Abstract
Seasonal tropical forests exhibit a great diversity of leaf exchange patterns. Within these forests variation in the timing and intensity of leaf exchange may occur within and among individual trees and species, as well as from year to year. Understanding what generates this diversity of phenological behaviour requires a mechanistic model that incorporates rate-limiting physiological conditions, environmental cues, and their interactions. In this study we examined long-term patterns of leaf flushing for a large proportion of the hundreds of tree species that co-occur in a seasonal tropical forest community in western Thailand. We used the data to examine community-wide variation in deciduousness and tested competing hypotheses regarding the timing and triggers of leaf flushing in seasonal tropical forests. We developed metrics to quantify the nature of deciduousness (its magnitude, timing and duration) and its variability among survey years and across a range of taxonomic levels. Tree species varied widely in the magnitude, duration, and variability of leaf loss within species and across years. The magnitude of deciduousness ranged from complete crown loss to no crown loss. Among species that lost most of their crown, the duration of deciduousness ranged from 2 to 21 weeks. The duration of deciduousness in the majority of species was considerably shorter than in neotropical forests with similar rainfall periodicity. While the timing of leaf flushing varied among species, most (∼70%) flushed during the dry season. Leaf flushing was associated with changes in photoperiod in some species and the timing of rainfall in other species. However, more than a third of species showed no clear association with either photoperiod or rainfall, despite the considerable length and depth of the dataset. Further progress in resolving the underlying internal and external mechanisms controlling leaf exchange will require targeting these species for detailed physiological and microclimatic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of new leaves in a tree requires a significant investment of carbon, water and nutrients, the availability of which is directly and indirectly influenced by intra- and interannual variations in environmental conditions. In the temperate zone, intra-annual temperature seasonality exerts the dominant control over the timing of leaf flushing (e.g. Kramer and Kozlowski 1979; Valentine 1983; Lechowicz 1984). In tropical forests, climatic seasonality is primarily a function of intra-annual variation in water availability (Borchert 1994a, 1994b, 1994c; Reich et al. 2004). In the seasonal or monsoonal tropical forests, where rainfall seasonality is most pronounced, this has led to a great diversity of leaf exchange patterns—trees may produce new leaves during the wet season, the dry season, or the transition between seasons; trees may lose all of their leaves, some of their leaves, or none of their leaves; and these patterns may vary within and among individuals and species and from year to year.
Variation in the timing and intensity of leaf exchange among sympatric tree species is influenced by a range of internal physiological rate-limiting thresholds (e.g. internal water status, nonstructural carbohydrate supply) and external environmental cues (e.g. photoperiod, soil water availability, vapour pressure deficit) that may interact in complex ways. In seasonal tropical forests around the world, the timing of leaf exchange varies within and among regions (Murphy and Lugo 1986). Leaf expansion requires cell enlargement—a water-intensive process that is highly susceptible to water stress (Borchert 1994a, 1994b). Intuitively, then, leaf flushing should occur when water and carbon availability are high (Chabot and Hicks 1982)—that is, during the wet season. Indeed, wet season leaf flushing is common in dry forests in Africa (Lieberman 1982; Lieberman and Lieberman 1984) and the neotropics (Frankie et al. 1974; Bullock and Solis-Magallanes 1990). However, the occurrence of leaf flushing during the dry season, which would appear to contravene this rationale, is well-documented in tropical savannas of north Australia (Williams et al. 1997) and Brazil (Rivera et al. 2002), dry lowland forest in Costa Rica (Borchert 1994a), deciduous forest in India (Elliott et al. 2006), and seasonal tropical forests in Thailand (Rivera et al. 2002; Elliott et al. 2006), Argentina and Java (Rivera et al. 2002).
While dry season leaf exchange may seem counterintuitive, the production of new leaves during a period of high water stress implies that the tree has access to or has accumulated sufficient water and carbon reserves for leaf expansion. Given sufficient reserves, the timing of leaf expansion may then depend upon the appropriate environmental cue, such as rainfall (Walter 1971; Lieberman 1982; Reich and Borchert 1984; Borchert 1994b), temperature (Walter 1971; Morellato et al. 2000) or photoperiod (Rivera et al. 2002; Borchert et al. 2005; Elliott et al. 2006). Many phenological studies of seasonal tropical forests have focused on rainfall as a proximate cue for leaf flushing because of the rate-limiting threshold imposed by water availability (Reich and Borchert 1984; Borchert 1994a, 1994b, 1994c). However, the results have been equivocal. Rainfall is clearly not the only factor triggering leaf exchange (Wright and Cornejo 1990; Rivera et al. 2002; Kushwaha and Singh 2005; Elliott et al. 2006) and irrigation studies have failed to induce leaf flushing consistently among species within a seasonal tropical forest community (Rivera et al. 2002). Recent attention has focused on photoperiod, in particular the rapid change in day-length that occurs around the spring equinox, as a trigger of leaf flushing (Borchert et al. 2005). However, these studies have also failed to account for a considerable proportion of the variation in leaf flushing behaviour. Where sufficient water or carbon reserves are not present, leaf flushing will not occur despite the occurrence of the requisite environmental cue. For example, Shorea siamensis in western Thailand experience bud break several weeks before the spring equinox, but leaf expansion is limited to those individuals with ready access to subsurface soil water reserves (Elliott et al. 2006). For those individuals with small root systems or that are distant from a ready source of subsurface soil water, leaf expansion occurs only after the first significant rains, when water availability is no longer rate-limiting.
Reconciling the range of leaf flushing behaviours among diverse biogeographic regions and tree flora requires a general mechanistic model of foliar phenology that integrates both internal and external factors and the interactions among them. In this study we use “deciduousness” as an organizing concept to evaluate the range of leaf exchange behaviour from an extensive, decade-long phenology survey of a seasonal tropical forest in western Thailand. In this area where forest types dominated by deciduous or evergreen tree species occur in mosaic fashion across the landscape, leaf exchange patterns at the community level are particularly complex and poorly documented (Elliot et al. 2006). However, the wide range of leaf exchange patterns, species-specific physiologies, and local environmental heterogeneity provide a unique opportunity to investigate the relative importance of specific internal and external factors on leaf exchange.
In this study we examine variation in the timing, magnitude, and consistency of leaf flushing behaviour within and among conspecific trees, within and among years, and among different tree species. We use these data to define the range of variation in leaf flushing behaviour among a diverse set of species and test the role of photoperiod and rainfall as potential external cues for leaf flushing.
Methods
Study site
The study was conducted at the Huai Kha Khaeng (HKK) Wildlife Sanctuary, Uthai Thani Province in central-west Thailand (15°N, 100°E). Huai Kha Khaeng is dominated by three forest types that occur in a landscape mosaic: seasonal dry evergreen forest, mixed deciduous forest, and deciduous dipterocarp forest. Foliar phenology was monitored in and around a 50-ha forest dynamics plot dominated by seasonal dry evergreen forest. Seasonal evergreen forest at HKK is structurally complex, with trees reaching heights of 60 m, and species-rich, with >250 tree species. While the seasonal evergreen forest has a complex history of natural disturbances, the area of forest in which we conducted the phenological study has no history of human disturbance (Baker et al. 2005).
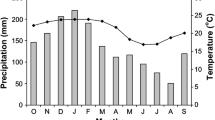
This part of Thailand has a distinct monsoon climate. The study area receives ca. 1,500 mm of rainfall each year, with >90% occurring between April and October. While the dry season is typically considered to extend from November to April, there is interannual variation in the timing of the onset of the monsoons. In some years the first significant rains may arrive as early as mid-March, while in other years the rains may arrive as late as mid-May. The early dry season is comparatively cool (mean minimum and maximum January temperatures are 18.3 and 32.5°C, respectively), with maximum daily temperatures reaching a peak during the late dry season (mean minimum and maximum April temperatures are 25.6 and 37.9°C, respectively) (Rundel and Boonpragob 1995).
Data collection
The full dataset includes observations of the phenological conditions of 1,383 individual trees from 218 species. Because some species are only represented in the dataset by one or a few individuals we have restricted our analysis to the 85 tree species with >5 individuals, Table 1). These species include all of the common species and many of the less common species on the 50-ha plot. For these 85 species, observations were made at two-week intervals from April 1999 to April 2004. For 28 species, observations were also made at two-week intervals from April 1994 to 1997 (Table 1). Phenological activity of each tree was recorded using an incremental scale (“phenology score”) of 0–4 to record the proportions of the tree crown present as new (light green leaves), mature (dark-green leaves), old (brown, red, or yellow leaves) or fallen leaves; 0 = 0%, 0.1 = rare, 1 = up to 25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100%, such that each tree’s phenology score summed to four. Analyses of the spatial distribution of tree species on the 50-ha plot relative to known edaphic and topographic variations have shown little evidence of habitat specialization among the tree species. All trees surveyed were reproductively mature individuals and were not suppressed. In those cases in which a tree died during the study period it was replaced with a nearby individual of the same species. However, this was relatively uncommon (<1% of the study trees).
Data preparation
Phenological observations were tabulated and checked for missed phenophases where consecutive observations showed the crown changing from fallen to mature leaves without an intervening observation of leaf flushing. In instances where a leaf-flushing event was missed between observation periods, an observation of new leaves (of phenological intensity equivalent to the change from fallen to mature leaves) was inserted on the date halfway between two observation periods. Where a tree died during the study period, data preceding the tree’s death were excluded from analysis, based on a qualitative assessment of typical phenological behaviour, to remove atypical phenological observations related to the health of the tree.
Data analysis
Characteristics of deciduousness
We examined the nature of deciduousness within and among species in the community using two quantitative metrics: the magnitude and duration of deciduousness. The magnitude of deciduousness for each species was determined by calculating the maximum leaflessness of each individual during each phenological year (November–November) and averaging these values for the species to give the mean maximum magnitude of deciduousness (D m ) for each species. We estimated the duration of deciduousness for those species with D m scores > 2 (i.e. moderate to high magnitudes of deciduousness). For each species, the number of weeks that each tree was at or near the species-specific D m was calculated for each phenological year and then averaged to obtain the mean duration of deciduousness (D d ). For both metrics, standard errors were calculated to provide an index of interannual and intraspecific variability.
Timing of leaf flushing
Circular statistics were used to analyse the timing of leaf flushing (Batchelet 1981; Milton 1991; Davies 1999; Morellato et al. 2000). Initially, dates of leaf flushing were converted to angles from 0 to 360°. Observations of leaf flushing were weighted according to their intensity (i.e. phenology score of 0.1, 1–3, or 4). As data were collected on a linear scale, statistical weighting was performed by substituting intensity with frequency—for example, an observation of phenology score 2 was presented as two observations of leaf flushing on that date (Dan Bebber, personal communication). The mean angle \( (\ifmmode\expandafter\bar\else\expandafter\=\fi{\theta }), \) which represents the mean date of leaf flushing, and the vector length (r), which represents the temporal concentration of data around the mean angle, were then calculated using the formulae:
where n is the number of observations of phenological activity and θ i is the angle representing the date of phenological activity (Davies and Ashton 1999). The vector length, r, is a value between 0 and 1; 0 if all data are evenly distributed around the circle and 1 if all data occur on a single point (Batchelet 1981; Milton 1991; Zar 1999). We tested whether the mean angle was nonrandom using Rayleigh’s test (Batchelet 1981; Zar 1999). We then back-transformed the mean angle to determine the mean date of flushing.
Proximate causes of leaf flushing
To determine the relative importance of rainfall and photoperiod as triggers of leaf flushing, we compared the timing of leaf flushing of 24 species with sufficient phenology data to daily rainfall data for the period 1994–1997. In principle, distinguishing between these two factors as leaf flushing triggers should be relatively straightforward. Rainfall varies substantially in timing and intensity during the dry season and the onset of the monsoons, whereas photoperiod is invariant at temporal and spatial scales relevant to community ecology. Thus if photoperiod triggers leaf flushing, there should be both intraspecific and interannual synchrony in the emergence of new leaves. In contrast, if rainfall triggers leaf flushing, new leaves should not emerge before the first substantial rains (defined as ≥ 20 mm; Rolf Borchert, personal communication). However, this assumes that the internal water status is directly correlated with rainfall availability. Where trees have access to deep soil water (e.g. Nepstad et al. 1994) or are capable of storing water in their stems during the dry season (e.g. Chapotin et al. 2006), this correlation may be weak or nonexistent. Nonetheless, it is reasonable to expect that the internal water status of the tree, which is a function of species-specific physiology, soil–water availability, rainfall, temperature, and relative humidity, will show considerably more interannual variation than photoperiod.
Results
Characteristics of deciduousness
The magnitude of deciduousness (D m ) among the 85 species covered the full range of potential scores, from no loss of foliage (D m = 0) to complete canopy loss (D m = 4) (Fig. 1). Most species displayed either a low magnitude of deciduousness (49% of species had a D m < 1) or a high magnitude of deciduousness (32% of species had a D m >3; Fig. 1). For species with an intermediate magnitude of deciduousness there was a gradient of D m scores (Fig. 1).
Mean magnitude of deciduousness (D m )—the mean proportion of crown loss for each of the 85 species (solid symbols)—and mean duration of deciduousness (D d )—the mean number of consecutive months spent at or near maximum leaf fall, for the 34 species showing an average maximum leaf fall of phenology score >2 (open symbols). Error bars represent standard error
Most species showed interannual variation in D m (represented by the error bars in Fig. 1). While eight species lost their entire crown each year, Persea sp. was the only species to remain completely evergreen. Interannual variability in Dm was lowest for species with high scores. For example, Pterocarpus macrocarpus had a D m of 3.9 and showed high synchrony among years in the magnitude and timing of crown loss (Fig. 2). In contrast, interannual variability in D m was greatest for species with intermediate scores. For example, Acer oblongum had a D m of 1.6 and showed considerable variability among years in the magnitude and timing of crown loss (Fig. 2). The highest interannual variability was observed in Shorea obtusa, with a D m of 2.4 and a standard deviation of 1.9; individuals in some years lost their entire crown and in other years did not lose any of their crown (Fig. 1).
For the 34 species with a D m > 2, the duration of deciduousness (D d ) ranged from <3 weeks in Shorea obtusa, to ∼5 months in Tetrameles nudiflora (Fig. 1). The majority of species (70%) had D d values of <2 months (Fig. 1). Only four species were deciduous for more than 2.5 months (T. nudiflora, Spondias pinnata, Garuga pinnata and Vitex glabrata). Interannual variation in D d was evident, with standard deviations ranging from 0.1 months in V. glabrata to 1.5 months in Lithocarpus ceriferus. However, the variation in D d scores was not correlated with the means of the D d scores.
In general, species with low D m scores had low D d values (R 2 = 0.39; Fig. 3); however, this relationship was not consistent. For example, both S. pinnata and V. peduncularis generally lost their entire crown. However, S. pinnata on average was leafless for four months, whereas V. peduncularis was leafless for 1.3 months.
Timing and patterns of leaf flushing
In all species, the timing of leaf flushing was significantly nonrandom (Rayleigh’s test, P < 0.005). Within the community, the timing of leaf flushing varied widely among the 85 species (Fig. 4); however, the majority of species (∼70%) had their mean flushing date during the dry season and 90% of these flushed during the last two months of the dry season (Fig. 4). Of the species flushing new leaves during the wet season, most flushed in the early wet season, from mid-April to mid-May. Only four species, Dipterocarpus obtusifolius, Cyathocalyx martabanicus, Persea sp., and Chionanthus callophyllus, flushed after June (Fig. 4). All species with mean flushing dates at less common times, such as the late wet season and early dry season, were evergreen species.
Summary of foliar phenology for the 85 species analysed and average rainfall in mm at the study site (presented as cumulative over the previous 14 days) for four years during the study period (1995, 1996, 1999 and 2000; error bars represent standard error). Species are grouped as either deciduous (magnitude of deciduousness of phenological score >2) or evergreen, with deciduous stem succulent species (sensu Borchert 1994a; Borchert and Rivera 2001) grouped separately. Mean date of leaf flushing is indicated by a filled square and error bars represent the standard deviation of interannual variation in the mean flushing date (note that the standard deviation for Dipterocarpus obtusifolius is 72 and is truncated in the figure). Hatched lines represent the duration of deciduousness (shown only for species grouped as deciduous). The broken line represents the spring equinox
The degree of interannual synchrony in leaf flushing varied among species (error bars, Fig. 4). In some species, the mean date of leaf flushing occurred within the same 14-day period in all years of observation (e.g. Dalbergia cochinchinensis, D. oliveri and Pterospermum grandiflorum). In other species the mean date of leaf flushing differed among years by >5 months (e.g. Champereia manillana, L. ceriferus, and Memecylon ovatum). In general, greater interannual synchrony was observed in species with a greater D m (Fig. 4).
The degree of intraspecific synchrony in the timing of leaf flushing varied among species. Some species, for example Premna villosa, S. obtusa and Ardisia polycephala, had synchronous leaf flushing, with all conspecific individuals flushing within a 14-day period. Other species, for example Duabanga grandiflora and Memecylon ovatum, showed asynchrony in leaf flushing with the mean date of leaf flushing differing among conspecific individuals by >5 months.
Four genera were examined that each contained three species (Lagerstroemia, Terminalia, Diospyros, and Shorea). Terminalia and Lagerstroemia showed relatively high congeneric synchrony in the timing of leaf flushing (r = 0.999; Fig. 5a, b). The Lagerstroemia species (L. macrocarpa, L. tomentosa, and L. villosa) had mean flushing dates within five days in mid-April, while the Terminalia species (T. bellirica, T. glaucifolia and T. mucronata) had mean flushing dates within eight days in early April (Fig. 5a, b). The three Diospyros species were less synchronous, with mean flushing dates spread over ∼1.5 months (Fig. 5c). Despite relatively high intraspecific synchrony in Shorea species (r ≥ 0.87), flushing of Shorea congeners was generally asynchronous with mean flushing dates spread over ∼2 months (Fig. 5d).
Mean patterns (from September 1999 to 2003) in leaf flushing for congeneric species from a Terminalia, b Lagerstroemia, c Diospyros, and d Shorea, and for confamilial species from e Meliaceae and f Dipterocarpaceae. Note that two Dipterocarpaceae species, S. roxburghii and S. siamensis, are excluded from Fig. 5f for clarity but are shown in Fig. 5d. Note also that the scale of the y-axis varies among figures. The broken line represents the spring equinox
The degree of synchrony in the timing of leaf flushing varied among families. In some families, such as the Meliaceae, species all had mean flushing dates within 1.5 months (Fig. 5e). In contrast, other families showed much less synchronous interspecific flushing. For example, the eight species of Dipterocarpaceae had mean dates of leaf flushing ranging from October to April, spanning the entire dry season (Fig. 5f).
Proximate causes of leaf flushing
The synchrony of flushing among conspecifics, across years and relative to the timing of rainfall, varied among species. The first substantial rainfall occurred later in 1995 than in 1996 and 2000. Six of the 24 species analysed had mean dates of leaf flushing that were relatively asynchronous among years and conspecifics, but that were consistent with interannual variation in the timing of rainfall and always occurred after the first rain of ≥20 mm. Nine species had mean dates of leaf flushing that were relatively asynchronous among years and individuals, and did not correlate with rainfall patterns. Nine species showed consistency among years and conspecifics in the timing of leaf flushing, despite the varied rainfall patterns.
Discussion
The nature of deciduousness in a seasonal tropical forest
Substantial within-stand variation in the nature of deciduousness has been reported in many forests, including tropical deciduous forest in India (Kushwaha and Singh 2005) and subtropical deciduous forest in Argentina (Rivera et al. 2002). In this study, the magnitude, duration and interannual variation of deciduousness was found to vary greatly among species. Only a weak positive association was observed between duration and magnitude of deciduousness. Accordingly, there was a continuum in the magnitude, duration and interannual variability of deciduousness among the 85 species that we examined from this forest community. Within this forest, the duration of deciduousness was generally shorter than in many neotropical forests with comparable rainfall periodicity. Only four species were observed to be deciduous for >2.5 months, compared with many species in the seasonal dry tropical forest of Costa Rica (Frankie et al. 1974; Borchert 1994a) and Mexico (Bullock and Solis-Magallanes 1990) that are deciduous for >4 months. This suggests that foliar phenology is not reliably predicted by rainfall periodicity (Elliott et al. 2006).
Timing and patterns of leaf flushing
Among species within HKK, leaf flushing was not randomly distributed throughout the year. Leaf flushing during the dry season, and especially the late dry season, was predominant. The presence and prevalence of dry season leaf flushing is not a uniform feature of tropical deciduous forests. However, the prevalence of dry season leaf flushing in this study is comparable to a subtropical forest in Argentina where 94% of the 50 species analysed flushed new leaves during the dry season (Rivera et al. 2002), and a Northern Australian savanna (Williams et al. 1997) where 80% of dominant species flushed leaves during the dry season.
In general, less synchrony in the timing of leaf flushing was observed within and among species that had a lower magnitude of deciduousness. A lack of synchrony in foliar phenology within and among species and years has been observed in aseasonal tropical rainforest (e.g. Frankie et al. 1974; Reich 1995; Reich et al. 2004). Selection for synchrony in a species’ foliar phenology may relate to the intensity of seasonality (Reich 1995; Reich et al. 2004) and therefore be strongest in species with marked intra-annual changes in phenological attributes, namely more deciduous habits.
Given the severity of seasonal drought and the physiological dependence of leaf flushing upon water, the existence of dry season leaf flushing—let alone its prevalence—seems counterintuitive. Physiologically, this phenomena implies that the tree must have access to or have accumulated sufficient reserves of water for leaf expansion. For some individuals subsoil water reserves may provide the requisite water source for flushing leaves during the dry season (Borchert 1994a; Nepstad et al. 1994; Elliott et al. 2006). Despite flushing during the dry season, most species in this study did not flush leaves before the first significant rainfall event. This rainfall is unlikely to influence soil water availability for the trees, but may facilitate leaf flushing by increasing relative humidity and reducing vapour pressure deficit (Duff et al. 1997; Myers et al. 1998). Water storage within plants may also influence phenological patterns (Borchert 1994a; Borchert and Rivera 2001; Rivera et al. 2002; Chapotin et al. 2006). In this study, however, water storage ability was not observed to facilitate dry season leaf flushing, with the two stem succulent species (Spondias pinnata and Tetrameles nudiflora) observed to consistently flush leaves during the early wet season. Ecologically, the prominence of dry season leaf flushing suggests an adaptive advantage of this phenological behaviour over evolutionary time. Dry season leaf flushing may be advantageous in avoiding or minimising damage from herbivores because young, vulnerable leaves are not being produced during the wet season when insects may be abundant (Aide 1992; Rivera et al. 2002). Leaf flushing during the late dry season may also be advantageous in maximising the length of the growing season by producing young, photosynthetically efficient leaves before the onset of the wet season (Rivera et al. 2002; Elliott et al. 2006).
Proximate causes of leaf flushing
The proximate cause of leaf flushing varied among the 24 species that we examined in detail. Of these species, 38% had intraspecifically and interannually synchronous phenological patterns indicating photoperiodic control of leaf flushing and 25% had phenological patterns indicating rainfall as the proximate cause. The remaining 37% of the species did not have phenological patterns consistent with either photoperiod or rainfall as the proximate trigger of leaf flushing. The phenological behaviour of these species requires further analysis. However, phenological patterns in some of these species may be opportunistic and consistent with the “irregular leaf exchangers” described in Elliott et al. (2006). In irregular leaf exchangers, leaf flushing is primarily controlled by internal factors, with the timing of leaf flushing related to leaf aging and soil water availability (Rivera et al. 2002; Elliott et al. 2006).
Disentangling the proximate cause of leaf flushing is complicated by the difficulty of defining synchrony and confounding environmental factors. The degree to which an external trigger may drive intraspecific and interannual synchrony is dependent on the physiological requirements of an individual being met (Reich 1995; Elliott et al. 2006). For all species, the availability of water acts as a physiological threshold for leaf flushing. Environmental variation (e.g. variation in soil and topography) may influence water availability; therefore, the degree of synchrony across the population and among years may be lower than theoretically expected for photoperiodic control. Correlation between phenology and rainfall patterns may also be complicated by environmental heterogeneity and microsite-specific variation that may influence the association between rainfall and the availability of water to an individual (Reich and Borchert 1984; Bullock and Solis-Magallanes 1990; Rivera et al. 2002; Elliott et al. 2006). In addition, cloud cover associated with rainfall may reduce the availability of nonstructural carbohydrates that are necessary for leaf formation (Graham et al. 2003). These complications may indicate the need to integrate intensive physiological and microclimatological studies with correlative analyses to determine the proximate causes of leaf flushing.
Phenology and phylogeny
An examination of phenological patterns at successive phylogenetic levels may provide a framework for examining community dynamics and the evolution of phenologies. Rivera et al. (2002) suggested that functional phenological types that include characteristics of deciduousness and the proximate triggers of leaf flushing might be conserved within congeneric taxa. Among the 24 species analysed to determine their proximate cause of leaf flushing, phenological patterns of confamilial species varied in similarity. For example, the three Fabaceae species (Afzelia xylocarpa, Cassia fistula and Pterocarpus macrocarpus) all appeared to have leaf flushing triggered by photoperiod. In contrast, the three Dipterocarpaceae species (Dipterocarpus obtusifolius, Hopea odorata and Vatica cinerea) showed no consistent pattern in the timing of their leaf flushing. The existence of phylogenetic similarities in proximate triggers may provide an indication of the physiological basis of external cues. However, in our study phylogenetic patterns in proximate triggers were inconsistent despite the large number of related sympatric species that we examined.
Further research
To further understand dry season leaf flushing, there is a need to integrate the findings of observational and physiological studies. Several recent studies have investigated aspects of the physiological controls and constraints on foliar phenology. For example, studies of trees in seasonal dry tropical forest have investigated water relations and gas exchange (Choat et al. 2006), hydraulic architecture (Choat et al. 2005), and relationships between hydraulics and photosynthesis (Brodribb et al. 2002) across seasons and in species with differing foliar phenologies. An extensive field survey such as ours can provide the necessary context for species selection to target specific phenological comparisons or contrasts that may be informative and help bridge the gap between correlative and physiological analyses. Our dataset identifies a number of species that are brevideciduous (e.g. L. ceriferus, S. obtusa, Litsea semicarpifolia) but show considerable interannual and intraspecific variation in the timing of leaf flushing. A more intensive study focusing on microsite conditions and stem and leaf physiology could be developed to address the underlying causes of this variability.
Developing a conceptual model of functional types that encompasses deciduousness and other phenological patterns may advance understanding of community patterns and processes (Borchert 1994a; Williams et al. 1997). However, the diversity of phenological patterns observed within this study indicates the need to develop an integrated view of foliar phenology using a mechanistic model consistent with the underlying physiological principles of carbon, water, and nutrient investment in leaf exchange and the continuum of phenological attributes at a community scale (Wurth et al. 2005). For example, the apparently continuous spectrum of deciduousness among sympatric species observed in this study is inconsistent with the conventional approach of delineating discrete phenological groups and with associating a degree or type of deciduousness with a particular climatic regime. Instead, our data suggest that developing a functional typology based around continuous trait variation as suggested by Wright et al. (2004) would be more appropriate.
Conclusions
We observed a wide range of foliar phenologies within a seasonal tropical forest community in western Thailand. The character of deciduousness, as defined by the magnitude, duration and interannual variability of crown loss, varied among the study species in an almost continuous spectrum from completely deciduous to completely evergreen. Foliar phenology is controlled by intra-annual patterns of drought and water availability—leaf flushing cannot occur unless the underlying physiological requirements of cell expansion are met. However, the complex interactions among species-specific physiologies, local edaphic conditions, and intra-annual variation in the timing of rainfall lead to considerable variation in the timing, duration and magnitude of leaf exchange among the tree species within this community. This within-community variation in deciduousness, combined with marked differences in the duration of deciduousness when compared to neotropical forests of similar rainfall seasonality, demonstrate that foliar phenology is a poor indicator of climate. Further advances in understanding the internal and external factors that influence deciduousness within forest communities must integrate detailed physiological studies to complement the growing body of correlative and descriptive studies of foliar phenology in tropical forests.
References
Aide TM (1992) Dry season leaf production: an escape from herbivory. Biotropica 24:532–537
Baker PJ, Bunyavejchewin S, Oliver CD, Ashton PS (2005) Disturbance history and historical stand dynamics of a seasonal tropical forest in western Thailand. Ecol Monogr 75:317–343
Batchelet E (1981) Circular statistics in biology. Academic Press, London
Borchert R (1994a) Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 75:1437–1449
Borchert R (1994b) Induction of rehydration and bud break by irrigation or rain in deciduous trees of a tropical dry forest in Costa Rica. Trees 8:198–204
Borchert R (1994c) Water status and development of tropical trees during seasonal drought. Trees 8:115–125
Borchert R, Rivera G (2001) Photoperiodic control of seasonal development and dormancy in tropical stem succulent trees. Tree Physiol 21:221–231
Borchert R, Rivera G, Hagnauer W (2002) Modification of vegetative phenology in a tropical semi-deciduous forest by abnormal drought and rain. Biotropica 34:27–39
Borchert R, Renner SS, Calle Z, Navarrete D, Tye A, Gautler L, Spichiger R, von Hildebrand P (2005) Photoperiodic induction of synchronous flowering near the equator. Nature 433:627–629
Brodribb TJ, Holbrook NM, Gutierrez MV (2002) Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ 25:1435–1444
Bullock SH, Solis-Magallanes JA (1990) Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica 22:22–35
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Annu Rev Ecol Syst 13:229–259
Choat B, Ball MC, Luly JG, Holtum JAM (2005) Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees 19:305–311
Choat B, Ball MC, Luly JG, Donnelly CF, Holtum JAM (2006) Seasonal patterns of leaf gas exchange and water relations in dry rain forest trees of contrasting leaf phenology. Tree Physiol 26:657–664
Chapotin SM, Razanameharizaka JH, Holbrook NM (2006) Baobab trees (Adansonia) in Madagascar use stored water to flush new leaves but not to support stomatal opening before the rainy season. New Phytologist 169:549–559
Daubenmire R (1972) Phenology and other characteristics of tropical semi-deciduous forest in north-western Costa Rica. J Ecol 60:147–170
Davies SJ, Ashton PS (1999) Phenology and fecundity in 11 sympatric pioneer species of Macaranga (Euphorbiaceae) in Borneo. Am J Bot 86:1786–1795
Duff GA, Myers BA, Williams RJ, Eamus D, O’Grady A, Fordyce IR (1997) Seasonal patterns in soil moisture, vapour pressure deficit, tree canopy cover and pre-dawn water potential in a northern Australian savanna. Aust J Bot 45:211–224
Elliott S, Baker PJ, Borchert R (2006) Leaf flushing during the dry season: the paradox of Asian monsoon forests. Glob Ecol Biogeogr 15:248–257
Frankie GW, Baker HG, Opler PA (1974) Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 62:881–919
Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ (2003) Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc Natl Acad Sci USA 100:572–576
Kramer PJ, Kozlowski TT (1979) Physiology of woody plants. Academic, New York
Kushwaha CP, Singh KP (2005) Diversity of leaf phenology in a tropical deciduous forest in India. J Trop Ecol 21:47–56
Lechowicz MJ (1984) Why do temperate deciduous trees leaf out at different times? Adaptation and ecology of forest communities. Am Nat 124:821–842
Lieberman D (1982) Seasonality and phenology in a dry tropical forest in Ghana. J Ecol 70:791–806
Lieberman D, Lieberman M (1984) The causes and consequences of synchronous flushing in a dry tropical forest. Biotropica 16:193–201
Milton K (1991) Leaf change and fruit production in six neotropical Moraceae species. J Ecol 79:1–26
Morellato LPC, Talora DC, Takahasi A, Bencke CC, Romera EC, Zipparro VB (2000) Phenology of Atlantic rainforest trees: a comparative study. Biotropica 32:811–823
Murphy PG, Lugo AE (1986) Ecology of tropical dry forest. Annu Rev Ecol Syst 17:67–88
Myers BA, Williams RJ, Fordyce I, Duff GA, Eamus D (1998) Does irrigation affect leaf phenology in deciduous and evergreen trees of the savannas of northern Australia? Aust J Ecol 23:329–339
Nepstad DC, de Carvalho CR, Davidson EA, Jipp P, Lefebvre PA, Negreiros GH, da Silva ED, Stone TA, Trumbore SE, Vieira S (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:666–669
Reich PB (1995) Phenology of tropical trees: patterns, causes, and consequences. Can J Bot 73:164–174
Reich PB, Borchert R (1984) Water stress and tree phenology in a tropical dry forest in the lowlands of Costa Rica. J Ecol 72:61–74
Reich PB, Uhl C, Walters MB, Prugh L, Ellsworth DS (2004) Leaf demography and phenology in Amazonian rain forest: a census of 40,000 leaves of 23 tree species. Ecol Monogr 74:3–23
Rivera G, Elliott S, Caldas LS, Nicolossi G, Coradin VTR, Borchert R (2002) Increasing day-length induces spring flushing of tropical dry forest trees in the absence of rain. Trees 16:445–456
Rundel PW, Boonpragob K (1995) Dry forest ecosystems of Thailand. In: Bullock SH, Mooney HA, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 93–123
Singh KP, Kushwaha CP (2005) Paradox of leaf phenology: Shorea robusta is a semi-evergreen species in tropical dry deciduous forests in India. Curr Sci 88:1820–1824
Valentine HT (1983) Budbreak and leaf growth functions for modeling herbivory in some gypsy-moth hosts. For Sci 29:607–617
Walter H (1971) Ecology of tropical and subtropical vegetation. Oliver and Boyd, Edinburgh
Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D (1997) Leaf phenology of woody species in a north Australian tropical savanna. Ecology 78:2542–2558
Woodward FI (1987) Climate and plant distribution. Cambridge University Press, New York
Wright I, Reich P, Westoby M, Ackerly D, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen J, Diemer M, Flexas J, Garnier E, Groom P, Gulias J, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov V, Roumet C, Thomas S, Tjoelker M, Venekiaas E, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright SJ, Cornejo FH (1990) Seasonal drought and leaf fall in a tropical forest. Ecology 71:1165–1175
Wurth MKR, Pelaez-Riedl S, Wright SJ, Korner C (2005) Non-structural carbohydrate pools in a tropical forest. Oecologia 143:11–24
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River, NJ
Acknowledgments
We would like to thank the various staff at the Khlong Phuu Research Station and Kapook Kapiang Ranger Station at Huai Kha Khaeng who have taken the phenology measurements every fortnight over much of the past decade. The management staff at Huai Kha Khaeng have supported the 50-ha plot and the research associated with it since its inception in 1991. This research has been funded by USAID, the National Science Foundation (USA), and the Smithsonian Institution’s Center for Tropical Forest Science Small Grants program. We thank Dan Bebber for his suggestion regarding circular statistics. Comments from Christian Körner, Marilyn Ball, Jenny Read, and two anonymous reviewers improved the quality of the manuscript. The research complies with the current laws governing the conductance of research in Thailand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marilyn Ball.
Rights and permissions
About this article
Cite this article
Williams, L.J., Bunyavejchewin, S. & Baker, P.J. Deciduousness in a seasonal tropical forest in western Thailand: interannual and intraspecific variation in timing, duration and environmental cues. Oecologia 155, 571–582 (2008). https://doi.org/10.1007/s00442-007-0938-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0938-1