Abstract
Global environmental changes can have immediate impacts on plant growth, physiology, and phenology. Long-term effects that are only observable after one or more generations are also likely to occur. These transgenerational effects can result either from maternal environmental effects or from evolutionary responses to novel selection pressures and are important because they may alter the ultimate ecological impact of the environmental change. Here, we show that transgenerational effects of atmospheric carbon dioxide (CO2) and soil nitrogen (N) treatments influence the magnitude of plant growth responses to elevated CO2 (eCO2). We collected seeds from Lupinus perennis, Poa pratensis, and Schizachyrium scoparium populations that had experienced five growing seasons of ambient CO2 (aCO2) or eCO2 treatments and ambient or increased N deposition and planted these seeds into aCO2 or eCO2 environments. We found that the offspring eCO2 treatments stimulated immediate increases in L. perennis and P. pratensis growth and that the maternal CO2 environment influenced the magnitude of this growth response for L. perennis: biomass responses of offspring from the eCO2 maternal treatments were only 54% that of the offspring from the aCO2 maternal treatments. Similar trends were observed for P. pratensis and S. scoparium. We detected some evidence that long-term N treatments also altered growth responses to eCO2; offspring reared from seed from maternal N-addition treatments tended to show greater positive growth responses to eCO2 than offspring from ambient N maternal treatments. However, the effects of long-term N treatments on offspring survival showed the opposite pattern. Combined, our results suggest that transgenerational effects of eCO2 and N-addition may influence the growth stimulation effects of eCO2, potentially altering the long-term impacts of eCO2 on plant populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environment is rapidly changing on a global scale, largely as a result of anthropogenic pressures. Plant populations are likely to respond to global changes in the short-term with plastic changes in phenotype. For example, numerous studies have documented immediate increases in plant growth in response to elevated atmospheric carbon dioxide (eCO2) concentrations and nitrogen (N) fertilization (Bazzaz 1990; Reich et al. 2001a; Poorter and Navas 2003; Niklaus and Körner 2004; Reich et al. 2006). Long-term responses that only become apparent after one or more generations are also possible. These transgenerational effects can result from maternal environmental effects, which occur when the environmental conditions experienced by parents influence offspring traits (Roach and Wulff 1987; Rossiter 1996), or from genetic changes in response to altered patterns of natural selection. Regardless of the underlying cause, transgenerational effects may either increase (e.g., Bezemer et al. 1998; Reale et al. 2003) or decrease (e.g., Huxman et al. 1998, 2001) the initial phenotypic response to the environmental change. Thus, the two types of transgenerational effects (maternal environmental and genetic) may alter the long-term predicted effects of environmental change on natural communities.
Maternal environmental effects commonly occur in both plant and animal species when the maternal environment influences resource allocation to offspring. One example of this is when parents occupy resource-rich habitats: offspring size and subsequent growth and fitness are often increased relative to the offspring of parents inhabiting more stressful environments (e.g., Durrant 1958; Aarssen and Burton 1990; Trombulak 1991; reviewed in Rossiter 1996). Maternal environmental conditions can also influence a wide range of other offspring characters, including anti-predator defenses (Agrawal et al. 1999) and physiological traits (Huxman et al. 2001; reviewed in Rossiter 1996). As a result, maternal effects can impact population dynamics (e.g., Ginzburg and Taneyhill 1994), responses to abiotic stress (Aarssen and Burton 1990), and interactions with other community members, such as herbivores and competitors (Agrawal et al. 1999; Platenkamp and Shaw 1993). Maternal environmental effects also can influence plastic responses to variation in the offspring environment (e.g., Aarssen and Burton 1990; Bezemer et al. 1998; Huxman et al. 2001). For example, offspring from maternal plants that had experienced nutrient stress were smaller and germinated later in both low and high nutrient environments, but they also exhibited greater tolerance to low nutrient conditions (Aarssen and Burton 1990). In another example, offspring of plants grown in eCO2 environments showed an increased growth response to eCO2 compared to offspring from plants grown in ambient CO2 (aCO2) environments (Bezemer et al. 1998). As these studies illustrate, the maternal effect can result in reduced or heightened plastic responses to offspring environmental conditions.
Populations also might evolve in response to the novel selection pressures that may accompany environmental change, resulting in a very different type of long-term effect. While maternal environmental effects typically decrease over time and often become non-detectable after one or a few generations or even over the course of the offspring life cycle (Roach and Wulff 1987), genetic changes in response to novel environmental conditions are likely to persist longer, even if organisms are no longer exposed to that environment. Adaptive evolutionary changes are expected to reduce any negative fitness effects of the environmental perturbation; however, these evolutionary changes also have the potential to influence population dynamics (e.g., Yoshida et al. 2003, 2004), ecosystem processes (Wieneke et al. 2004; Collins et al. 2006), interactions with other community members (Snaydon and Davies 1982; Lau et al. 2008), and, most importantly, the magnitude of response to the environmental change itself (Snaydon 1970; Snaydon and Davies 1972, 1982) (reviewed in Hairston et al. 2005).
We have assessed how transgenerational effects of long-term CO2 and N treatments influence the magnitude of the growth response to eCO2 concentrations. We first tested how offspring eCO2 treatments impact plant growth and survival and then examined how maternal CO2 and N environments affect plant growth and growth responses to offspring CO2 treatments. Finally, we investigated how maternal CO2 and N treatments impact resource allocation to seeds and whether differences in seed mass can explain any of the observed transgenerational effects.
Materials and methods
Study system and experimental design
We investigated transgenerational effects of eCO2 and soil N deposition on the growth of Schizachyrium scoparium (Poaceae), Poa pratensis (Poaceae), and Lupinus perennis (Fabaceae). These three species represent different functional groups (C4 grass, C3 grass, and legume, respectively) that are expected to respond differently to eCO2 and N-addition. We performed a partial reciprocal transplant experiment in which we planted seeds collected from populations growing in long-term atmospheric CO2 and soil N treatments into aCO2 and eCO2 environments. Long-term CO2 and N treatments were manipulated in a modified split-plot design as part of the BioCON experiment at Cedar Creek Natural History Area, Minnesota, USA (Reich et al. 2001a). In the BioCON experiment, biodiversity (plant species richness), CO2, and N are all experimentally manipulated. The two CO2 treatments (aCO2 approx. 370 μmol/mol; eCO2 approx. 560 μmol/mol) were applied to whole plots (three 20-m-diameter rings per treatment) using Free Air CO2 Enrichment. Nitrogen treatments (0 or 4 g N m2/year) were applied to half of the 2 × 2-m sub-plots within each whole plot (i.e., ring). Carbon dioxide and N treatments have been applied annually since 1998. Only seeds from the high-diversity (16 plant species) treatment plots were used in this study (n = 48 plots, four plots per N treatment per ring). In 1997, each high-diversity plot was seeded with 12 g/m2 of seed, divided equally among 16 species that were native or naturalized to the site. All seeds were bulked collections, obtained from local nurseries: S. scoparium seed were obtained from Prairie Restorations (Princeton, MN); L. perennis seeds were obtained from the Prairie Moon Nursery (Winona, MN).
Seeds used in this study were collected from a single maternal plant per species from each plot, resulting in 12 half-sib families from each maternal CO2 × N treatment. All seeds were collected in the fall of 2002, after treatments had been applied for five full growing seasons. Even though all plants used in this experiment are perennial species, because plots were initially established from seed, evolutionary responses could result from differential survival of the initial stand of seedlings as well as from genotypic differences in sexual reproduction and recruitment from seed in subsequent years.
Planting design and plant measurements
Seeds were initially planted into 164-ml conetainers (Ray Leach Conetainers; Stuewe & Sons, Corvallis, OR) that had been filled with potting mix (Sunshine Mix #5; Sun Gro Horticulture Canada, Spruce Grove, AB) and bottom-watered until saturated. For S. scoparium and L. perennis, we planted four seeds (all from a single maternal family) per conetainer on 24 April 2006, placed the conetainers in a dark cold-room for 8 days at 4°C, and then moved them to a greenhouse (ambient CO2) where they remained until the seeds began to germinate. For L. perennis, we planted one seed per conetainer on 5 May 2006. Lupinus perennis seeds were scarified and soaked overnight prior to planting to improve germination, but L. perennis individual plants were not inoculated with rhizobia. Because we germinated all plants under aCO2 conditions, we unfortunately missed eCO2 effects on germination and very early seedling growth; however, germinating the plants in this way was necessary for facilitating the germination of a fourth species used in a separate experiment conducted simultaneously. All plants were moved to the field and placed into the appropriate offspring CO2 treatment in the BioCON facility on 7 May 2006. We placed two conetainers from each maternal family into each ring (six conetainers per family per offspring CO2 treatment). A total of 1728 conetainers were planted (12 conetainers per family × 48 maternal families per species × 3 species); however, because of poor germination, which was likely due to the poor quality and limited numbers of some seed, actual sample sizes were much lower (final n = 236, 135, or 264 conetainers for L. perennis, S. scoparium, and P. pratensis, respectively). Sample sizes were roughly equivalent across most maternal environment × offspring environment treatment combinations for most species, and there were no missing cells (i.e., treatment combinations with no data; n = 20–41 plants/treatment combination for L. perennis; n = 25–39 for P. pratensis, and n = 7–26 for S. scoparium). However, we report statistics based on Type 3 sums of squares to further minimize biases due to unequal sample sizes between treatments (Shaw and Mitchell-Olds 1993).
We weighed each individual L. perennis seed prior to planting. Additionally, we obtained family mean seed mass for S. scoparium and P. pratensis by weighing all collected seeds from a given maternal plant and dividing by seed number. For all three species, we recorded emergence date, and on 10 July 2006 we measured the height of the tallest culm and culm number for S. scoparium and P. pratensis and leaf number and leaf size (length of longest leaflet) for L. perennis. After 3 months of growth, when foliage was beginning to turn brown, we harvested aboveground biomass. For S. scoparium and L. perennis, we also harvested the belowground biomass (P. pratensis roots were difficult to separate from the soil) and measured the length of longest root (L. perennis) or belowground biomass (S. scoparium). All biomass was dried at 60°C for at least 2 days prior to weighing. Because four grass seeds were planted into each conetainer, many conetainers contained multiple individuals; all plants in a conetainer were weighed together to obtain total biomass, and heights and culm numbers were averaged for all analyses. Survival was assessed at the final harvest.
Statistical analysis
All analyses were performed using SAS 2001 statistical software (SAS Institute, Cary N.C.).
We used mixed model multivariate analysis of variance (MANOVA; PROC GLM) and ANOVA (PROC MIXED) to determine how both the offspring and maternal growing environments influenced plant growth and plant responsiveness to eCO2. Data for each species were analyzed separately. The N and CO2 treatments that the seeds were collected from (“maternal N treatment” and “maternal CO2 treatment”), the CO2 treatment in which the experimental plants were reared (“offspring CO2 treatment”), and all interactions were included in the model as fixed factors. Ring nested within the offspring CO2 treatment and maternal family nested within the maternal N × maternal CO2 interaction were included as random factors. Emergence date and seed mass were included as covariates in the L. perennis analyses. Mean emergence date, family mean seed mass, and number of individuals per conetainer were included as covariates in the P. pratensis and S. scoparium analyses. Preliminary analyses revealed that seed mass did not significantly influence any P. pratensis or S. scoparium growth measures; therefore, it was not included in the final analyses. Results from models including covariates were qualitatively similar to results from additional analyses that did not include the seed character covariates, so only results from the full model are presented. For L. perennis, final aboveground biomass, root length, leaf number, and leaf length were included as response variables. For the grasses, final aboveground biomass, root biomass (S. scoparium only), culm number, and height were included as response variables.
The effects of maternal CO2 and N treatments and offspring CO2 treatment on survival were tested with logistic ANOVA (Proc GENMOD). In these analyses, maternal CO2 treatment, maternal N treatment, offspring CO2 treatment, and all interactions were included as predictor variables, and the proportion of germinants in each pot surviving to harvest was the response variable. For L. perennis, however, survival was scored as a binomial response variable since only one seed was planted per pot. Emergence date and seed mass were included as covariates. As above, data for each species were analyzed separately.
Because maternal environmental effects often result from differences in resource allocation to offspring, we used ANOVA (PROC MIXED) to investigate how maternal CO2 and N treatments influenced the mass of field-collected seeds used in the reciprocal transplant experiment. For L. perennis, the model included maternal CO2 and maternal N treatments as fixed factors, and maternal family nested within the maternal CO2 × maternal N interaction as a random factor. Individual seed mass was the response variable. For S. scoparium and P. pratensis, the mean family seed mass was the response variable, and maternal CO2 and maternal N treatments were included as predictor variables.
Results
Direct phenotypic effects of CO2
Compared to aCO2 treatments, offspring eCO2 treatments increased L. perennis biomass by 42%, leaflet length by 17%, and leaf number by 12% (Table 1; Fig. 1a). Similarly, eCO2 increased P. pratensis biomass, height, and culm number by 116, 44, and 31%, respectively (Table 1; Fig. 1b). In contrast, S. scoparium, the C4 grass, did not respond significantly to eCO2 (Table 1; Fig. 1c). These results are consistent with prior results for these three species in BioCON (Reich et al. 2001b) as well as with those from other studies demonstrating that species in the C4 functional group tend to show weaker responses to eCO2 (Poorter and Navas 2003). On average, offspring eCO2 treatments increased P. pratensis survival by 21% but did not significantly influence L. perennis or S. scoparium survival (P > 0.3) (Table 2; Fig. 2).
Effects of offspring CO2 treatments on aboveground biomass of Lupinus perennis (a), Poa pratensis (b), or Schizachyrium scoparium (c) populations collected from maternal aCO2 (open symbols) vs. eCO2 (filled symbols) and N-addition (squares) vs. ambient N (circles) treatments. Values shown are least-squares means ± 1SE. Symbols are offset so that non-overlapping error bars can be easily observed
Effects of offspring CO2 treatment on survival of L. perennis (a), P. pratensis (b), or S. scoparium (c) populations collected from maternal ambient CO2 (aCO2; open symbols) vs. elevated CO2 (eCO2; filled symbols) and N-addition (squares) vs. ambient N (circles) treatments. Values shown are raw means ± 1SE. Symbols are offset so that non-overlapping error bars can be easily observed
Transgenerational effects of CO2 and N
Effects of maternal CO2 environment
Offspring response to eCO2 was strongly influenced by the maternal CO2 environment in L. perennis (significant maternal CO2 × offspring CO2 interaction; Table 1). Offspring from aCO2 maternal treatments showed greater biomass increases in response to eCO2 than offspring from eCO2 maternal treatments (Fig. 3). While this difference was only statistically significant for L. perennis, P. pratensis and S. scoparium showed similar trends (Fig. 3). The biomass stimulation effect of offspring eCO2 treatments on plants from eCO2 maternal environments (averaged across N treatments) was only 54, 26, or 72% that of offspring from aCO2 maternal treatments for L. perennis, S. scoparium, and P. pratensis, respectively (Fig. 3). A significant maternal CO2 environment × offspring CO2 environment interaction also was detected for P. pratensis culm number in the univariate analysis, where genotypes from eCO2 maternal treatments showed only 34% of the growth response to offspring eCO2 compared to genotypes from aCO2 maternal treatments (Table 1). The maternal CO2 environment had minimal impacts on other traits (Table 1). We also did not detect any significant effects of maternal CO2 environment or interactions between maternal CO2 and offspring CO2 treatments on the survival of any species (Table 2).
Effects of maternal CO2 environment on the percentage biomass stimulation effect of offspring eCO2 treatments for L. perennis, S. scoparium, or P. pratensis. For all species, genotypes from aCO2 maternal environments (white bars) tended to respond more to offspring eCO2 treatments than genotypes from eCO2 maternal environments (black bars)
Effects of maternal N environment
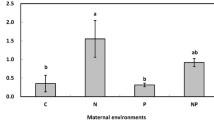
Although the multivariate analyses revealed little evidence that maternal N environment affects L. perennis and S. scoparium growth or growth responses to eCO2, maternal N environment × offspring CO2 environment interactions were detected for L. perennis root length and for S. scoparium height (Table 1). For both species, offspring from maternal N-addition treatments exhibited greater positive growth responses to offspring eCO2 treatments. Lupinus perennis individuals reared from seed collected from ambient N maternal treatments had shorter root lengths when reared under eCO2 than when reared under aCO2, but root lengths of plants reared from N-addition maternal treatments responded positively to eCO2 offspring treatments (a 3% decrease compared to a 9% increase). Similarly, S. scoparium from ambient maternal N environments responded to offspring eCO2 treatments with decreases in height (a 6% decrease), but individuals from N-addition maternal environments were 16% taller when grown in offspring eCO2 than when grown in the aCO2 treatments. Greater positive biomass responses to eCO2 for S. scoparium from N-addition maternal treatments were also observed (Fig. 1c), although this interaction was not statistically significant (P > 0.15). Additionally, maternal N and maternal CO2 treatments interacted to influence the growth response of P. pratensis to offspring eCO2 treatments. A significant three-way interaction between maternal CO2 environment, maternal N environment, and offspring CO2 environment was detected in the multivariate analysis of P. pratensis growth traits (Table 1), but because no significant three-way interactions were detected in the univariate analyses, this effect was likely due to joint effects on multiple traits. In general, the effects of maternal CO2 environment on the response to offspring CO2 environment tended to be greater for P. pratensis from N-addition maternal environments. For P. pratensis from ambient N maternal treatments, plants from aCO2 and eCO2 maternal treatments showed similar increases in biomass in response to offspring eCO2 treatments. For P. pratensis from N-addition maternal treatments, however, the growth stimulation effects of offspring eCO2 treatments were greater on plants from aCO2 maternal treatments than on those from eCO2 maternal treatments, and a marginally significant maternal CO2 × offspring CO2 interaction on biomass was detected for offspring from the maternal N-addition treatment (F 1,54 = 3.55, P = 0.065), but not for offspring from the ambient N maternal treatment (F 1,60 = 0.08, P = 0.78).
Averaged across offspring and maternal CO2 treatments, L. perennis offspring reared from seeds collected from N-addition maternal environments had a lower survival than offspring from seeds from ambient N maternal environments (mean ± SD: N-addition 0.81 ± 0.40; ambient N 0.90 ± 0.30, Table 2). Poa pratensis survival showed similar, but non-significant, patterns (N-addition 0.48 ± 0.49; ambient N 0.60 ± 0.49, P < 0.12). No significant main effect of maternal N environment on survival was detected for S. scoparium (P > 0.5); however, we detected significant interactions between maternal N environment and offspring CO2 environment on S. scoparium survival (Table 2). Genotypes from maternal N-addition treatments showed reduced survival in eCO2 offspring environments compared to aCO2 offspring environments, whereas genotypes from ambient N maternal treatments showed increased survival in offspring eCO2 treatments (Fig. 2c). Furthermore, these effects were heightened when plants were collected from eCO2 maternal environments (Fig. 2c; significant CO2 offspring × CO2 maternal × N maternal interaction, Table 2).
Effects of maternal CO2 and N environments on seed mass
We did not detect any evidence that maternal N treatment affected seed mass of our study species; however, maternal CO2 treatment affected L. perennis and S. scoparium seed mass. Lupinus perennis seeds from eCO2 maternal environments were heavier than those from aCO2 maternal environments (mean seed mass ± SE: aCO2 8.0 ± 0.9 mg; eCO2 11.0 ± 0.9 mg; F 1,44 = 6.13, P = 0.017). Schizachyrium scoparium showed the opposite pattern, and seeds from aCO2 environments were heavier than seeds from eCO2 environments (mean seed mass ± SE: aCO2 1.6 ± 0.06 mg; eCO2 1.3 ± 0.07 mg; F 1,5 = 7.62, P = 0.04). Differences in seed mass that resulted from the maternal environment did not, however, explain the differences in offspring responses to CO2. For L. perennis, seed mass was positively correlated with biomass, but seed mass did not affect biomass response to offspring CO2 treatments (no seed mass × offspring CO2 interaction was detected, F 1,182 = 0.74, P = 0.39), and significant maternal CO2 × offspring CO2 interactions were detected even when seed mass was included in the analyses as a covariate (Table 1). Mean family seed mass did not significantly influence any growth trait in S. scoparium or P. pratensis and also did not affect biomass responses to offspring eCO2 treatments (seed mass × offspring CO2 interaction: S. scoparium F 1,86 = 0.26, P = 0.61; P. pratensis F 1,119 = 2.01, P = 0.16), although seed mass affected P. pratensis survival (Table 2). Results from analyses including germination date and seed mass as covariates were very similar to those from analyses that did not include these covariates. Thus, these results are consistent with differences in seed mass between maternal treatments not being responsible for the differences in offspring responses to CO2.
Discussion
Increases in resource availability as a result of rising atmospheric CO2 concentrations and increased N deposition are expected to stimulate primary productivity. This prediction has been supported by field studies in a variety of natural communities, although biomass increases often depend on community composition and the availability of other nutrients (Reich et al. 2001a; Niklaus and Körner 2004; Norby et al. 2005; Rasse et al. 2005; see also Körner 2003). Because many growth chamber, greenhouse, and even field experiments involve only a single generation of plants, most studies are not of long enough duration to include the contribution of transgenerational effects to the magnitude of treatment response. However, as we have demonstrated here, increased resource availability can cause maternal effects or evolutionary responses that could either increase or decrease the plastic growth responses observed in single generation experiments.
Lupinus perennis and P. pratensis plants grown in eCO2 were larger than those grown in aCO2, but plants grown from seeds collected from maternal eCO2 environments responded less to offspring eCO2 treatments than those grown from seeds collected from maternal aCO2 environments. Thus, the transgenerational effect reduced the plastic phenotypic growth response to eCO2. Similar trends were observed for S. scoparium. Huxman et al. (2001) also found that maternal environmental effects of eCO2 reduced the positive effects of offspring eCO2 treatments on the growth of Bromus madritensis ssp. rubens. While we observed few statistically significant transgenerational effects of N-addition on plant growth or survival, we detected some evidence that plant genotypes collected from N-addition maternal treatments showed greater (more positive) growth responses to offspring eCO2 treatments than genotypes collected from ambient N maternal treatments. For P. pratensis survival, however, the opposite pattern was observed, and genotypes from N-addition maternal treatments exhibited lower survival under offspring eCO2 treatments, while genotypes from ambient N maternal treatments experienced increased survival in eCO2 offspring treatments.
Transgenerational effects may influence long-term responses to global environmental changes. Based on within-generation plastic growth responses to eCO2, eCO2 is predicted to increase primary productivity (i.e., the “eCO2 fertilization effect”). This increased productivity is expected to increase the effectiveness of the terrestrial CO2 sink, thereby slowing the rate of increase in atmospheric CO2 concentration (Hungate et al. 1997; Cramer et al. 2001). The transgenerational effects that we observed, however, suggest that the magnitude of the growth response to eCO2 will decrease over time. Thus, estimates of biomass increases based on short-term plastic growth responses may overestimate the magnitude of the CO2 fertilization effect by as much as 50%. If N-deposition continues to increase along with atmospheric CO2 concentrations, however, the positive transgenerational effects of N-addition on plant growth response to offspring eCO2 treatments may ameliorate some of the decrease in CO2 responsiveness—although the magnitude and direction of transgenerational effects differed across traits (growth vs. survival), and some of the positive transgenerational effects of N-addition on growth responses to eCO2 may be offset by the negative transgenerational effects of N-addition on survival in eCO2 offspring environments.
Because all seeds were germinated in a common greenhouse environment, the effects of offspring CO2 environment on germination and early growth may have been missed. It is our belief, however, that germinating all seeds in a common, relatively benign environment likely had minimal impacts on the observed maternal environment × offspring environment interactions, especially since the magnitude of the maternal effects increased over time (data not shown). Additionally, because we used field-collected seeds that were not propagated for one or more generations in a common environment, we were unable to differentiate between maternal environmental effects and evolutionary responses. Including seed mass and germination date in our analyses did not qualitatively alter the patterns that we observed, suggesting that the observed transgenerational effects were not primarily driven by differences in seed provisioning or germination timing. Seed nutrient concentrations were not measured, however, and are also potential sources of maternal environmental effects. Seeds produced from plants reared under eCO2 and ambient N conditions might be expected to have lower nutrient (N) concentrations that could result in reduced responsiveness to eCO2. It is also plausible that genetic changes that have occurred over the five growing seasons of enhanced CO2 and N also contributed to changes in eCO2 responsiveness: plant populations growing in the long-term CO2 and N treatments could have diverged genetically if N or CO2 alter patterns of natural selection or the expression of genetic variation. While limited work has investigated how soil N influences predict evolutionary trajectories, a few examples suggest that eCO2 can influence plant evolution, although the magnitude of these evolutionary effects is often weak (Bazzaz et al. 1995; Steinger et al. 2007). In contrast, a study by Lau et al. (2007) detected little evidence for direct evolutionary effects of eCO2.
In our experiment, species representing different functional groups seemed to differ in the magnitude of the transgenerational effect. There are at least three potential explanations for these differences among species. First, species differ in the magnitude of ecological response to eCO2 (reviewed in Poorter and Navas 2003). In our experiment, we detected no evidence that eCO2 stimulated the growth of S. scoparium, a C4 plant. In contrast, both L. perennis and P. pratensis responded positively to eCO2. If there is no plastic growth response to eCO2, then it seems less plausible that eCO2 will alter resource allocation to seeds, thus minimizing the potential for maternal environmental effects. Second, species differed in the expression of family-level variation of growth traits and growth response to CO2. Maternal family explained significant amounts of variation in three of four measured L. perennis growth traits (Table 1); in contrast, significant family effects were detected in P. pratensis and S. scoparium only for culm heights (Table 1). These results suggest that while L. perennis may possess the genetic variation required for rapid evolutionary responses, genetic variation for most P. pratensis and S. scoparium growth traits may be lacking in these populations. Finally, interspecific differences in mating system and gene flow, life history, and generation time could all have influenced the likelihood of evolutionary change occurring over the relatively small temporal and spatial scales that these treatments have been applied.
Interestingly, Bezemer et al. (1998) document transgenerational effects of eCO2 on the C3 grass Poa annua that are in the opposite direction of the transgenerational effects found in our study. While basic biological differences between P. annua and the species used in this study may contribute to these contrasting results, methodological differences, including differences in the duration of maternal treatments, could also play a role. In the Bezemer et al. (1998) study, maternal treatments were applied for a single generation, and seeds used in the offspring generation were collected from all plants in the population (not just the most fecund). Thus, the intensity of selection was relatively low, the potential for genetic change during the experiment was minimal, and the observed transgenerational effect was likely primarily a maternal environmental effect. In our study, genetic changes that may have occurred in response to five growing seasons of eCO2 and N-addition treatments also could have contributed to the observed transgenerational effects and potentially could have counteracted any maternal environmental effects that were in the opposing direction.
In conclusion, our results suggest that transgenerational effects of maternal CO2 environments may decrease plant growth responses to future eCO2 conditions. Predictions of the long-term effects of eCO2 on natural communities are typically based on biomass responses to short-term treatments that cannot assess transgenerational effects. Ignoring these multi-generation effects, however, could potentially bias predictions of future biomass and ecosystem responses to eCO2 or other global changes.
References
Aarssen LW, Burton SM (1990) Maternal effects at four levels in Senecio vulgaris (Asteraceae) grown on a soil nutrient gradient. Am J Bot 77:1231–1240
Agrawal AA, Laforsch C, Tollrian R (1999) Transgenerational induction of defences in animals and plants. Nature 401:60–63
Bazzaz FA (1990) The response of natural ecosystems to the rising global CO2 levels. Annu Rev Ecol Syst 21:167–196
Bazzaz FA, Jasienski M, Thomas SC, Wayne P (1995) Microevolutionary responses in experimental populations of plants to CO2-enriched environments: parallel results from two model systems. Proc Natl Acad Sci USA 92:8161–8165
Bezemer TM, Thompson LJ, Jones TH (1998) Poa annua shows inter-generational differences in response to elevated CO2. Glob Chang Biol 4:687–691
Collins S, Sultemeyer D, Bell G (2006) Changes in C uptake in populations of Chlamydomonas reinhardtii selected at high CO2. Plant Cell Environ 29:1812–1819
Cramer W, Bondeau A, Woodward FI, Prentice IC, Betts RA, Brovkin V, Cox PM, Fisher V, Foley JA, Friend AD, Kucharik C, Lomas MR, Ramankutty N, Sitch S, Smith B, White A, Young-Molling C (2001) Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Glob Chang Biol 7:357–373
Durrant A (1958) Environmental conditioning of flax. Nature 181:928–929
Ginzburg LR, Taneyhill DE (1994) Population cycles of forest lepidoptera: a maternal effect hypothesis. J Anim Ecol 63:79–92
Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA (2005) Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett 8:1114–1127
Hungate BA, Holland EA, Jackson RB, Chapin FS, Mooney HA, Field CB (1997) The fate of carbon in grasslands under carbon dioxide enrichment. Nature 388:576–579
Huxman TE, Hamerlynck EP, Jordan DN, Salsman KJ, Smith SD (1998) The effects of parental CO2 environment on seed quality and subsequent seedling performance in Bromus rubens. Oecologia 114:202–208
Huxman TE, Charlet TN, Grant C, Smith SD (2001) The effects of parental CO2 and offspring nutrient environment on initial growth and photosynthesis in an annual grass. Int J Plant Sci 162:617–623
Körner C (2003) Ecological impacts of atmospheric CO2 enrichment on terrestrial ecosystems. Philos Trans R Soc Lond B 361:2023–2041
Lau JA, Shaw RG, Reich PB, Shaw RG, Tiffin P (2007) Strong ecological but weak evolutionary effects of elevated CO2 on a recombinant inbred population of Arabidopsis thaliana. New Phytol 175:351–362
Lau JA, Strengbom J, Stone LR, Reich PB, Tiffin P (2008) Direct and indirect effects of CO2, nitrogen, and community diversity on plant–enemy interactions. Ecology 89:226–236
Niklaus PA, Körner C (2004) Synthesis of a six-year study of calcareous grassland responses to in situ CO2 enrichment. Ecol Monogr 74:491–511
Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R, De Angelis P, Finzi AC, Karnosky DF, Kubiske ME, Lukac M, Pregitzer KS, Scarascia-Mugnozza GE, Schlesinger WH, Oren R (2005) Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci USA 102:18052–18056
Platenkamp GAJ, Shaw RG (1993) Environmental and genetic maternal effects on seed characters in Nemophila menziesii. Evolution 47:540–555
Poorter H, Navas M-L (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157:175–198
Rasse DP, Peresta G, Drake BG (2005) Seventeen years of elevated CO2 exposure in a Chesapeake Bay Wetland: sustained but contrasting responses of plant growth and CO2 uptake. Glob Chang Biol 11:369–377
Reale D, McAdam AG, Boutin S, Berteaux D (2003) Genetic and plastic responses of a northern mammal to climate change. Proc R Soc Lond B 270:591–596
Reich PB, Knops J, Tilman D, Craine J, Ellsworth D, Tjoelker M, Lee T, Wedin D, Naeem S, Bahauddin D, Hendrey G, Jose S, Wrage K, Goth J, Bengston W (2001a) Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 410:809–812
Reich PB, Tilman D, Craine J, Ellsworth D, Tjoelker MG, Knops J, Wedin D, Naeem S, Bahauddin D, Goth J, Bengston W, Lee TD (2001b) Do species and functional groups differ in acquisition and use of C, N and water under varying atmospheric CO2 and N availability regimes? A field test with 16 grassland species. New Phytol 150:435–448
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Syst 18:209–235
Rossiter MC (1996) Incidence and consequences of inherited environmental effects. Annu Rev Ecol Syst 27:451–476
Shaw RG, Mitchell-Olds T (1993) ANOVA for unbalanced data: an overview. Ecology 74:1638–1645
Snaydon RW (1970) Rapid population differentiation in a mosaic environment. I. The response of Anthoxanthum odoratum populations to soils. Evolution 24:257–269
Snaydon RW, Davies MS (1972) Rapid population differentiation in a mosaic environment. II. Morphological variation in Anthoxanthum odoratum. Evolution 26:390–405
Snaydon RW, Davies TM (1982) Rapid divergence of plant populations in response to recent changes in soil conditions. Evolution 36:289–297
Steinger T, Stephan A, Scmid B (2007) Predicting adaptive evolution under elevated atmospheric CO2 in the perennial grass Bromus erectus. Glob Chang Biol 13:1028–1039
Trombulak SC (1991) Maternal influence on juvenile growth rates in Belding ground squirrel (Spermophilus beldingi). Can J Zool 69:2140–2145
Wieneke S, Prati D, Brandl R, Stocklin J, Auge H (2004) Genetic variation in Sanguisorba minor after 6 years in situ selection under elevated CO2. Glob Chang Biol 10:1389–1401
Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG Jr (2003) Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424:303–306
Yoshida T, Hairston NG Jr, Ellner SP (2004) Evolutionary tradeoff between defense against grazing and competitive ability in a simple unicellular alga, Chlorella vulgaris. Proc R Soc Lond B 271:1947–1953
Acknowledgements
We thank A. Mueller for her assistance in field and R. Shaw, J. Dechaine, and two anonymous reviewers for providing helpful comments on an earlier draft of this manuscript. This project was funded primarily by NSF IOB 0417094 to P. Tiffin, R. Shaw, and P. Reich and secondarily by NSF LTER (DEB 0080382) and Biocomplexity (0322057) programs, and a University of Minnesota Initiative on Renewable Energy and the Environment seed grant. This is KBS contribution #1462. The experiments described herein comply with current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Manuel Lerdau.
Rights and permissions
About this article
Cite this article
Lau, J.A., Peiffer, J., Reich, P.B. et al. Transgenerational effects of global environmental change: long-term CO2 and nitrogen treatments influence offspring growth response to elevated CO2 . Oecologia 158, 141–150 (2008). https://doi.org/10.1007/s00442-008-1127-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1127-6