Abstract
Animals in social aggregations often spend more time foraging than solitary conspecifics. This may be a product of the relative safety afforded by aggregations: group members can devote more time to foraging and less time to antipredator behaviors than solitary animals (the “risk reduction” effect). All else being equal, risk reduction should result in higher food intake for grouped animals. However, intragroup competition may force group members to spend more time foraging in order to obtain the same food ration as solitary individuals (the “resource competition” effect). We compared these opposing explanations of foraging time allocation in a coral reef fish, bluehead wrasse (Thalassoma bifasciatum). Aggregations of juvenile bluehead wrasse experience safety-in-numbers, and preliminary observations suggested that juveniles in aggregations spent more time foraging for copepods in the water column than solitary juveniles. However, the risk reduction and resource competition hypotheses are indistinguishable on the basis of behavioral observations alone. Therefore, we collected behavioral, dietary, and growth data (using otolith growth rings) for bluehead wrasse at multiple reefs around a Caribbean island. Despite spending more time foraging in the water column, grouped fish did not capture more prey items and had slower growth rates than solitary fish. Thus, the increased foraging time of grouped fish appears to reflect resource competition, not risk reduction. This competition may limit the size and frequency of aggregations among juvenile bluehead wrasse, which have been shown to experience reduced mortality rates in larger groups. Bluehead wrasse recruits also spent less time foraging but grew faster at sites where planktonic copepod prey were more abundant. This suggests the possibility that large-scale spatiotemporal variability in the abundance of planktonic copepods over coral reefs may produce corresponding variability in the dynamics of reef fish populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many animal species, individuals that aggregate into groups can experience a range of advantages over solitary conspecifics, including reduced mortality risk, improved reproductive success and more efficient foraging (Magurran 1990; Courchamp et al. 1999; Gascoigne and Lipcius 2004). In birds and fish, the foraging benefits of group membership have generally been documented with behavioral observations showing increases in foraging activity and decreases in per capita vigilance or other antipredator behaviors with increased group size (reviewed by Bednekoff and Lima 1998). This phenomenon is sometimes referred to as the group-size effect, and is thought to reflect the greater safety of larger groups due to some combination of simple risk dilution and collective threat detection (Beauchamp 2003). For clarity we refer to this as the “risk reduction” effect. However, the increased foraging time resulting from risk reduction can be indistinguishable from increases in foraging activity by individuals scrambling to obtain a minimum food ration in the face of competition from fellow group members (Bednekoff 2003), as has been observed in some fishes (Grand and Dill 1999; Johnsson 2003). We refer to this alternative phenomenon as “resource competition.” For animals enjoying risk reduction, net per capita energy intake could increase with group size as a result of increased time spent foraging, foraging effort (number of feeding attempts per unit time), or foraging efficiency (number of successful prey captures per attempt). Animals experiencing resource competition in large groups, however, may not increase their energetic intake despite foraging at a higher rate (Clark and Mangel 1986). Consequently, distinguishing between these opposing effects is important in understanding the consequences of group membership and predicting their impact on population dynamics (Lima 1998; Parrish and Edelstein-Keshet 1999). This problem could be resolved by simultaneously measuring the effects of group membership on foraging activity, food consumption, and growth rate. This approach has not been taken previously, but we used it to examine the energetic effects of group membership in a gregarious coral reef fish.
Aggregation is a common strategy among coral reef fishes (Connell and Gillanders 1997), but reef fish ecologists have historically focused on the numeric consequences of group membership (i.e., mortality; reviewed by Hixon and Webster 2002) despite the striking changes in foraging behavior that often accompany increases in group size (Booth 2004; Morgan and Kramer 2004). For example, aggregating surgeonfish (Acanthuridae) and parrotfish (Scaridae) forage more efficiently in larger groups (Wolf 1987; Clifton 1991), as do many schooling freshwater fishes (Cyprinidae and Poeciliidae; Pitcher et al. 1982). By contrast, in several damselfish (Pomacentridae) species, growth rates decrease with group size (Jones 1987; Forrester 1990; Booth 1995; but see Booth 2004). For site-attached planktivores like these damselfish, near-bottom depletion of zooplankton prey (Motro et al. 2005) may force fish in groups to forage higher in the water column, where they may be more exposed to predation (Eckert 1987; Sackley and Kaufman 1996) and are further from their benthic refuges (Holbrook and Schmitt 2002). As a result, fish in high-density aggregations may forage less effectively or simply choose to spend less time foraging, and they may also experience interference competition while foraging (Buckel and Stoner 2004), especially if there is a dominance hierarchy in the aggregation (Clark and Mangel 1986). Whether positive or negative, the effects of group membership on growth rates are not strictly nonlethal: fast-growing fish may become invulnerable to gape-limited predators more quickly and have higher survivorship (Sogard 1997). Because fecundity increases nonlinearly with body size in fish (Weatherley 1972), changes in growth associated with group membership could also have important consequences for both individual fitness and population dynamics.
Coral reef fishes are well suited for examining the behavioral and energetic effects of group membership because their otoliths—accretionary calcium carbonate structures in the inner ear—provide a daily record of individual growth rate. Otolith growth rate analysis should reveal whether increases in foraging effort with group size lead to increased energetic intake (indicative of a risk reduction effect) or to constant or declining energetic intake (indicative of resource competition among groupmates).
We examined the effects of group size on foraging activity, diet, and growth rate in juvenile bluehead wrasse (Thalassoma bifasciatum). Unlike the damselfish mentioned above, bluehead wrasses are habitat generalists. Juveniles occur both singly and in groups, but they are found in groups more frequently than expected by chance, possibly because larger groups experience reduced mortality (White and Warner 2007). Like the damselfish, though, bluehead wrasses are microcarnivores. Preliminary observations indicated that newly settled juveniles feed primarily on planktonic copepods and occasionally on benthic copepods, and members of groups spend a greater fraction of their time feeding in the water column and less time sheltering from predators in crevices than solitary fish. Planktonic copepods can be more energy-rich than those in the benthos (Hartney 1989; Clarke 1999), so juvenile bluehead wrasse may face a choice between a risky, high-profit food source and a safer, less profitable one. The safety of group membership could reduce the risk associated with foraging in the water column, allowing grouped fish to spend more time feeding there. Therefore, we hypothesized that, like minnows and guppies (Magurran and Pitcher 1983), grouped bluehead wrasse enjoy risk reduction. We tested this hypothesis by quantifying the time budget, diet, and otolith-derived growth rate of solitary and grouped fish. We also explored whether among-reef variability in food availability or predator abundance altered the effect of group size on behavior, diet, and growth rate by conducting observations during three settlement pulses at five sites where abundance of predatory fish and copepod prey were quantified.
Materials and methods
Study species and sites
The bluehead wrasse, Thalassoma bifasciatum, is a small planktivore common on reefs throughout the Caribbean. Like most reef fishes, the species has pelagic larvae that settle to the reef in monthly pulses centered on the new moon (Caselle and Warner 1996). After settlement, bluehead wrasses spend several days buried in the substrate while completing metamorphosis (Victor 1986). After emerging from the substrate, juveniles remain highly site-attached for approximately 7 days, sheltering in crevices in the reef pavement and coral rubble. After 1 week, fish become more mobile, often joining large, loose schools of adults; all observations and collections were made on fish <7 days old (“settlers”).
Settlers are small (<15 mm total length) and occur singly and in groups of from two to 20 individuals. We defined a group as fish that shared shelter spaces in the reef substrate, occupied the same cylinder of water above the reef, and exhibited concerted behavior (i.e., group members either all fed or all sheltered in the reef at the same time). A preliminary tagging study indicated that bluehead wrasse settlers rarely leave or move between groups (J. W. White, unpublished data). Both grouped and solitary fish are highly site-attached and occupy an area of the reef roughly 15 cm in diameter.
We conducted this study at three sites (Butler Bay, Northstar, and Cane Bay) on the leeward northwestern shore and two sites (Wood Cottage and Jacks Bay) on the windward southeastern shore of St Croix, US Virgin Islands [17.75°N, 64.75°W; see White and Warner (2007) for map of site locations]. At each site, the study area was located at 5–10 m depth in areas primarily composed of flat coral pavement with sparse patches of living and dead coral heads; see Caselle and Warner (1996) for details.
Estimation of foraging time budgets of solitary and grouped individuals
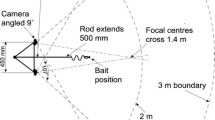
We observed the behavior of haphazardly selected bluehead wrasse settlers for 10-min intervals at each site during three settlement pulses in July–September 2005. Fish were observed by a diver resting on the reef pavement approximately 1 m distant; this was the smallest distance at which observations could be made without visibly altering settler behavior. After the diver was positioned on the bottom, fish were allowed approximately 1 min to acclimate and resume normal activity; fish were then observed for 10 min. For fish in groups, the diver noted the group size and observed the behavior of one haphazardly selected focal individual. In most cases, two solitary fish and two grouped fish were observed per day at a site.
We recorded the amount of time fish spent in the water column versus near the reef substrate or in crevices. We considered fish to be in the water column if they were more than four body lengths (∼6 cm) above the reef substrate (the same distance criterion is often used to define the proximity of a fish to a shoal; Magurran and Pitcher 1987); individuals commonly ranged up to 30 cm above the reef. While in the water column, fish should have greater access to planktonic prey but are also further from shelter. When not in the water column, fish had access to benthic prey (and could be seen foraging by the diver) and were closer to shelter. Aggressive interactions between the focal fish and conspecifics were also noted. We recorded time budget data in 5-s increments, then analyzed time budgets in terms of the proportion of time spent in the water column; proportions were arcsine-square root transformed to improve normality.
Estimation of diets of solitary and grouped individuals
After each behavioral observation, the focal fish was collected with an aquarium dipnet, held in an individual plastic bag and returned to the surface (<45 min) where fish were euthanized with an overdose of clove oil and fixed in 10% formalin (Clarke 1999). To augment sample size, we collected additional solitary and grouped settlers at each site during each settlement pulse. Individual fish and entire groups were haphazardly selected, then collected and preserved in the manner described above. All collections were made between 0900 and 1600 hours, avoiding the near-dawn and near-dusk periods of reduced prey capture observed in other zooplanktivores (Clarke 1999).
For dietary analysis, we dissected the entire gut of each individual and classified food items to the lowest taxonomic level possible (usually order). Most items were copepod exoskeletons, which were commonly disarticulated into cephalothorax and multiple abdomen fragments. We counted only cephalothoraces as individual prey items and estimated their length as <200 μm, 200–500 μm, or >500 μm; more precise length estimates were not possible given the degraded state of many items. Count data were square-root transformed to improve normality and homoscedasticity.
Copepod taxa differ somewhat in body shape, so length may not be the most informative measure of dietary value. It was not possible to obtain precise estimates of copepod mass from these samples, but we calculated approximate masses for copepods of each size class using published length-weight (L–W) relationships for tropical copepods (cyclopoids, ln W = 1.98 × ln L−11.38; harpacticoids, ln W = 1.03 × ln L−6.38; Chisholm and Roff 1990; Satapoomin 1999). For the purposes of these calculations, we estimated the arithmetic mean length of copepods in each of the size class to be 100, 350, and 600 μm. This method is somewhat crude but should produce conservative estimates of prey mass, since copepods at the upper extreme of each size class will have masses much greater than predicted by the mean length in that size class. Mass data were square-root transformed to improve normality and homoscedasticity.
Estimation of growth of solitary and grouped individuals
We estimated growth using otolith analysis. We removed both sagittal otoliths from fish captured for dietary analysis, allowed them to clear in type B immersion oil for 30 days, then examined one sagitta from each fish using transmission light microscopy at 200×. We captured digital images of each sagitta with Image Pro Plus 4.5 (MediaCybernetics, Bethesda, Md.). Using these images, we counted the number of daily post-settlement growth rings manually and estimated the distance along the postrostral radius between the final visible ring and the mark corresponding to settlement; we divided this distance by postsettlement age to obtain a growth rate. We examined multiple focal planes for each otolith to ensure all rings were counted. Fish <1 day post-settlement or for which both otoliths were difficult to read accurately were excluded from analysis. Bluehead wrasse produce daily otolith rings as well as a settlement mark indicating the transition from planktonic larva to demersal settler, and supplemental feeding increases the width of daily rings in wild fish (Victor 1982). To confirm that otolith growth is positively correlated with somatic growth, we performed the residual analysis suggested by Thorrold and Hare (2002).
Estimation of distribution and abundance of predators
During June–September 2004 and 2005, we censused predator population densities at each site bimonthly. We performed visual censuses of all predatory fish within 30 × 6-m belt transects centered on three permanent 30-m transects at each site. The most common piscivores on St Croix and those we have observed feeding on small bluehead wrasse are small serranids (Cephalopholis fulva, Cephalopholis cruentata, Epinephelus guttatus, and Serranus tigrinus), snappers (Lutjanus sp. and Ocyurus chrysurus) and the jack Caranx ruber (White and Warner 2007), so our censuses and analyses focused on these species. To lessen the influence of occasional observations of large schools of jacks and snappers, we treated the total predators observed in each census as an independent observation and used median abundances in our analyses.
Estimation of spatial and temporal patterns of food availability
We obtained time-integrated samples of near-reef planktonic prey items during each settlement pulse using fixed tube trap collectors containing a bottom layer of 100 ml formalin (Yund et al. 1991). At each site, three tube traps on rigid frames were placed approximately 20 m apart on small sand patches within the study area. The tube mouth was 1 m above the reef surface. We deployed tubes for 5 days during the settlement pulse; at the end of this period, tubes were capped in situ and brought to the surface. We allowed the contents to settle into the formalin layer for fixation for 24 h, after which all contents that did not pass through a 150-μm mesh were preserved in 75% ethanol.
To confirm that the tube trap samples of near-reef plankton were comparable to samples obtained using more traditional methods, we performed two tows with a 150-μm-mesh plankton net (0.5 m diameter × 1.5 m long) at each site during the August and September pulses. Each tow was a timed, 3-min round-trip swim 0.5 m over a 50-m transect tape placed along the line formed by the tube traps. After each tow, the contents of the cod-end were filtered through 150-μm mesh; all material that did not pass through the mesh was preserved in 10% formalin.
We sampled the benthic meiofauna at each site by collecting three small (5 × 5-cm) fragments of coral pavement and coral rubble at each site prior to both the August and September lunar settlement pulses. Rocks were sealed in plastic bags on the bottom; at the surface, 100 ml of 10% formalin was added to each bag. After 48 h all organisms and sediment were removed from the rocks with a wire brush. The resulting slurry of sediment and liquid was gently agitated to allow the heaviest particles to settle out, then the supernatant was filtered through 150-μm mesh and items remaining on the filter were preserved in 75% ethanol. Subsequent examination of the settled material confirmed that <5% of organisms were discarded with this material.
For analysis of planktonic and benthic samples, we agitated a 25-ml vial containing the sample, removed a 1-ml subsample by pipette and counted the subsample in a Sedgwick-Rafter counting cell under 50× magnification. For extremely high-density plankton samples, we diluted the 1-ml subsample to 10% with tapwater before counting. We identified all organisms to the lowest taxonomic level possible and placed copepods in the same size categories used for gut content analysis. We used the mean of two subsamples as the count for each sample; the mean of these sample counts for each site–month combination was used in all subsequent analyses. We reported plankton samples as number of items per sample (tow or tube); benthic samples were standardized by the surface area of each rock and reported as number of items per square meter. All rocks were approximately spherical, conical, or rectangular prismatic in shape, and all had similar surface rugosity, so we estimated surface area using the geometrical formula for the three-dimensional solid each rock most closely resembled.
Data analysis
We hypothesized a positive effect of group size on time spent in the water column (i.e., foraging), food intake, and growth rate, and tested for these effects using linear regressions. We also anticipated that these three response variables would not vary independently: food intake is likely to depend strongly on the amount of time spent foraging, which may in turn be affected by the abundance of food and predators. Growth rate is not likely to be affected by the amount of time a fish spent foraging immediately prior to capture, but may be related to time-integrated measures of food and predator abundance. To test for the additional effects of planktonic and benthic food abundance and predator density, we added terms for these covariates and their interaction with group size to the regression models for food intake and growth rate (for food intake we also included a term for time spent foraging; the specific data used for each covariate term are explained in “Results”). Unfortunately, median predator density, planktonic copepod abundance and benthic copepod abundance were all collinear in pairwise comparisons (sites with high predator densities tended to have high densities of planktonic copepods and low densities of benthic copepods; Fig. 1). Multiple regressions with collinear regressors can give spurious results, so we only compared the independent effects of each of those covariates. We performed multiple regressions with group size and each of the collinear covariates separately and selected the regression with the highest r 2 as the best explanatory model. Because we only compared models with equal numbers of parameters, this is a valid method for model comparison (Montgomery et al. 2001). All analyses were performed in JMP 5.1.1 (SAS Institute, Cary, N.C.). We inspected normal quartile plots of residuals and residual versus predicted plots to confirm that data (or transformed data, in some cases) met the distributional assumptions of regression (Montgomery et al. 2001).
Abundance of bluehead wrasse (Thalassoma bifasciatum) predators and prey at five study sites during July, August (Aug), and September (Sept) 2005 at St Croix, US Virgin Islands. a Median density of predatory fish (serranids, lutjanids, carangids; n = 3 transects per site), b mean number of cyclopoid copepods per tube trap (n = 3 traps per site), and c mean density of benthic harpacticoid copepods (n = 3 samples per site). Planktonic samples were not collected in August at Jacks Bay (JB) or Wood Cottage (WC) and no benthic samples were collected in July. Error bars are 1 SE. BB Butler Bay, NS Northstar, CB Cane Bay
Our sampling scheme for predators, plankton, and benthos produced estimates of abundance for each site–month combination, and all multiple regressions involving these factors were performed at this spatiotemporal scale. That is, for behavioral, dietary, and growth data, we treated the mean value for each group size for each site–month combination as a single observation. Some group sizes were not sampled at all sites in all months, and in some cases only a single individual from a particular group size was sampled, but a range of group sizes were sampled for each site–month combination. For analyses involving behavioral observations in which environmental factors were not used as covariates, individual fish were considered to be independent observations.
Results
Spatial and temporal patterns of food availability and predator abundance
Plankton tow and tube-trap samples were composed primarily of cyclopoid copepods, while benthic samples were mostly harpacticoid copepods, although both types of copepods appeared in both types of sample (Fig. 2a, b). Since these two taxa comprised almost the entire diet of bluehead wrasse, the densities of planktonic cyclopoids and benthic harpacticoids were used as proxies for planktonic and benthic food abundance, respectively, in all subsequent analyses.
Mean proportions of invertebrate taxa in a planktonic tube trap samples (n = 36), b benthic samples (n = 30), and c in diets of bluehead wrasse recruits (n = 391). d Mean number (±SE) and estimated mass of copepod taxa in diets of bluehead wrasse recruits (n = 391). Copepod taxa are grouped into three size classes by cephalothorax length. Total mass was obtained by multiplying approximate mass (calculated using published length–mass relationships) by the mean number of copepods in that size class (see text for details). Error bars were not calculated for mass. All means were taken across all sites and dates
The mean abundance of cyclopoid copepods in plankton tows was highly correlated with the mean abundance of cyclopoid copepods in the tube-traps in each site and month (r = 0.92, n = 9, P = 0.0005; Fig. S1). Plankton tows were only made in a subset of months, so we used the tube-trap data in all analyses.
There was considerable spatial and temporal variation in the abundance of predators, planktonic cyclopoid copepods, and benthic harpacticoid copepods (Fig. 1). Predator and planktonic cyclopoid abundances were positively correlated (r = 0.58, n = 13, P = 0.037), but neither of these variables was significantly correlated with the abundance of benthic harpacticoid copepods (n = 9, r = −0.35, −0.15, respectively; P > 0.14 for both comparisons) although statistical power was low for the latter comparisons (power = 0.34, 0.12, respectively). Despite this spatiotemporal variability, the differences between the planktonic and benthic prey communities remained relatively consistent. Cyclopoid copepods made up <42% of benthic copepods in all sites and months (median = 20%, n = 10), while they made up >47% of planktonic copepods in all sites and months (median = 95%, n = 13).
It should be noted that the estimation procedure for benthic copepods was obtained at the scale of small rocks that were similar in rugosity across sites. However, when measured at a larger spatial scale (1 m), rugosity was higher at Jacks Bay and Wood Cottage than at the other sites (White 2007). Consequently, those sites might have greater total reef surface areas, slightly amplifying the trend for higher benthic copepod abundances there (Fig. 1c).
Diets of solitary and grouped individuals
A total of 391 fish were used for dietary and growth (otolith) analysis: 156 solitary and 235 in groups ranging in size from three to ten individuals. Fish diets were composed almost exclusively of cyclopoid and harpacticoid copepods (Fig. 2c), and only 17 fish had empty guts (seven solitary, ten grouped). There was no significant effect of group size on the relative proportions of gut items, (P > 0.9, power = 0.05), so we pooled data from all group sizes for ease of presentation. There was also no significant effect of group size on the mean number of gut items in fish diets (n = 67, P = 0.29, power = 0.18; Fig. 3a), nor on the estimated total mass of copepods in fish diets (n = 67; P = 0.41, power = 0.13, Fig. 3c). The power of these tests was low, but in all three cases the (nonsignificant) least-squares estimate of the regression slope was either negative (for the effect of group size on number of items and total mass) or very near zero (slope = 0.0001 for the effect of group size on proportion of cyclopoid copepods). As such it is unlikely that we failed to detect a positive effect of group size on diet composition.
Mean a, b number of prey items and c, d estimated total mass of copepods in diets of bluehead wrasse recruits as a function of a, c group size and b, d the proportion of time an individual spent in the water column. Data in a, c are the means for each group size in each site–month (n = 67); data in b, d are for individual solitary recruits only (n = 75). Only regression lines with slopes significantly different from zero are shown. Data were square-root transformed to improve normality
We observed a similar range of lengths of cyclopoid and harpacticoid copepods in fish diets, but these taxa differ in body form: harpacticoids are roughly cylindrical, while cyclopoids are more spheroid, with greater mass per unit length. Consequently, cyclopoid copepods represented approximately 5 times more mass than harpacticoid copepods in bluehead wrasse diets (4.15 and 0.85 μg, respectively; Fig. 2d).
Foraging time budgets of solitary and grouped individuals
We observed 152 fish: 78 solitary (group size = 1) and 74 in groups ranging in size from three to ten individuals. On average, fish spent 47.2 ± 2.7% (mean ± SE) of the observation period swimming and foraging in the water column and 26.4 ± 3.2% of the observation period foraging on the benthos; fish spent the remainder of their time sheltering in crevices or engaging in brief chases with conspecifics.
Eighteen grouped fish were involved in at least one aggressive chase with another group member (either as the aggressor or the victim). By contrast, only seven solitary fish participated in chases, usually with neighboring bluehead wrasse settlers (both solitary and grouped). This represents a significantly lower incidence of aggressive behavior (χ 2 = 6.51, df = 1, P = 0.01).
Although fish regularly appeared to strike at prey while in the water column and “foraging” over the benthos, we were unable to count or evaluate the success of individual bites, and we cannot report foraging rates or foraging success for either habitat. Instead, we examined the effect of time spent in the water column and time spent feeding on the benthos on the number of prey items in an individual’s gut. For the entire dataset of 152 fish, there was no effect of the fraction of time spent in either activity on the total number of items consumed. The absence of a relationship between foraging time and gut fullness could reflect competition within groups: fish in larger groups might spend more time foraging in order to obtain a constant food ration. For solitary fish, however, there should be a positive relationship between time spent foraging and the number of prey items captured. When we excluded grouped fish from the analysis, we found a significant, positive effect of proportion of time in the water column on the total number of items (linear regression on square-root-transformed data: r 2 = 0.07, F 1,76 = 5.28, P = 0.024; Fig. 3b) and estimated total copepod mass (linear regression on square-root-transformed data: r 2 = 0.11, F 1,76 = 9.24, P = 0.003; Fig. 3d). This effect appeared to represent the combination of significant positive effects of the proportion of time in the water column on the number of cyclopoid copepods in the 200- to 500-μm size class (r 2 = 0.13, F 1,76 = 11.57, P = 0.001) and the number of harpacticoid copepods in the <200 (r 2 = 0.05, F 1,76 = 4.42, P = 0.039) and 200- to 500-μm (r 2 = 0.11, F 1,76 = 9.08, P = 0.004) size classes; there were no significant relationships with other diet items. We tested for the additional effects of cyclopoid, harpacticoid, or predator abundance on total number of gut items in multiple regressions but found none. There was no relationship between the proportion of time spent feeding on the benthos on the number of gut items, even when excluding grouped fish from the analysis. As such, time spent in the water column appears to be the best proxy available for time spent foraging.
Given this result, we then examined factors explaining variance in the proportion of time fish spent in the water column. For this analysis, data were grouped by site, month, and group size as described in the methods, giving a sample size of 53. The best-fit regression model had terms for group size and planktonic cyclopoid density (Table 1, Fig. 4a, b); the interaction term was not significant. In this model, the proportion of time spent in the water column increased with group size and decreased with increasing planktonic cyclopoid density.
Effect of group size and prey availability on bluehead wrasse a, b foraging behavior and c, d growth. a, b Partial regression plots for mean proportion of time in the water column (a proxy for time spent foraging; grand mean = 0.60) as a function of a group size and b planktonic cyclopoid abundance. Regression analysis was performed on arcsine-square-root-transformed proportions; untransformed residual values are shown here. c, d Partial regression plots for mean post-settlement otolith growth rates of bluehead wrasse (grand mean = 6.44 μm days−1) as a function of c group size and d combined prey abundance. Combined prey abundance is the linear combination of standardized (by Z-transformation) planktonic cyclopoid and benthic harpacticoid densities (see text for details). Each panel shows the effect of one regressor on the response variable independent of the effect of the second regressor; this is achieved by obtaining the residuals of the relationship between the second regressor and the response variable. Each data point is the mean for each group size in each site–month. Error bars are ± 1 SE; points without error bars were samples with a single observation
Growth of solitary and grouped individuals
Of the 391 fish captured, otoliths from 120 solitary fish and 160 grouped fish were suitable for analysis. Fish ranged in age from 1 to 6 days post-settlement; there was no effect of group size on post-settlement age. A positive relationship between residual somatic lengths and residual otolith lengths indicated that otolith growth rate is a reliable predictor of somatic growth (Fig. S2). There was no significant relationship between group size and either otolith size-at-settlement or metamorphic band width, two common indicators of pre-settlement condition (Searcy and Sponaugle 2001).
The mean otolith growth rate for all fish was 6.44 ± 0.21 μm days−1. To examine factors affecting variation around this value using multiple regression, the data were grouped by site, month, and group size to yield a sample size of 56. No combination of group size and planktonic cyclopoid, benthic harpacticoid, or predator abundance yielded a significant relationship with growth rate, although there were positive, nonsignificant effects of both benthic harpacticoid and planktonic cyclopoid abundance on growth. These two variables were negatively correlated with each other, but bluehead wrasse growth rates might be positively related to the combined abundance of planktonic and benthic prey. Unfortunately, a simple linear combination of two-dimensional (per square meter, benthic) and three-dimensional (per cubic meter, planktonic) prey density was not possible. Instead, to approximate the effect of combined prey abundance, we Z-transformed both benthic harpacticoid and planktonic cyclopoid abundances and then took the mean of each pair of standardized values. This value, combined prey abundance, had a significant positive effect on mean growth rate in the best fit model, while group size had a significant negative effect (Table 2, Fig. 4c, d). The latter relationship was driven primarily by low growth rates at the highest group sizes (Fig. 4c).
Discussion
Foraging is an inherently risky endeavor, and animals use a variety of behavioral tactics to adjust the balance of risks to rewards in order to maximize fitness (Stephens and Krebs 1986). Aggregation with other foragers is a common risk-reduction strategy, allowing more time to be spent foraging without incurring a higher probability of being eaten. However, it is difficult to assess the prevalence of this tactic, since animals also commonly aggregate in response to nonrandom distributions of resources, in which case resource competition may force animals to spend more time foraging (Bednekoff 2003). Foraging time increases in both scenarios, but only risk reduction yields an increase in energetic intake. In practice, both processes may operate simultaneously (Clark and Mangel 1986), but the costs and benefits of group membership will depend on whether risk reduction or resource competition predominates. One approach to distinguishing between these alternatives is indirect: examine the relationship between group size and foraging activity under different levels of predation risk. Many animals minimize risky foraging behavior when predators are more abundant (Lima and Dill 1990; Brown et al. 1999). If group membership reduces predation risk, grouped individuals should reduce foraging activity less than solitary individuals in the face of increased predator densities, but if resource competition is occurring, group size will have no effect on the response to predators. Several authors have applied this indirect test (Grand and Dill 1999; Johnsson 2003), but they have been criticized on statistical grounds (Bednekoff 2003) and for inferring energetic effects from behavioral observations alone (Barbosa 2003). We avoided these difficulties by taking a more direct approach: in addition to observing foraging behavior, we quantified the effect of group size on diets and growth rates. This revealed that bluehead wrasse recruits do not enjoy an energetic benefit from group membership: grouped individuals spent a greater fraction of their time foraging in the water column but captured no more prey, and fish in large groups grew more slowly than those in smaller groups. These results suggest that grouped bluehead wrasse increase risky foraging behavior in response to resource competition from their groupmates.
Bluehead wrasse diets included both benthic and planktonic prey items, so the fraction of time spent in the water column may not have been strictly equivalent to total foraging effort. However, foragers appeared to strike at zooplankters continuously while in the water column (J. W. White, personal observation), and the majority of their diet was cyclopoid copepods, which were found at a much greater frequency in the plankton than in the benthos, so we believe that time in the water column was a reasonable proxy for foraging effort. Moreover, among solitary fish that were not competing for prey, individuals that were observed spending more time in the water column had fuller guts and had consumed significantly more of some sizes of cyclopoid and harpacticoid copepods. Though statistically significant, these relationships were weak, likely because we observed fish for only 10 min, during which time they would have caught only a fraction of the items we found in their guts.
Leaving shelter to swim in the water column tends to be a risky endeavor on coral reefs (Sackley and Kaufman 1996; Connell and Gillanders 1997; Holbrook and Schmitt 2002), and foraging bluehead wrasse quickly retreat to crevices in the benthos when startled by predators (J. W. White, personal observation). Bluehead wrasse may be willing to brave the water column to obtain cyclopoid copepods for their nutritional advantages: cyclopoids have thinner exoskeletons and greater interior volume than harpacticoids of the same length (Chisholm and Roff 1990; Satapoomin 1999), so they may be more digestible and contain more energy per individual (Clarke 1999; see Fig. 2d). We are not aware of any direct evaluations of the energetic content of nearshore copepods, but planktivores with high energetic demands tend to prefer planktonic copepods to benthic harpacticoids (Hartney 1989; Clarke 1999).
Despite spending more time foraging in the water column, the guts of grouped fish did not contain more items than those of solitary fish. This pattern could represent exploitative competition among groupmates: on coral reefs, near-bottom copepod densities can be severely depressed by fish predation (Motro et al. 2005). The higher incidence of aggressive chases in groups than among solitary bluehead wrasse suggests that grouped fish might also experience interference competition, as do some other shoaling fishes (Buckel and Stoner 2004).
Competition for food is a potential explanation for the slower growth rate documented for fish in larger groups. While they captured the same number of prey as solitary fish, grouped fish spent more time swimming in the water column, so they may have had a higher rate of energy consumption than solitary fish, leaving them a lower net amount of energy available for growth. The observed reduction in growth rate was small but striking given that the difference arose after only a few days on the reef. After bluehead wrasse abandon the site-attached settler lifestyle for more mobile adult foraging behavior, fish that had been in groups as settlers may experience higher mortality rates than those that were solitary as settlers and grew faster. Size-selective mortality is pervasive among fish (Sogard 1997) and under-fed coral reef fish can exhibit impaired antipredator behavior and higher mortality rates (Mesa et al. 1994; Booth and Beretta 2004). However, bluehead wrasse settlers may opt for group membership initially in order to avoid mortality in their first days on the reef (White and Warner 2007). Group membership may be an effective strategy for avoiding heavy post-settlement mortality, but the associated energetic costs may ultimately limit the number of fish in groups.
Several other studies have documented food competition among social groups of coral reef fish, all of them damselfish (Jones 1987; Forrester 1990; Booth 1995). These results and those of the present study stand in contrast to the conventional wisdom that shoaling fish enjoy foraging benefits that solitary fish do not (Pitcher and Parrish 1993). The difference may lie in the mobility of the group: surgeonfish, parrotfish, and minnows are mobile foragers, and additional group members can improve their ability to locate patches of food or overcome competitors to a degree that compensates for the increased competition for resources in a given patch; these species aggregate largely for foraging purposes alone. Damselfish and bluehead wrasse recruits, however, are highly site attached and must compete for passing zooplankton that are easy to locate but limited in number; these species aggregate not for foraging reasons but to avoid predation (White and Warner 2007) or to compete for specific microhabitats (Booth 1992).
Data from this study matched one major prediction of foraging theory (Stephens and Krebs 1986; Werner and Anholt 1993): fish spent less time foraging when planktonic cyclopoid prey were more abundant. In contrast, we did not find an effect of predator abundance on foraging behavior, despite widespread evidence from other systems that increases in predation risk also reduce foraging activity (Lima and Dill 1990; Brown et al. 1999). Detecting this effect is not always straightforward because behavioral decisions can reflect the combined effects of predation risk and prey availability (Holbrook and Schmitt 1988). Unfortunately we were unable to examine the simultaneous effects of predator and copepod abundance in our multiple regression framework because these factors were strongly collinear. Although no best-fit model contained a term for predator abundance, this does not rule out the possibility of a predator effect. Predation risk may influence bluehead wrasse behavior, but prey abundance simply explained a greater fraction of the variance in all cases considered here.
For the subset of fish with both behavioral and dietary data, there was no effect of cyclopoid abundance on diet once the effect of time spent in the water column had been accounted for. Ultimately, our ability to elucidate patterns in the behavioral and dietary data (which might reflect hourly or daily variation in copepod densities) was limited by the resolution of our sampling scheme, which integrated estimates of cyclopoid abundance over multiple days. However, our estimates of post-settlement growth were also integrated over several days, and the positive effect of planktonic cyclopoid abundance on growth rate is much easier to interpret. When prey were at higher densities, bluehead wrasse grew faster, presumably because they were able to forage with greater efficiency. There were striking spatial differences in copepod abundance around St Croix (Fig. 1), so the growth of bluehead wrasse and other planktivores may be consistently higher at the reefs with higher prey density (see also Caselle et al. 2003).
These results suggest that increased foraging time in groups of bluehead wrasse is a response to intragroup resource competition and does not result in higher food intake. In fact, there appears to be an energetic cost associated with group membership in bluehead wrasse and perhaps other site-attached foragers. Our results also indicate that large-scale spatial and temporal variation in zooplankton prey availability can shape the behavior and growth rates of reef fish. Given the strong influence body size has on both survival and fecundity in reef fishes, spatial variation in growth and behavior may prove to play a strong role in the dynamics of reef fish metapopulations.
References
Barbosa A (2003) Group size effects on vigilance: we need more bricks in the wall. Behav Processes 63:133–134
Beauchamp G (2003) Group-size effecs on vigilance: a search for mechanisms. Behav Processes 63:111–121
Bednekoff PA (2003) Testing explanations of the group size effect on vigilance: let’s be direct. Behav Processes 63:135–136
Bednekoff PA, Lima SL (1998) Randomness, chaos and confusion in the study of antipredator vigilance. Trends Ecol Evol 13:284–287
Booth DJ (1992) Larval settlement patterns and preferences by domino damselfish Dascyllus albisella Gill. J Exp Mar Biol Ecol 155:85–104
Booth DJ (1995) Juvenile groups in a coral-reef damselfish: density-dependent effects on individual fitness and population demography. Ecology 76:91–106
Booth DJ (2004) Synergistic effects of conspecifics and food on growth and energy allocation of a damselfish. Ecology 85:2881–2887
Booth DJ, Beretta GA (2004) Influence of recruit condition on food competition and predation risk in a coral reef fish. Oecologia 140:289–294
Brown JS, Laundre JW, Gurung M (1999) The ecology of fear: optimal foraging, game theory and trophic interactions. J Mammal 80:385–399
Buckel JA, Stoner AW (2004) Negative effects of increasing group size on foraging in two estuarine piscivores. J Exp Mar Biol Ecol 307:183–196
Caselle JE, Warner RR (1996) Variability in recruitment of coral reef fishes: the importance of habitat at two spatial scales. Ecology 77:2488–2504
Caselle JE, Hamilton SL, Warner RR (2003) The interaction of retention, recruitment, and density-dependent mortality in the spatial placement of marine reserves. Gulf Carib Res 14:107–118
Chisholm LA, Roff JC (1990) Size-weight relationships and biomass of tropical neritic copepods off Kingston, Jamaica. Mar Biol 106:71–77
Clarke RD (1999) Diets and metabolic rates of four Caribbean tube blennies, genus Acanthemblemaria (Teleostei: Chaenopsidae). Bull Mar Sci 65:185–199
Clark CW, Mangel M (1986) The evolutionary advantages of group foraging. Theor Popul Biol 30:45–75
Clifton KE (1991) Subordinate group members act as food-finders within striped parrotfish territories. J Exp Mar Biol Ecol 145:141–148
Connell SD, Gillanders BM (1997) Mortality and abundance of a schooling reef fish. Proc Eighth Int Coral Reef Symp 1:1035–1038
Courchamp F, Clutton-Brock T, Grenfell BT (1999) Inverse density dependence and the Allee effect. Trends Ecol Evol 14:405–410
Eckert GJ (1987) Estimates of adult and juvenile mortality for labrid fishes at One Tree Reef, Great Barrier Reef. Mar Biol 95:167–171
Forrester GE (1990) Factors influencing the juvenile demography of a coral reef fish. Ecology 71:1666–1681
Gascoigne JC, Lipcius RN (2004) Allee effects in marine systems. Mar Ecol Prog Ser 269:49–59
Grand TC, Dill LM (1999) The effect of group size on the foraging behavior of juvenile coho salmon: reduction of predation risk or increased competition? Anim Behav 58:443–451
Hartney KB (1989) The foraging ecology of two sympatric gobiid fishes: importance of behavior in prey type selection. Environ Biol Fishes 26:105–118
Hixon MA, Webster MS (2002) Density dependence in marine fishes: coral-reef populations as model systems. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, Calif., pp 303–325
Holbrook SJ, Schmitt RJ (1988) The combined effects of predation risk and food reward on patch selection. Ecology 69:125–134
Holbrook SJ, Schmitt RJ (2002) Competition for shelter space causes density-dependent predation mortality in damselfishes. Ecology 83:2855–2868
Johnsson JI (2003) Group size influences foraging effort independent of predation risk: an experimental study on rainbow trout. J Fish Biol 63:863–870
Jones GP (1987) Competitive interactions among adults and juveniles in a coral reef fish. Ecology 68:1534–1547
Lima SL (1998) Nonlethal effects in the ecology of predator–prey interactions. BioScience 48:25–35
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Magurran AE (1990) The adaptive significance of schooling as an anti-predator defence in fish. Ann Zool Fenn 27:51–66
Magurran AE, Pitcher TJ (1983) Foraging, timidity and shoal size in minnows and goldfish. Behav Ecol Sociobiol 12:147–152
Magurran AE, Pitcher TJ (1987) Provenance, shoal size and the sociobiology of predator-evasion behaviour in minnow shoals. Proc R Soc B 229:439–465
Mesa MG, Poe TP, Gadomski DM, Petersen JH (1994) Are all prey created equal? A review and synthesis of differential predation on prey in substandard condition. J Fish Biol 45:81–96
Montgomery DC, Peck EA, Vining GG (2001) Introduction to linear regression analysis, 3rd edn. Wiley, New York
Morgan IE, Kramer DL (2004) The social organization of adult blue tangs, Acanthurus coeruleus, on a fringing reef, Barbados, West Indies. Environ Biol Fishes 71:261–273
Motro R, Ayalon I, Genin A (2005) Near-bottom depletion of zooplankton over coral reefs. III. vertical gradient of predation pressure. Coral Reefs 24:95–98
Parrish JK, Edelstein-Keshet L (1999) Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284:99–101
Pitcher TJ, Parrish JK (1993) Functions of shoaling behaviour in teleosts. In: Pitcher TJ (ed) Behaviour of teleost fishes, 2nd edn. Chapman & Hall, London, pp 363–440
Pitcher TJ, Magurran AE, Winfield IJ (1982) Fish in larger shoals find food faster. Behav Ecol Sociobiol 10:149–151
Sackley PG, Kaufman LS (1996) Effect of predation on foraging height in a planktivorous coral reef fish, Chromis nitida. Copeia 1996:726–729
Satapoomin S (1999) Carbon content of some common tropical Andaman Sea copepods. J Plankton Res 21:2117–2123
Searcy SP, Sponaugle S (2001) Selective mortality during the larval-juvenile transition in two coral reef fishes. Ecology 82:2452–2470
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60:1129–1157
Stephens DW, Krebs JR (1986) Foraging Theory. Princeton University Press, Princeton, N.J.
Thorrold SR, Hare JA (2002) Otolith applications in reef fish ecology. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, Calif., pp 243–264
Victor BC (1982) Daily otolith increments and recruitment in two coral-reef wrasses, Thalassoma bifasciatum and Halichoeres bivittatus. Mar Biol 71:203–208
Victor BC (1986) Larval settlement and juvenile mortality in a recruitment-limited coral reef fish population. Ecol Monogr 56:145–160
Weatherley AH (1972) Growth and ecology of fish populations. Academic Press, London
Werner EE, Anholt BR (1993) Ecological consequences of the trade-off between growth and mortality mediated by foraging activity. Am Nat 142:242–272
White JW (2007) Spatially correlated recruitment of a marine predator and its prey shapes the large-scale pattern of density-dependent prey mortality. Ecol Lett (in press)
White JW, Warner RR (2007) Safety in numbers and the spatial scaling of density dependence in a coral reef fish. Ecology (in press)
Wolf NG (1987) Schooling tendency and foraging benefit in the ocean surgeonfish. Behav Ecol Sociobiol 21:59–63
Yund PO, Gaines SD, Bertness MD (1991) Cylindrical tube traps for larval sampling. Limnol Oceanogr 36:1167–1177
Acknowledgements
We thank J. Barr, S. Brander, J. M. Ecker, and C. Grigsby for expert assistance in the field. J. Godwin, E. Marsh, P. Munday, and St Croix Ultimate Bluewater Adventures provided invaluable logistical support. S. Hamilton and J. Samhouri kindly provided instruction in otolith analysis and, with S. Sandin, plankton tube traps. We are grateful to K. Klose and S. Cooper for providing equipment and assistance with plankton and benthos analysis. Ideas and comments from D. Booth, S. Brander, L. Dill, S. Hamilton, D. Kramer, the Sih lab group, and especially S. Gaines and S. Holbrook greatly improved this work and the manuscript. J. W. W. was supported by National Science Foundation Predoctoral and University of California Regents fellowships. This is contribution 260 from the Partnership for Interdisciplinary Studies of Coastal Oceans, funded primarily by the Gordon and Betty Moore Foundation and the David and Lucile Packard Foundation. This work complied with the current laws of the United States Virgin Islands (USVI); all fieldwork was performed in accordance with University of California Santa Barbara Institutional Animal Care and Use Committee permit no. 4-04-403 and USVI Department of Planning and Natural Resources permit nos. STX-024-04 and STX-025-05.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marc Mangel.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
White, J.W., Warner, R.R. Behavioral and energetic costs of group membership in a coral reef fish. Oecologia 154, 423–433 (2007). https://doi.org/10.1007/s00442-007-0838-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0838-4