm, 0.62 g cm−3, due to the influence of large low-density species, e.g. Cecropia, Ficus and Tachigali spp.
Additionally, the analysis was repeated using only trees for which species-level ρ values were available. This provides results based on the most reliable ρ data, albeit a significantly reduced sample size, for comparison with where generic- and family-level ρ means have been included.
Finally, multiple statistical tests can lead to spurious results, due to the increased probability of type I error, i.e. falsely accepting an insignificant result. We used Hochberg (1988) sequential adjustments (table- or sub table-wide) to produce more stringent P-values, reducing the risk of type I error, without increasing the risk of falsely rejecting a significant result (type II error).
Results
Spatial and environmental variation
No distance decay is evident in the similarity of annual diameter and AGB increments (of trees d ≥ 20 cm, CII = 4 or 5, n = 743) among plots (n = 36 plot pairs, diameter increment, y = 1.3 − 0.001x, r 2 = 0.001; AGBChave increment, y = 5.7 − 0.005x, r 2 = 0.002). On average, closer plots have less similar growth rates than distant plots, although the relationship is not significant (P > 0.05). Variation in both the diameter and AGB increments within plots is greater than variation between plots of differing soil types (Mann–Whitney U-test, P < 0.05; Fig. S1). For example, there is no significant difference between the AGB increments in ALP-30 (“less fertile”, white sand plot) and those in YAN-01 (“more fertile”), which has the second highest median increment, or SUC-03 (“less fertile”, seasonally flooded plot) which has the lowest median increment (Fig. S1). However, when plots are grouped by soil fertility, trees (d ≥ 20 cm, CII = 4 or 5) in more fertile plots have significantly greater median annual diameter (more fertile, 4.0 mm year−1; less fertile, 2.7 mm year−1; Mann–Whitney U-test, P < 0.001) and significantly greater AGB growth (more fertile, AGBChave 21.7 kg year−1, AGBChambers 28.4 kg year−1; less fertile, AGBChave 14.9 kg year−1, AGBChambers 20.5 kg year−1; P < 0.001).
Annual diameter increment
There is a weak, negative relationship between taxon ρ and annual diameter increment after controlling for light availability and tree size (multivariate rank regression, supplementary material; Table S1). Also, separate rank regression analyses show ρ to have a significant negative effect for both soil fertility groups (Table S1). However, at the plot level, ρ is only significant in the “less fertile” white sand plots (ALP-21 and ALP-30) after Hochberg adjustments (Table S1). CII is a significant positive factor in all regression models, although the interaction variable CII × ρ is only significant when analysing the whole data set and for ALP-30 (Table S1).
Variation with light availability only
The relationship between ρ and annual diameter increment strengthens as light availability increases. In low-light environments, diameter growth is invariant with ρ, but in intermediate light there are weak negative correlations between ρ and diameter increments for trees d < 40 cm (Table 1) and low-density species have significantly greater median increments in size class 10 ≥ d < 20 cm (Table S2). In high-light environments, there are slightly stronger correlation coefficients (Table 1) and greater differentiation between the median increments of low- and high-ρ species, for all size classes (Table S2). Low-density species also have the greatest maximum annual diameter increment in all light environments, with the difference in maximum increment between low- and high-density groups increasing with increasing light (Table S2). However, overall, any ρ influence always remains weak.
Variation with light availability and soil fertility
There is some correlation between annual diameter increments and ρ in both soil fertility groups, which strengthens with increasing light availability. In more fertile plots, ρ is immaterial in low-light environments (Table 1, S2). In intermediate light, there is only a significant (negative) correlation between ρ and diameter increment in size class 10 ≥ d < 20 cm (Table 1), for which low-density species also have the greatest median increment (Table S2). In high-light environments, low-density species have the greatest median increments in all size classes (although insignificant for trees ≥ 20 cm, Table S2) even though only trees 10 ≥ d < 20 cm show a significant negative correlation between ρ and diameter increment after applying Hochberg adjustments (Table 1).
In less fertile plots, there is no significant correlation between ρ and diameter increments in low light (Table 1), although in the smallest size class low-density species have the greatest median and maximum increments (Table S2). In intermediate light, only trees 20 ≥ d < 40 cm show a significant negative correlation after the Hochberg adjustment (Table 1) and, although maximum diameter increments are greater for low-density species in all size classes, median diameter increments only differ significantly for trees 20 ≥ d < 40 cm (Table S2). In high light, again only trees 20 ≥ d < 40 cm show a significant negative correlation after Hochberg adjustments (Table 1), with low-density species showing greater median and maximum growth rates (Table S2). Small sample sizes limit analyses for individual size classes in high light.
Mean AGB increment
Despite the two allometric models producing differing absolute AGB estimates, very similar results were obtained throughout the subsequent analyses. For trees < 60 cm, Chambers et al.’s (2001) model consistently estimated more AGB (median: 1.14 kg greater), while for trees > 60 cm, the Chave et al. (2005) model estimated slightly more AGB (median: 4.24 kg greater). Since conclusions with respect to our questions are consistent between models, we present only the results using the Chave et al. (2005) model as this is based on forests climatically similar to ours (Tables 1, S2, S3, S4). Results using the Chambers et al. (2005) AGB model are presented in the supplementary material for comparison (Tables S5, S6, S7).
Multivariate rank regressions of all the data, controlling for light availability and tree size, suggest that ρ may have a very weak positive effect, but this is statistically insignificant (Table S3). Separate analyses show ρ to have an insignificant negative effect in the “less fertile” group, although a significant weak positive effect is evident in the “more fertile” group (Table S3). A plot-level analysis of the “more fertile” group consistently shows a weak positive density effect, although after Hochberg adjustments no plot-level effect was individually significant. CII positively affects AGB increment, whereas the interaction variable CII × ρ is insignificant after Hochberg adjustments for all plots (Table S3).
Variation in light availability only
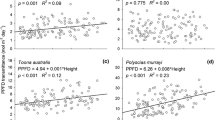
It is unclear whether the association between ρ and biomass increment is influenced by light availability in these analyses. A relationship between density and AGB increment is only evident in intermediate light, where there is a significant positive correlation for trees with d ≥ 40 cm (Table 1). High-density species in this group also have significantly greater median and maximum AGB increments (Fig. 1, Table S4). In low and high light, there are no statistically significant patterns.
Median annual AGB increments [kg year−1, calculated following Chave et al. (2005)] for three ρ groups, under low, intermediate and high light; for trees of all sizes, each size class individually and species-level wood density subset only; for all plots, more fertile plots, and less fertile plots. a, b Denote significant differences under common light environments (Mann–Whitney U-test, P < 0.05, after Hochberg adjustments)
Variation in light availability and soil fertility
Separate analyses for “more fertile” and “less fertile” plots produced contrasting results. In less fertile plots, there is no significant difference between the median growth rates of ρ groups, although low-density species have greater maximum growth rates (Table S4), and ρ and AGB increment are negatively correlated for the larger size classes, in intermediate and high light (Table 1). By contrast, in more fertile plots, ρ and annual AGB increments are positively associated, in particular for the larger, better lit individuals (Fig. 1, Table S4). In low light, there is a weak positive correlation between AGB increments and ρ for small trees, although median increments are invariant with ρ (Table 1, Fig. 1). In intermediate light, there is a significant positive correlation between density and AGB increments for all size classes (Table 1) and high-density species have greater median increments (“all size classes” subset) and have greater maximum increments in trees ≥ 20 cm (Table S4). In high light, there is a significant positive correlation for trees 20 ≥ d < 40 cm (Table 1) and high-density species also have greater median and maximum increments in this size class (Fig. 1).
Results are consistent for both methodological approaches (rank regression and categorisation) and also when including only trees for which species-level ρ means are available. Also, the soil-associated differences in ρ effects are not explained by differences in ρ ranges among the plots. ρ does vary more in the more fertile (0.11–1.21 g cm−3) than the less fertile plots (0.34–0.97 g cm−3), but when the extreme density values in the former are excluded to give an equal range, the significant positive ρ effect remains evident in multivariate rank regression analysis (Table S3). The range and median of light level in both soil fertility groups are equal (CII: range 1–5, median 3).
Discussion
Spatial and environmental variation in the data set
Spatial autocorrelation is unlikely to confound our results. There is no distance decay in the similarity of plot data, and within-plot variation in diameter and AGB increments greatly exceeds between-plot variation. Within tropical forests, light is typically the single most limiting factor for growth (Chazdon and Fetcher 1984; Whitmore 1996). Sizeable differences in light availability throughout the vertical forest profile imply that growth rates can vary significantly between individuals—for example, between highly shaded understorey trees and fully illuminated emergent trees, and therefore within-plot variation can be considerable. Soils do vary between plots and are accounted for by undertaking separate analyses for two broadly defined soil fertility groups. However, growth rates varied only weakly with soil type.
Variation in allocation strategies between functional groups
The negative relationship between mean taxon ρ and annual diameter increment observed in this study and others (e.g. Lieberman and Lieberman 1987; Muller-Landau 2004; King et al. 2005) is likely to be driven by differences in allocation between species’ functional groups. Compared to high-density species, low-density species allocate more fixed carbon to diameter growth than to tissue density and therefore diameter growth rates are higher and these species respond disproportionately to greater light. For example, in low light the maximum annual diameter increment of low-ρ species is 56% greater than high-density species and in high light, 111% greater (results for “all data”).
Variation in annual AGB increments between functional groups
Our findings are not fully consistent with the predictions of any of the three hypotheses proposed to represent the AGB gain rates of differing functional types. H1 predicted that rates of biomass gain would be invariant with shade tolerance (after Enquist et al. 1999). Although no strong patterns are evident in the less fertile plots, a significant positive relationship between ρ and AGB is shown in the more fertile plots. H2 predicted a trade-off between functional traits that promote greater rates of biomass gain in the shade, and those that maximise biomass gain in the sun. We did not observe this in any plots. Finally, H3 predicted that inherently greater photosynthetic capacity would always lead to greater rates of biomass gain by light-demanding species. There is no evidence for this in these Amazonian forests, although on less fertile soils, low-ρ species may tend towards slightly faster rates of AGB gain in the highest light environments.
Our observation that species’ ρ has little effect on AGB gain, or even a positive effect on more fertile soils, sits uncomfortably with the knowledge that light-demanding species have inherently faster rates of leaf-level carbon assimilation (Boardman 1977). We suggest two possible explanations for this outcome.
Explanation 1: greater whole-plant carbon gain
Shade-tolerant (high density) species develop deeper crowns than light-demanding species (Reich et al. 1992; Aiba and Kohyama 1997; Lusk and Contreras 1999; Bohlman and O’Brien 2006). The cost of crown maintenance is minimised through long leaf life spans (Reich et al. 1991; Poorter and Bongers 2006) which enable the accumulation of a large area for light capture, without requiring a large investment in foliage biomass (Lusk 2002). Whereas light-demanding species cannot utilise heavily shaded leaves at the canopy base for carbon assimilation, these leaves do contribute to whole-plant carbon gain in shade-tolerant species (Lusk and Contreras 1999). Consequently, although light-demanding (low density) species may have faster leaf-level carbon assimilation rates, the large crown volume of shade-tolerant species may compensate so that tree-level carbon assimilation rates are similar (Reich et al. 1992; Grubb 1998) and perhaps even surpass those of low-density species with continued crown development. Indeed in our Amazonian study, the positive relationship between ρ and biomass increment is mainly observed in trees larger than 20 cm diameter, possibly reflecting the time required for species with long-lived foliage to accumulate a large enough foliage mass.
However, in the less fertile sites, ρ may have a weak negative effect on biomass gain in high light (although is largely insignificant overall). Also, there is greater differentiation between the diameter increments of high- and low-density species for these sites. Other work has observed that infertile sites favour species with denser wood, long leaf life spans and slow metabolism (Escudero et al. 1992; Reich et al. 1992; Veenendaal et al. 1996; Muller-Landau 2004), but nutrient limitation appears to restrict the whole-plant carbon gain of shade-tolerant species in this forest. Nutrient limitation may drive greater allocation of biomass below-ground due to higher root costs for nutrient acquisition (King 2003), limiting potential investment in leaf biomass and therefore restricting crown development in shade-tolerant species. Also, shade-tolerant species with long leaf life spans may be less effective in reabsorbing nitrogen during leaf senescence, leading to higher nitrogen losses in leaf litter and higher crown nitrogen losses for a given diameter, which consequently constrains crown maintenance and expansion on nutrient-poor sites (Reich et al. 1995; Lusk and Contreras 1999, but see Reich et al. 1992). Thus, nutrient constraints on crown development may prevent shade-tolerant species from achieving the canopy volume necessary for higher rates of carbon gain.
Explanation 2: equal whole-plant carbon gain, with unmeasured carbon fluxes
Alternatively, the positive relationship between ρ and AGB increments may be explained by low-density species losing carbon at a faster rate than high-density species. Light-demanding species typically have faster foliage turnover, which acts as a significant biomass “drain” (Poorter and Bongers 2006), and therefore greater biomass production is necessary to maintain sufficient leaf biomass (Givnish et al. 2004). This carbon flux as leaf turnover is not accounted for in our calculations of annual AGB increment, as losses to litterfall are unmeasured. Additionally, light-demanding species prioritise radial and height growth at the expense of allocation to defence and storage, increasing the risk of (unquantified) biomass loss due to herbivory and pathogen attacks (Coley and Barone 1996) and the risk of mechanical damage (Bohlman and O’Brien 2006), which could all restrict biomass gain.
Light-demanding species may also experience greater carbon losses through higher respiration rates. High leaf nitrogen content, associated with light-demanding species (Poorter and Evans 1998; Lusk and Contreras 1999) promotes faster plant metabolism and correlates positively with both assimilation and respiration (Wright et al. 2004; Poorter et al. 2006; Reich et al. 2006). Greater leaf-level respiration rates have been shown for light-demanding species (Bazzaz and Pickett 1980).
Carbon allocation below-ground is unmeasured in our study and theoretically could also explain differences in AGB increment with shade tolerance. For example, high leaf nitrogen concentrations in light-demanding species, to support greater photosynthetic rates, may imply greater root costs for nitrogen acquisition (Mooney and Gulman 1979; Reich et al. 1998). However, shade-tolerant species generally prioritise allocation to establishment and survival rather than leaf area and rapid growth and so, at least as seedlings and saplings, allocate disproportionately below-ground (Walters et al. 1993; Kitajima 1994; Cao and Ohkubo 1998; but see Reich et al. 1998).
Whether our results are driven by greater whole plant biomass gain by high-density species, unmeasured carbon fluxes, or a combination of both, requires further investigation. However, although functional traits play a role in determining annual AGB increments and allocation strategies (i.e. diameter increments), their influence is weak compared to that of light and soil resources. Our results show environmental variation is the key driver of AGB gain rates, with environmental variation mediating the strength of any effect of functional traits.
Our findings contrast with seedling studies which show light-demanders to have the greatest relative biomass growth rates in all light environments (e.g. Kitajima 1994; Poorter 1999). Functional traits associated with shade tolerance may be more important in driving biomass gain during early juvenile stages, which were excluded a priori from this study of trees ≥ 10 cm diameter. At the seedling and sapling stage, life strategy trade-offs are vital for survival and establishment in the canopy, although once trees have reached maturity, allocation strategies may tend to converge. Also, there is some debate over whether short-term seedling studies can adequately represent patterns associated with long-term natural establishment of juvenile trees (Sack and Grubb 2001; Kitajima and Bolker 2003; Sack and Grubb 2003), casting further doubt over the use of these seedling studies to describe mature tree processes.
ρ as an indicator of shade tolerance
In this hyper-diverse forest, where species-level trait information is limited, taxon ρ means provide a valuable method for categorising light demand across whole forest stands. However, there are some limitations associated with the use of taxon-averaged values. Although ρ is known to be strongly conserved phylogenetically (Baker et al. 2004; Chave et al. 2006) there is also evidence of inter-specific variation between individuals, attributed to environmental conditions (Nogueira et al. 2007; Patino et al. 2008). Also, while in general species’ light requirements as small and large trees are significantly correlated (e.g. Hawthorne 1995; Sheil et al. 2006), the light demand of some species has been observed to “crossover” during ontogeny (e.g. Dalling et al. 2001; Poorter et al. 2005). Nevertheless, taxon-averaged ρ data provide a viable method for generalising species’ light requirements, and offers the best way to tackle these hypotheses on this scale (nearly 4,000 study trees, 792 different species). A sub-analysis that included only trees with species-level mean ρ data confirmed the results based partially on genus- and family-level means, supporting the inclusion of these higher-taxon averages.
Application of general allometric models
Stand-level studies in such diverse forests necessitate the use of general allometric models for calculating tree-level biomass, inevitably leading to error in estimates due to species-specific variation in allometry. In particular, any systematic error relating to species’ functional type could bias the results presented here. For example, crown architecture is known to vary according to light demand with shade-tolerant species typically having deeper crowns (Lusk and Contreras 1999; Bohlman and O’Brien 2006). However, leaf biomass is generally a small component of total AGB in adult trees (e.g. Takyu et al. 2003; Liddell et al. 2007) and furthermore, other studies show that the proportion of total AGB allocated to the crown may not differ significantly between functional types. For example, there was no relationship between percentage crown biomass and species’ ρ in a study of an Australian forest by Liddell et al. (2007). We explored this further, using data in the literature, and again found no significant difference between the percentage crown biomass of low, medium or high ρ genera in a Brazilian forest (90 trees, DBH 10–39.9 cm, one-way ANOVA, F = 0.93, P = 0.398; % crown biomass from Araujo et al. 1999, genus ρ from Chave et al. 2006).
These studies suggest that differences in allometry are small across functional groups, supporting the assumption made in this and other studies (e.g. Chambers et al. 2004; Chave et al. 2008) that general allometric models give valid estimates of individual tree biomass across a range of functional types. If, however, any allometric differences did bias results, it is likely that this would result in an under-estimation of shade-tolerant species’ AGB, due to their larger than average crown size. For example, although Chave et al. (2005) found no significant difference between the diameter/biomass relationships for mature and successional tropical forest stands, Nelson et al. (1999) showed that when published mixed species allometric equations from primary forest were applied to successional species, AGB tended to be over-estimated, even when ρ was taken into account. Therefore, the allometric models used in our study, based on old growth forests, may in fact produce conservative estimates of AGB for high-density species. As a result, our conclusion that high-ρ species have higher rates of biomass gain on fertile soils than low-ρ species would be robust to this possible bias.
Amazonian-scale drivers of above-ground wood productivity
The macro-scale pattern of stand-level above-ground coarse wood production in Amazonia follows a gradient in mean stand-level ρ, with greater wood productivity in the west, where lower density species dominate, and lower productivity in central and eastern regions, where higher density species dominate (Baker et al. 2004; Malhi et al. 2004; ter Steege et al. 2006). This negative association between ρ and AGB increments is surprising in the light of our findings. However, the more productive western forests are also associated with the younger and least weathered soils in Amazonia (Malhi et al. 2004) and so environmental variation may be of more fundamental importance than compositional variation in driving the Amazonian productivity gradient. Consequently, abiotic factors may be the dominant drivers of Amazonian productivity at both the tree- and stand-level (T. R. Baker et al. unpublished manuscript). However, we have demonstrated that functional traits do affect tree-level biomass production, and therefore functional traits could also influence future changes in Amazonian carbon dynamics.
References
Aiba SI, Kohyama T (1997) Crown architecture and life-history traits of 14 tree species in a warm-temperate rain forest: significance of spatial heterogeneity. J Ecol 85:611–624
Araujo TM, Higuchi N, de Carvalho Junior JA (1999) Comparison of formulae for biomass content determination in a tropical rain forest site in the state of Para, Brazil. For Ecol Manage 117:43–52
Augspurger CK (1984) Light requirements of neotropical tree seedlings: a comparative study of growth and survival. J Ecol 72:777–795
Baker TR, Phillips OL, Malhi Y, Almeida S, Arroyo L, Di Fiore A, Erwin T, Killeen TJ, Laurance SG, Laurance WF, Lewis SL, Lloyd J, Monteagudo A, Neill DA, Patino S, Pitman NCA, Silva JNM, Martinez RV (2004) Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob Change Biol 10:545–562
Bazzaz FA, Pickett STA (1980) Physiological ecology of tropical succession: a comparative review. Annu Rev Ecol Syst 11:287–310
Boardman NK (1977) Comparative photosynthesis of sun and shade plants. Ann Rev Plant Physiol 28:355–377
Bohlman S, O’Brien S (2006) Allometry, adult stature and regeneration requirement of 65 tree species on Barro Colorado Island, Panama. J Trop Ecol 22:123–136
Bunker DE, DeClerck F, Bradford JC, Colwell RK, Perfecto I, Phillips OL, Sankaran M, Naeem S (2005) Species loss and aboveground carbon storage in a tropical forest. Science 310:1029–1031
Burslem DFRP, Whitmore TC (1996) Silvics and wood properties of the common timber tree species on Kolombangara. Tropical forest papers 34. Oxford Forestry Institute
Cao KF, Ohkubo T (1998) Allometry, root/shoot ratio and root architecture in understorey saplings of deciduous dicotyledonous trees in central Japan. Ecol Res 13:217–227
Chambers JQ, Higuchi N, Schimel JP (1998) Ancient trees in Amazonia. Nature 391:135
Chambers JQ, dos Santos J, Ribeiro RJ, Higuchi H (2001) Tree damage, allometric relationships, and above-ground net primary production in central Amazon forest. For Ecol Manage 152:73–84
Chambers JQ, Higuchi N, Teixeira LM, dos Santos J, Laurance SG, Trumbore SE (2004) Response of tree biomass and wood litter to disturbance in a Central Amazon forest. Oecologia 141:596–611
Chao KJ, Phillips OL, Gloor E, Monteagudo A, Torres-Lezama A, Martinez RV (2007) Growth and wood density predict tree mortality in Amazon forests. J Ecol 96:281–292
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Riera B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Chave J, Muller-Landau HC, Baker TR, Easdale TA, Ter Steege H, Webb CO (2006) Regional and phylogenetic variation of wood density across 2,456 neotropical tree species. Ecol Appl 16:2356–2367
Chave J, Condit R, Muller-Landau HC, Thomas SC, Ashton PS, Bunyavejchewin S, Co LL, Dattaraja HS, Davies SJ, Esufali S, Ewango CEN, Feeley KJ, Foster RB, Gunatilleke N, Gunatilleke S, Hall P, Hart TB, Hernandez C, Hubbell SP, Itoh A, Kiratiprayoon S, LaFrankie JV, Loo de Lao S, Makana J-R, Noor MNS, Kassim AR, Samper C, Sukumar R, Suresh HS, Tan S, Thompson J, Tongco MDC, Valencia R, Vallejo M, Villa G, Yamakura T, Zimmerman JK, Losos EC (2008) Assessing evidence for a pervasive alteration in tropical tree communities. PLOS Biol 6:e45
Chazdon RL, Fetcher N (1984) Photosynthetic light environments in a lowland tropical rain forest in Costa Rica. J Ecol 72:533–564
Clark DA, Clark DB (1992) Life history diversity of canopy and emergent trees in a neotropical rainforest. Ecol Monogr 62:315–344
Coley PD, Barone JA (1996) Herbivory and plant defences in tropical forests. Annu Rev Ecol Syst 27:305–335
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Dalling JW, Winter K, Nason JD, Hubbell SP, Murawski DA, Hamrick JL (2001) The unusual life history of Alseis blackiana: a shade-persistent pioneer tree. Ecology 82:933–945
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annu Rev Ecol Syst 18:431–451
Enquist BJ, Niklas KJ (2001) Invariant scaling relations across tree-dominated communities. Nature 410:655–660
Enquist BJ, West GB, Charnov EL, Brown JH (1999) Allometric scaling of production and life-history variation in vascular plants. Nature 401:907–911
Escudero A, del Arco JM, Sanz IC, Ayala J (1992) Effects of leaf longevity and retranslocation efficiency on the retention time of nutrients in the leaf biomass of different woody species. Oecologia 90:80–87
Gentry AH (1988) Tree species richness of upper Amazonian forests. Proc Natl Acad Sci USA 85:156–159
Givnish TJ (1988) Adaptation to sun and shade: a whole plant perspective. Aust J Plant Physiol 15:63–92
Givnish TJ, Montgomery R, Goldstein G (2004) Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses and whole-plant compensation points. Am J Bot 91:228–246
Grubb PJ (1998) A reassessment of the strategies of plants which cope with shortages of resources. Perspect Plant Ecol 1:3–31
Hawthorne WD (1995) Ecological profiles of Ghanaian forest trees. Oxford Forestry Institute. Tropical forestry papers no. 29. Nuffield Press, Oxon
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802
Hollander M, Wolfe DA (1999) Non-parametric statistical methods. Wiley, New York
Keeling HC, Phillips OL (2007) A calibration method for the crown illumination index for assessing forest light environments. For Ecol Manage 242:431–437
King DA (1994) Influence of light level on the growth and morphology of saplings in a Panamanian forest. Am J Bot 81:948–957
King DA (2003) Allocation of above-ground growth is related to light in temperate deciduous saplings. Funct Ecol 17:482–488
King DA, Davies SJ, Supardi MNN, Tan S (2005) Tree growth is related to light interception and wood density in two mixed dipterocarp forests of Malaysia. Funct Ecol 19:445–453
King DA, Davies SJ, Tan S, Noor NSM (2006) The role of wood density and stem support costs in the growth and mortality of tropical trees. J Ecol 94:670–680
Kitajima K (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428
Kitajima K, Bolker BM (2003) Testing performance rank reversals among coexisting species: crossover point irradiance analysis. Funct Ecol 17:276–281
Kobe RK (1997) Carbohydrate allocation to storage as a basis of interspecific variation in sapling survivorship and growth. Oikos 80:226–233
Laurance WF, Oliveira AA, Laurance SG, Condit R, Nascimento HEM, Sanchez-Thorin AC, Lovejoy TE, Andrade A, D’Angelo S, Ribeiro JE, Dick CW (2004) Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 428:171–175
Liddell MJ, Nieullet N, Campoe OC, Freiberg M (2007) Assessing the above-ground biomass of a complex tropical rainforest using a canopy crane. Aust Ecol 32:43–58
Lieberman D, Lieberman M (1987) Forest tree growth and dynamics at La Selva, Costa Rica (1969–1982). J Trop Ecol 3:347–358
Lusk CH (2002) Leaf area accumulation helps juvenile evergreen trees tolerate shade in a temperate rainforest. Oecologia 132:188–196
Lusk CH, Contreras O (1999) Foliage area and crown nitrogen turnover in temperate rain forest juvenile trees of differing shade tolerance. J Ecol 87:973–983
Malhi Y, Baker TR, Phillips OL, Almeida S, Alvarez E, Arroyo L, Chave J, Czimczik CI, Di Fiore A, Higuchi N, Killeen TJ, Laurance SG, Laurance WF, Lewis SL, Montoya LMM, Monteagudo A, Neill DA, Vargas PN, Patino S, Pitman NCA, Quesada CA, Salomao R, Silva JNM, Lezama AT, Martinez RV, Terborgh J, Vinceti B, Lloyd J (2004) The above-ground coarse wood productivity of 104 Neotropical forest plots. Glob Change Biol 10:563–591
Malhi Y, Phillips OL, Lloyd J, Baker T, Wright J, Almeida S, Arroyo L, Frederiksen T, Grace J, Higuchi N, Killeen T, Laurance WF, Leano C, Lewis S, Meir P, Monteagudo A, Neill D, Vargas PN, Panfil SN, Patino S, Pitman N, Quesada CA, Rudas-Ll A, Salomao R, Saleska S, Silva N, Silveira M, Sombroek WG, Valencia R, Martinez RV, Vieira ICG, Vinceti B (2002) An international network to monitor the structure, composition and dynamics of Amazonian forests (RAINFOR). J Veg Sci 13:439–450
Mooney HA, Gulman SL (1979) Environmental and evolutionary constraints on the photosynthetic characteristics of higher plants. In: Solbrig OT, Jain S, Johnson GB, Raven PH (eds) Topics in plant population biology. Columbia University Press, New York, pp 316–317
Muller-Landau HC (2004) Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica 36:20–32
Nelson BW, Mesquita R, Pereira JLG, de Souza SGA, Batista GT, Couto LB (1999) Allometric regressions for improved estimate of secondary forest biomass in the central Amazon. Forest Ecol Manage 117:149–167
Nogueira EM, Fearnside PM, Nelson BW, França MB (2007) Wood density in forests of Brazil's ‘arc of deforestation’: Implications for biomass and flux of carbon from land-use change in Amazonia. For Ecol Manage 248:119–135
Pacala SW, Canham CD, Silander JA, Kobe RK (1994) Sapling growth as a function of resources in a north temperate forest. Can J For Res 24:2172–2183
Patiño S, Lloyd J, Paiva R, Quesada CA, Mercado LM, Baker TR, Czimczik CI, Schwarz M, Schmerler J, Sota A, Santos A, Horna V, Peacock J, Wagner M, Arroyo L, Almeida S, Alvarez E, Aguilar A, Bonal D, Gallo J, Herrera R, Higuchi N, Hoyos EJ, Jimenez EM, Killeen T, Leal E, Luizão F, Malhi Y, Meir P, Monteagudo A, Neill D, Núñez Vargas P, Palomino W, Peña-Cruz A, Peñuela MC, Phillips OL, Pitman NL, Priante Filho N, Prieto A, Panfil SN, Rudas A, Salomão R, Silva N, Silveira M, Lezama A, Turriago JD, Vásquez-Martínez R, Vieira I, Villanueva B, Vitzthum P (2008) Branch xylem density variation across the Amazon Basin. Biogeosci Discuss 5:2003–2047
Phillips OL, Martinez RV, Arroyo L, Baker TR, Killeen T, Lewis SL, Malhi Y, Mendoza AM, Neill D, Vargas PN, Alexiades M, Ceron C, Di Fiore A, Erwin T, Jardim A, Palacios W, Saldias M, Vinceti B (2002) Increasing dominance of large lianas in Amazonian forests. Nature 418:770–774
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116:26–37
Poorter L (1999) Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743
Poorter L, Bongers F, Sterck FJ, Wöll H (2005) Beyond the regeneration phase: differentiation of height-light trajectories among tropical tree species. J Ecol 93: 256-267
Poorter L, Bongers L, Bongers F (2006) Architecture of 54 moist-forest tree species: traits, trade-offs, and functional groups. Ecology 87:1289–1301
Reich PB, Ellsworth DS, Uhl C (1995) Leaf carbon and nutrient assimilation and conservation in species of differing successional status in an oligotrophic Amazonian forest. Funct Ecol 9:65–76
Reich PB, Tjoelker MG, Machado JL, Oleksyn J (2006) Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439:457–461
Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Bushena C (1998) Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Funct Ecol 12:327–338
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia 86:16–24
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Roderick ML, Berry SL (2001) Linking wood density with tree growth and environment: a theoretical analysis based on the motion of water. New Phytol 149:473–485
Sack L, Grubb PJ (2001) Why do species of woody seedlings change rank in relative growth rate between low and high irradiance? Funct Ecol 15:145–154
Sack L, Grubb PJ (2003) Crossovers in seedling relative growth rates between low and high irradiance: analyses and ecological potential. Funct Ecol 17:281–287
Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T (2004) Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140:543–550
Swaine MD, Whitmore TC (1988) On the definition of ecological species groups in tropical rain forests. Vegetation 75:81–86
Takyu M, Aiba S-I, Kitayama K (2003) Changes in biomass, productivity and decomposition along topographic gradients under different geological conditions in tropical lower montane forests on Mount Kinabalu, Borneo. Oecologia 134:397–404
ter Steege H, Hammond DS (2001) Character convergence, diversity, and disturbance in tropical rain forest in Guyana. Ecology 82:3197–3212
ter Steege H, Pitman N, Sabatier D, Castellanos H, Van der Hout P, Daly DC, Silveira M, Phillips O, Vasquez R, Van Andel T, Duivenvoorden J, De Oliveira AA, Ek R, Lilwah R, Thomas R, Van Essen J, Baider C, Maas P, Mori S, Terborgh J, Vargas PN, Mogollon H, Morawetz W (2003) A spatial model of tree alpha-diversity and tree density for the Amazon. Biodivers Conserv 12:2255–2277
ter Steege H, Pitman NCA, Phillips OL, Chave J, Sabatier D, Duque A, Molino JF, Prevost MF, Spichiger R, Castellanos H, von Hildebrand P, Vasquez R (2006) Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443:444–447
Tuomisto H, Ruokolainen K, Kalliola R, Linna A, Danjoy W, Rodriguez Z (1995) Dissecting Amazonian biodiversity. Science 269:63–66
Vásquez-Martínez R, Phillips OP (2000) Allpahuayo: floristics, structure and dynamics of a high diversity forest in Amazonian Peru. Ann Mo Bot Gard 87: 499-527
Veenendaal EM, Swaine MD, Lecha RT, Walsh MF, Abebrese IK, Owusu-Afriyie K (1996) Responses of West African forest tree seedlings to irradiance and soil fertility. Funct Ecol 10:501–511
Vormisto J, Phillips OL, Ruokolainen K, Tuomisto H, Vasquez R (2000) A comparison of fine-scale distribution patterns of four plant groups in an Amazonian rainforest. Ecography 23:349–359
Walters MB, Reich PB (1996) Are shade tolerance, survival, and growth linked? Low light and nitrogen effects on hardwood seedlings. Ecology 77:841–853
Walters MB, Reich PB (2000) Trade-offs in low-light CO2 exchange: a component of variation in shade tolerance among cold temperate tree seedlings. Funct Ecol 14:155–165
Walters MB, Kruger EL, Reich PB (1993) Growth, biomass distribution and CO2 exchange of northern hardwood seedlings in high and low light-relationships with successional status and shade tolerance. Oecologia 94:7–16
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159
Whitmore TC (1989) Canopy gaps and the two major groups of forest trees. Ecology 70:536–538
Whitmore TC (1996) A review of some aspects of tropical rain forest seedling ecology with suggestions for further enquiry. In: Swaine MD (ed) The ecology of tropical forest tree seedlings, man and the biosphere series 17. UNESCO, Paris, pp 3–39
Woodward FI, Cramer W (1996) Plant functional types and climatic changes: introduction. J Veg Sci 7:306–308
Wright EF, Coates D, Canham CD, Bartemucci P (1998) Species variability in growth responses to light across climatic regions in northwestern British Columbia. Can J For Res 28:871–886
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright SJ, Muller-Landau HC, Condit R, Hubbell SP (2003) Gap-dependent recruitment, realised vital rates, and size distributions of tropical trees. Ecology 84:3174–3185
Wright JP, Naeem S, Hector A, Lehman C, Reich PB, Schmid B, Tilman D (2006) Conventional functional classification schemes underestimate the relationship with ecosystem functioning. Ecol Lett 9:111–120
Acknowledgements
We thank the School of Geography, University of Leeds, for financial support. This work was partially funded by NERC grant NE/B503384/1. We also thank the Instituto de Investigaciones de la Amazonia Peruana (IIAP) and Explorama for logistical support and Jérôme Chave for use of the ρ data base. Oliver Phillips was supported by a NERC grant and by a Leverhulme Trust Research Fellowship and Tim Baker acknowledges funding from NERC fellowship NE/C517484/1 and a RCUK fellowship at the University of Leeds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stephan Hättenschwiler.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keeling, H.C., Baker, T.R., Martinez, R.V. et al. Contrasting patterns of diameter and biomass increment across tree functional groups in Amazonian forests. Oecologia 158, 521–534 (2008). https://doi.org/10.1007/s00442-008-1161-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1161-4