Abstract
Many herbivores elicit biochemical, physiological, or morphological changes in their host plants that render them more resistant to co-occurring herbivores. Yet, despite the large number of studies that investigate how induced resistance affects herbivore preference and performance, very few have simultaneously explored the cascading effects of induction on higher trophic levels and consequences for prey suppression. In our study system, early-season herbivory by leafhoppers elevated plant resistance to subsequent attack by chrysomelid beetles sharing the same host plant. Notably, beetles feeding on leafhopper-damaged plants incurred developmental penalties (e.g., prolonged time in early larval instars) that rendered them more susceptible to predation by natural enemies. As a result, the combined bottom-up effect of leafhopper-induced resistance and the top-down effect of enhanced predation resulted in the synergistic suppression of beetle populations. These results emphasize that higher trophic level dynamics should be considered in conjunction with induced resistance to better understand how plants mediate interspecific interactions in phytophagous insect communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbivore-induced plant responses play an important role in determining the palatability of plant tissues for many phytophagous arthropods (Agrawal et al. 1999; Karban and Baldwin 1997). As a result, plants are thought to mediate interspecific interactions among herbivores and ultimately influence community structure (Hunter 1992; Denno et al. 1995; Van Zandt and Agrawal 2004; Viswanathan et al. 2005; Ohgushi 2005; Denno and Kaplan 2007). However, the bottom-up effect of plant quality is only one factor limiting the distribution and abundance of herbivores and the top-down effects of predators, parasites, and pathogens can be as or more important than plant defense (Hunter and Price 1992; Hunter et al. 1997; Denno et al. 2002). Moreover, in many instances bottom-up factors mediate the strength of top-down effects (Hunter and Price 1992; Stiling and Rossi 1997; Forkner and Hunter 2000; Denno et al. 2002; Lill et al. 2002). Therefore, to fully evaluate the ecological consequences of induced resistance, the impact of associated natural enemies must also be considered.
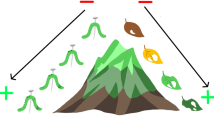
The relationship between herbivore-induced plant defenses and natural enemy effects can be described as: (1) antagonistic—expression of plant resistance traits reduces the effectiveness of top-down control, (2) additive—direct plant defenses and top-down effects function independently of one another, or (3) synergistic—plant resistance enhances top-down suppression of herbivores (Fig. 1). Of these, antagonistic interactions have been most widely reported in the literature. For example, several studies have documented the reduced performance of parasitoids reared from hosts developing on induced plants (Havill and Raffa 2000; Thaler 2002). Similarly, phytochemical induction by gypsy moth larvae feeding on red oak protects them from subsequent attack by an entomopathogenic polyhedrosis virus (Hunter and Schultz 1993). Furthermore, studies that have experimentally elevated constitutive levels of plant defense, thus simulating induction, often demonstrate negative effects that cascade up to higher trophic levels and disrupt top-down control (see reviews by Turlings and Benrey 1998; Hare 2002; Ode 2006). Such studies indicate that interference may be ubiquitous and thus challenge the notion of mutualism between the first and third trophic levels whereby plants encourage natural enemies for their own benefit (Takabayashi and Dicke 1996; Van der Meijden and Klinkhamer 2000).
A theoretical model predicting changes in herbivore density resulting from the individual and combined effects of herbivore-induced plant resistance and natural enemy presence (see Hare 1992). Both induced resistance and natural enemies alone are expected to reduce the performance and survival of individual herbivores, and thus generate a decline in the overall population size of herbivores. However, the combined impact of herbivory and natural enemy presence may promote additive or non-additive (i.e., synergistic or antagonistic) effects on herbivore population growth depending on the level of compatibility between direct and indirect plant defenses
Studies demonstrating additive or synergistic interactions between induced resistance and the top-down suppression of herbivores are rare in the ecological literature (but see Boege 2004; Kessler and Baldwin 2004). Nonetheless, several potential mechanisms could generate a synergistic interaction. First, many herbivore-induced phytochemicals are digestibility-reducing compounds (e.g., protease inhibitors and phenolics) that limit the assimilation of plant nutrients and thus delay herbivore growth (Karban and Baldwin 1997). As a result, induced plant defenses can increase the window of vulnerability that herbivores are susceptible to enemy attack, as predicted by the slow-growth-high-mortality hypothesis (Häggström and Larsson 1995; Benrey and Denno 1997; Williams 1999; but see Lill and Marquis 2001). Second, induced defenses frequently lead to greater herbivore movement, and consequently more dispersed patterns of plant damage (Schultz 1983; Edwards et al. 1991; Rodriguez-Saona and Thaler 2005). Such changes in feeding behavior are thought to make herbivores more apparent to visually-oriented enemies or increase the probability of encountering sit-and-wait predators that ambush their prey (Bergelson and Lawton 1988; Marquis and Whelan 1996; Kaitaniemi et al. 2004). Last, prior herbivory may prime plants to elicit stronger or more rapid expression of enemy-attracting plant cues (e.g., volatile organic compounds, extrafloral nectar), facilitating top-down effects on herbivores (Engelberth et al. 2004; Heil and Kost 2006).
We explored the possibility for synergistic (non-additive) interactions between induced plant resistance and top-down suppression from higher trophic levels by studying interactions between two dominant herbivores that share the same host plant, namely the potato leafhopper [Empoasca fabae (Harris)] and the Colorado potato beetle [Leptinotarsa decemlineata (Say)], and an important stinkbug predator [Podisus maculiventris (Say)] of beetle larvae. Both herbivores are foliar-feeding insects that frequently co-occur on potato (Solanum tuberosum L.) plants in the mid-Atlantic US and together inflict the vast majority of damage (>90%) from herbivorous insects in potato fields (G. Dively, unpublished data). The annual colonization sequence of these herbivores differs, with sap-feeding leafhoppers initially colonizing plants in early June followed by leaf-chewing potato beetles in mid-July (Lynch et al. 2006). As a result, leafhoppers colonize and initiate feeding on potato plants well in advance (5–6 weeks) of beetles. Early-season exploitation of host plants has been identified as a key factor that frequently leads to competitive dominance in multi-species herbivore assemblages (Denno and Kaplan 2007). Accordingly, early-season damage by leafhoppers in potato fields may generate asymmetrical plant-mediated effects on beetle performance. Existing evidence suggests this to be the case with prior leafhopper herbivory inducing resistance to co-occurring beetles (Tomlin and Sears 1992; Lynch et al. 2006). However, it remains unknown how such bottom-up resistance effects might interact with top-down suppression by beetle enemies.
Colorado potato beetle is persistently attacked by a diversity of natural enemies, but hemipteran predators (e.g., stinkbugs) inflict the highest levels of mortality on the egg and larval stages (Biever and Chauvin 1992; Cloutier and Bauduin 1995; Hough-Goldstein 1998). Moreover, several of these predators are attracted to volatile organic compounds (e.g., terpenoids) that are released from potato plants damaged by beetles (Weissbecker et al. 1999, 2000; Van Loon et al. 2000). As a result, this system provides an ideal tri-trophic assemblage of plants, herbivores, and predators for assessing how induced resistance influences enemy attack and ultimately prey suppression.
We conducted a series of field, greenhouse, and laboratory experiments to determine the impact of early-season leafhopper herbivory on potato beetles feeding later in the season. More specifically, our goal was to quantify the relative contribution of leafhopper-induced resistance, predation, and their interaction on beetle survival. We predicted that: (1) prior leafhopper herbivory would reduce the performance (e.g., increased development time), survival, and abundance of beetles sharing the same host plant; (2) leafhopper-induced resistance would not influence a predator’s preference for or consumption of beetle larvae via prey quality effects; and (3) suppression of beetle larvae by predators would be stronger on plants receiving prior leafhopper herbivory as a consequence of delayed larval development and increased exposure.
Materials and methods
Study organisms
Potato leafhopper (Empoasca fabae) is a sap-feeding herbivore that resides on the stems and undersides of leaves where it feeds on vascular tissues of potato plants. This multivoltine pest typically causes visual damage to plants, including foliar chlorosis and cupping (typical “hopperburn” symptoms), leaf necrosis, and premature senescence (Walgenbach and Wyman 1985; Backus et al. 2005). Colorado potato beetle (Leptinotarsa decemlineata) is a leaf-chewing herbivore native to Central America that is now the most damaging pest of potatoes throughout the US and worldwide (Hare 1990). Larvae of L. decemlineata develop through four larval instars, causing extensive defoliation to their host plants, before pupating in the soil and emerging as adults. The stinkbug Podisus maculiventris is an abundant predator of the immature stages of Colorado potato beetles throughout much of temperate North America and is considered one of the most effective biological control agents of beetle populations (Biever and Chauvin 1992; Cloutier and Bauduin 1995; Hough-Goldstein 1998).
Consequences of leafhopper-induced resistance for potato beetle colonization and abundance
In the summer of 2003 a field experiment was initiated at the Wye Research and Education Center in Queenstown, Maryland. The objective of this experiment was to measure the impact of varying levels of early-season leafhopper herbivory on the natural colonization and abundance of potato beetles in open field plots. The experiment was conducted in a single field that was planted with seed pieces of potatoes (var. Atlantic) in early April. The field was subsequently divided into 32 plots (each plot 3 × 8 m with 50 plants per plot) with 2 m separating all plots. Plants were managed with standard commercial agronomic inputs including frequent overhead irrigation and fertilizer applications as needed throughout the growing season.
Plots were assigned to one of four leafhopper herbivory treatments (none, low, moderate, or high) and arranged in a randomized complete block design (n = 8 plot replications per treatment). Consequently, each plot represents an independent experimental unit to which treatments were assigned. Herbivory treatments were achieved by manipulating natural leafhopper populations with selective applications of the foliar insecticide permethrin (FMC, Philadelphia, Pa.) to field plots. Permethrin is a synthetic pyrethroid that functions as a neurotoxin upon contact with the target herbivore, but produces no systemic activity within the plant. All data on beetles were collected long after the residual effects of insecticide application degraded (>2 weeks). We predicted that beetles would be most abundant in plots with no prior leafhopper herbivory (i.e., those plots receiving the most frequent insecticide applications). Thus, any unanticipated residual insecticide activity would not explain the expected pattern of enhanced beetle abundance and would only strengthen our results. Since leafhoppers are the only major early-season herbivore inhabiting potato fields, sprays primarily manipulated leafhopper abundance and minimized non-target effects. No-herbivory plots were maintained with weekly applications of permethrin at the rate of 0.2 lbs/acre, whereas high-herbivory plots remained untreated and achieved ambient densities of leafhoppers. Low- and moderate-herbivory plots were treated with insecticide when plant symptoms indicative of low and moderate leafhopper density appeared. Plant symptoms used to indicate the four levels of herbivory and initiate spray regimes were based on the percentage of potato leaves exhibiting curling and yellowing, symptoms indicative of leafhopper feeding: 0% curling/yellowing (no herbivory), 10–30% (low), 40–60% (moderate), and 70–90% (high). Spray regimes were initiated when leafhoppers colonized plots in early June and ended in early July when potato beetles begin colonizing fields (Lynch et al. 2006).
Once a week from early June up to and including mid-July plots were sampled for leafhopper adults and nymphs to verify the efficacy of sprays in generating the four desired herbivory levels. Adult density was estimated using sweep net sampling (ten sweeps per plot), whereas the density of nymphs was measured by visually searching the undersides of ten random leaves per plot. Plants were also rated for evidence of leafhopper damage (% leaf curling, yellowing, and necrosis) in all plots every 2 weeks. Two separate observers independently estimated the percentage of leaves in each plot displaying damage symptoms (% leaf curling, % yellowing, and % necrosis) and the average of these two observations was used as the plot mean. Densities of leafhopper adults and nymphs were compared between the four treatment plots using repeated measures ANOVA with spray regime and sample date as main effects. Plant damage on the final sampling date was assessed for differences between treatment plots using ANOVA (all statistical analyses were performed using SAS, version 9.1). An arcsine square-root transformation was performed on the proportion of plants displaying damage symptoms prior to statistical analysis.
To assess the impact of experimentally manipulated leafhopper herbivory on Colorado potato beetle colonization and survival, field plots were visually searched on 29 July for egg masses, beetle larvae and adults (no leafhopper herbivory, n = 8 plots; low leafhopper herbivory, n = 7 plots; moderate leafhopper herbivory, n = 7 plots; high leafhopper herbivory, n = 8 plots). Five randomly chosen plants per plot were searched and counts were used to calculate a plot mean (number per plant) for each beetle life stage. Plot means were compared between the four leafhopper-herbivory treatments using ANOVA, and differences among treatment means were assessed using Duncan’s multiple range test.
Interactive effects of leafhopper-induced resistance and predation on potato beetle survival
The potential interactive effects of leafhopper-induced resistance and predation on potato beetle survival was assessed in field cages using a 2 × 4 factorial experiment that manipulated beetle predators (presence or absent) and the level of prior leafhopper herbivory (none, low, moderate, or high). Cages were constructed with fine-mesh netting and enclosed five plants (2.5 × 1 × 1.5 m in height). Within each leafhopper-herbivory treatment plot, two cages were constructed and stinkbug predator treatments (present or absent) were randomly assigned to each cage. Stinkbug eggs were ordered from a biological supply company (The Bug Factory, Nanoose Bay, Canada) and reared to the third instar on Colorado potato beetle eggs prior to use in experiments. On 22 July plants were chosen for placement of field cages and all existing beetle eggs, larvae, and adults were manually removed from these sites. Subsequently, cages were erected and stocked with 200 newly eclosed first instar Colorado potato beetle larvae obtained from a laboratory colony. This larval density falls well within the density range commonly encountered in the field (G. Dively, unpublished data). Two days later, 25 stinkbug nymphs were placed in field cages designated for predator addition. Augmentative releases of five to ten stinkbugs per plant were shown to effectively suppress beetle larvae in the field (Biever and Chauvin 1992). Thus, our predator density of five per plant represents the lower end of this density spectrum.
When approximately 80% of the beetle larvae in each enclosure entered the pre-pupal stage, we removed cages and sampled the remaining insects between 30 July and 3 August. The effects of prior leafhopper herbivory and predator presence on the density of surviving beetle larvae were tested using a split-plot ANOVA with prior herbivory as the whole-plot factor and predator presence as the sub-plot factor. Data were log-transformed prior to analysis to meet assumptions of normality and homogeneity of variances. Moreover, log-transformed data are more appropriate for testing the multiplicative risk model of herbivore mortality from induced resistance and predation (see Sih et al. 1998).
Impact of prior leafhopper herbivory on beetle larval development
The effect of prior leafhopper feeding on the development of potato beetle larvae through the four larval instars was tested in a greenhouse experiment. Cohorts of beetle larvae were reared to pupation on plants that received prior leafhopper herbivory and plants that did not (n = 20 plants per treatment). Leafhopper damage was imposed by placing potted potato plants in large screen field cages and introducing leafhoppers at a rate of 20 adults per plant, which approximates densities that occur in mid-Atlantic potato fields (Lynch et al. 2006). Caged plants were exposed to leafhoppers for 2 weeks, after which potted plants were removed from cages and transferred to the greenhouse. Undamaged control plants were treated identically except they were not exposed to leafhoppers. Plants were blocked spatially on greenhouse benches to control for effects of small-scale environmental variation on beetle development. Each plant received one cohort of 15 newly eclosed first instar Colorado potato beetle larvae obtained from field-collected eggs. Cohorts were enclosed on plants in organdy-mesh cages that provided ample leaf material for larvae to complete development. Every second day, sleeve cages were temporarily removed from plants and the instar of each individual larva in the cohort was recorded. This sampling regime continued for 15 days, after which the majority of beetle larvae in both treatments entered the pupal stage. For each cohort, the proportion of beetle larvae in each larval instar was calculated for all sampling dates and data were arcsine square-root transformed prior to statistical analyses. Repeated-measures ANOVA was used to test the impact of prior leafhopper herbivory on beetle development through each larval instar (first up to and including the fourth). Only dates when larvae were found in a particular instar were used for subsequent analyses (i.e., day 2 was not used to assess the impact of leafhoppers on third instar beetles because no larvae in either treatment had reached that developmental stage yet).
Effect of leafhopper-induced resistance on predator preference and consumption of beetle prey
To determine if leafhopper-induced foliage negatively impacted predators via prey quality, we measured the preference and consumption rate of stinkbugs offered potato beetle larvae reared on leafhopper-induced or non-induced foliage by employing both choice and no-choice experiments. Beetle larvae used for both experiments were obtained from a laboratory colony with one sub-set of larvae reared to the third instar on undamaged potato plants while the other sub-set was reared to the same instar on induced plants created by exposure to leafhoppers for 5 weeks in field cages (leafhopper densities were comparable with those found in unsprayed potato fields). All stinkbugs used in experiments were obtained from the above-mentioned biological supply company, maintained in the laboratory on potato beetle eggs, and starved for 24 h before use in feeding trials.
In the choice experiment, two beetle larvae (one reared on undamaged plants and the other raised on leafhopper-damaged plants) were matched by size and placed together on a single excised potato leaf in a petri dish enclosure (16 cm diameter). After larvae settled and began feeding, a single third instar stinkbug nymph was introduced to the arena and was observed until one of the two larvae was attacked and killed. The first larva chosen by the predator was deemed the preferred prey. If no attack occurred after 15 min of observation, all insects were replaced with a new pair of beetle larvae and the trial was repeated. Altogether, there were 27 replicates. Predator preference was evaluated by comparing the ratio of larvae attacked from undamaged controls to larvae reared on leafhopper-induced plants. Deviation from a 1:1 attack ratio (i.e., no preference) was tested using a χ2 analysis.
In the no-choice consumption experiment, stinkbug nymphs were placed in petri dishes with either five third instar potato beetle larvae reared on undamaged plants or five larvae reared on leafhopper-induced plants (n = 15 treatment replications). Petri dish enclosures consisted of excised potato leaves placed on filter paper and maintained in a growth chamber (25°C, light:dark 14:10 h) for the duration of the experiment. After 72 h, larvae were removed from enclosures and the number of remaining larvae (dead and living) was counted. Predation-related mortality was easily distinguished from other sources of death because larvae consumed by stinkbugs appear “deflated” (hemipteran predators remove hemolymph from prey with piercing–sucking mouthparts). Differences in the consumption rate (no. prey killed/72 h) of stinkbugs offered larvae reared on undamaged and leafhopper-induced plants were assessed using a t-test.
Developmental changes in susceptibility of beetle larvae to predation
A functional response experiment was performed in simple petri dish enclosures to assess the relative susceptibility of the various larval stages of potato beetles to stinkbug predation. The experiment employed a 4 × 4 factorial design whereby each beetle instar (first up to and including the fourth) was offered at four different densities (3, 6, 12, and 24 larvae per enclosure) to a single third instar stinkbug nymph. All stinkbugs used in the experiment were reared on potato beetle eggs and starved for 24 h before use in feeding trials. Petri dish enclosures contained one to four excised potato leaves (greater numbers of leaves were supplied at the higher larval densities) placed on filter paper and maintained in a growth chamber (25°C, light:dark 14:10 h) for the duration of the experiment. After 24 h, larvae were removed from enclosures and the number of surviving and killed larvae was counted. Each treatment combination was replicated 10 times and four predator-free controls were established to determine background levels of mortality. The effects of prey density and larval instar on predation rate were assessed using two-way ANOVA.
Results
Consequences of leafhopper-induced resistance for potato beetle colonization and abundance
Foliar insecticide applications were successful at generating the desired variation in leafhopper density and herbivory across treatment plots. Spray regimes reduced the density of leafhopper adults by 85% (F 3,28 = 106.33, P < 0.001) and nymphs by >99% (F 3,28 = 50.05, P < 0.001) (unsprayed plots, 2.26 ± 0.53 adults per sweep, 0.925 ± 0.517 nymphs per leaf; sprayed plots, 0.35 ± 0.17 adults per sweep, 0.003 ± 0.008 nymphs per leaf). Furthermore, differences in leafhopper abundance translated into differences in plant-damage symptoms including leaf cupping (F 3,28 = 228.6, P < 0.001; no damage = 2.3%, low damage = 12.3%, moderate damage = 92.5%, high damage = 99.4%), yellowing (F 3,28 = 22.3, P < 0.001; no damage = 1.0%, low damage = 3.8%, moderate damage = 31.9%, high damage = 46.3%), and necrosis (F 3,28 = 22.9, P < 0.001; no damage = 0%, low damage = 0%, moderate damage = 9.5%, high damage = 25.8%).
Natural populations of adult potato beetles were less abundant in plots that received prior leafhopper herbivory (F 3,26 = 3.26, P = 0.037) (Fig. 2a), but this effect did not influence adult oviposition as there were no differences in the abundance of egg masses among the leafhopper herbivory treatments (F 3,26 = 0.62, P = 0.608) (Fig. 2b). However, beetle larvae were 2–3 times more abundant in undamaged and lightly damaged plots than they were in plots receiving ambient (high) levels of prior leafhopper herbivory (F 3,26 = 3.47, P = 0.031) (Fig. 2c).
Effects of early-season leafhopper (Empoasca fabae) herbivory treatments (none, low, moderate and high) in potato fields on the a colonization density of Colorado potato beetle (Leptinotarsa decemlineata) adults, b density of egg masses deposited by beetle colonists, and c density of beetle larvae (mean ± SE). Different letters above error bars indicate significant differences (P < 0.05)
Interactive effects of leafhopper-induced resistance and predation on potato beetle survival
The number of surviving beetle larvae in our field cage experiment was influenced by the main effects of predator presence (F 1,28 = 66.3, P < 0.001) and prior leafhopper herbivory (F 3,28 = 12.9, P < 0.001), as well as the interaction between predation and herbivory (F 3,28 = 7.86, P < 0.001) (Fig. 3a). The interaction was driven by the higher level of stinkbug predation on beetle larvae in leafhopper-damaged plots (95% prey suppression) compared with that in leafhopper-free plots (32% prey suppression), thus providing evidence for a synergistic relationship between induced resistance and predation (Fig. 3b).
a Interactive effects of early-season leafhopper herbivory (none, low, moderate and high) and stinkbug predation (absent or present) on the number of Colorado potato beetle larvae per field cage (mean + SE). Data from this experiment were subsequently used to calculate b beetle mortality resulting from stinkbug predation (no. beetle larvae surviving in predator-present versus predator-free cages on leafhopper-free plants) and induced resistance (no. beetle larvae surviving on plants incurring a high level of previous leafhopper herbivory versus undamaged plants in predator-free cages). As a result, beetle mortality was calculated relative to the undamaged, predator-free cages and thus background mortality in the absence of predation and induced resistance was accounted for. The expected and actual combined impact of both mortality factors on beetle survival is shown. The expected impact was calculated using the following multiplicative model (Sih et al. 1998): Pir + Pp − PirPp where Pir = proportion of larvae killed by induced resistance; Pp = proportion of larvae killed by predation
Impact of prior leafhopper herbivory on beetle larval development
Prior leafhopper herbivory resulted in delays in beetle development, as evidenced by a greater proportion of individuals remaining in the second instar stage (F 1,34 = 5.22, P = 0.029), and third instar stage (F 1,34 = 8.34, P = 0.007) on leafhopper-induced than on non-induced plants (Fig. 4b, c). Moreover, time to pupation was extended when larvae were fed induced foliage (F 1,34 = 19.29, P < 0.001) (Fig. 4e). Leafhopper-feeding had a marginally significant impact on first instar larvae (F 1,34 = 3.52, P = 0.069), and no detectable effect on fourth instar development (F 1,34 = 0.89, P = 0.351) (Fig. 4a, d). Date and block effects were significant in all cases.
Effects of leafhopper-induced resistance on predator preference and consumption of beetle prey
Larval food (leafhopper-induced vs. non-induced leaves) did not affect predator preference for beetle prey (14 attacks on larvae reared on leafhopper-induced plants vs. 13 attacks on larvae reared on non-induced plants; χ2 = 0.04, P = 0.850) or predator consumption rate (mean no. larvae consumed from leafhopper-damaged plants = 1.93 ± 0.21, mean no. larvae consumed from leafhopper-free plants = 1.8 ± 0.2; t = 0.46, P = 0.650).
Developmental changes in susceptibility of beetle larvae to predation
Prey density (F 3,144 = 10.17, P < 0.001), larval instar (F 3,144 = 27.67, P < 0.001), and the interaction between the two (F 9,144 = 2.97, P = 0.003) affected beetle consumption by the stinkbug predator, with first and second instar larvae experiencing far higher levels of predator-inflicted mortality than third and fourth instars (Fig. 5).
Discussion
The direct impact of induced plant resistance on phytophagous arthropods is thought to be compatible with indirect enemy-mediated defense as part of a plant protection strategy that exploits both bottom-up and top-down factors. Yet the interaction of these forces in generating population-level suppression of herbivores rarely has been tested. We predicted that leafhopper herbivory would: (1) reduce beetle performance, survival, and abundance; (2) have no impact on beetle enemies via prey quality effects; and (3) enhance natural enemy-mediated suppression of beetles through developmental delays. Our data provide robust support for all three predictions. Results from our open-plot and field-cage experiments demonstrate that plant resistance induced from early-season herbivory by sap-feeding leafhoppers had a negative impact on the density and survival of potato beetles feeding later in the season (Figs. 2, 3). Notably, leafhopper induction did not cascade up to adversely affect predators via prey quality effects. For instance, when offered a choice, the predaceous stinkbug P. maculiventris did not differentiate between beetle larvae reared on leafhopper-induced plants versus those raised on leafhopper-free foliage. Similarly, predator consumption rates did not differ between larvae fed induced and control plants. As a consequence, the top-down suppression of beetles was not influenced by induced resistance via any extended effects on prey palatability. Nonetheless, predation of beetle larvae was considerably greater on leafhopper-damaged plants in our field-cage experiment (Fig. 3a). Synergistic beetle suppression by predators on induced plants is invoked because actual beetle mortality was much greater than the expected sum of mortalities arising from induced resistance and predation alone (Fig. 3b). Thus, induced resistance from early-season leafhopper herbivory enhanced the impact of beetle enemies in this tri-trophic assemblage.
We suggest that the delay in beetle larval development on leafhopper-induced plants most likely explains the synergistic effects of induced resistance and predation on beetle mortality. Prior leafhopper herbivory significantly extended larval growth in early beetle instars (first up to and including the third), but not in the final instar (fourth) (Fig. 4). This finding is noteworthy because early larval instars are highly susceptible to predation as evidenced by the strong positive relationship between prey density and consumption by beetle predators (Fig. 5). Later instars, however, are not nearly as vulnerable with only one or two late-instar larvae consumed by predators over the range of prey densities offered. In the many hours of observing interactions between stinkbug nymphs and beetle larvae of various instars, we rarely witnessed evidence for prey rejection suggesting that ontogenetic changes in behavior or chemical defense do not likely explain instar-based differences in susceptibility to predation. The most striking difference among beetle instars is their size; late-instar larvae are several orders of magnitude larger than early-instar larvae (body mass of first instar larvae = 0.98 ± 0.05 mg, fourth instar larvae = 167.42 ± 5.45 mg). Consequently, it appears that older larvae quickly satiate stinkbug predators, whereas smaller-instar larvae do not easily quench the predator’s appetite and are frequently consumed. Our results correspond with life table analyses of Leptinotarsa beetle larvae in tropical forests, which found that predation-related mortality is greatest in the first and second instars, but rapidly decreases as larvae enter larger stage classes (Cappaert et al. 1991; Cañas et al. 2002).
In addition to exposing beetle larvae in their most vulnerable instars to predation, the window of vulnerability to predation is substantially extended by leafhopper-induced resistance. At day 12 of our greenhouse experiment virtually all larvae fed undamaged plants completed development, whereas only ∼50% of the cohort pupated on leafhopper-damaged plants (Fig. 4e). In fact, a substantial proportion (∼20%) of the beetle population on leafhopper-induced plants remained in the larval stage when the experiment was terminated. Consequently, larvae that develop on plants incurring leafhopper herbivory are more susceptible to potential predators. Such development-induced changes in risk of predation are predicted by the slow-growth-high-mortality hypothesis, which posits that extended development time increases exposure to natural enemies and results in greater enemy-inflicted mortality (see Benrey and Denno 1997). Moreover, many phytophagous insects are highly vulnerable to predation during their larval stages and this is certainly the case with Colorado potato beetle larvae that are heavily attacked by a diverse assemblage of predaceous arthropods (Cappaert et al. 1991; Biever and Chauvin 1992; Cloutier and Bauduin 1995; Hough-Goldstein 1998; Cañas et al. 2002).
Although we found a strong association between delayed larval development on leafhopper-induced plants and enhanced predation, other mechanisms may contribute to elevated attack rates of beetles on induced plants. For example, we cannot dismiss the possibility that leafhopper herbivory may prime plants for stronger or more rapid volatile emissions when beetle larvae defoliate leaves, given that hemipteran predators are known to respond to volatile cues induced from Colorado potato beetle herbivory (Weissbecker et al. 1999, 2000; Van Loon et al. 2000). However, leafhoppers had not been present on induced field plants for several weeks when beetles were exposed to predators. Thus, any priming of plant physiology by leafhoppers that contributed to the enhanced attractiveness of beetle feeding sites to predators would have to be quite persistent.
An important issue to consider is why our results differ from those of previous studies that frequently document interference between induced resistance and higher trophic level effects on herbivores (Havill and Raffa 2000; Thaler 2002). One factor that may contribute to this discrepancy is the identity of the inducing herbivore. The vast majority of investigations into herbivore-induced plant responses use leaf-chewing insects to elicit a resistance response (Karban and Baldwin 1997; Agrawal et al. 1999), whereas our study featured a sap-feeding herbivore. This difference is noteworthy because feeding guild can strongly influence how plants perceive and subsequently respond to herbivory (Karban and Baldwin 1997; Walling 2000; Denno and Kaplan 2007). For instance, leaf-chewers often elicit drastic changes in secondary metabolite expression, whereas many sap-feeders cause minimal tissue damage and therefore may act as “stealthy” herbivores (Raven 1983; Heidel and Baldwin 2004; Voelckel et al. 2004; but see Walling 2000). Sap-feeders, however, frequently induce nutritional changes (e.g., % nitrogen) in their host plant (Olmstead et al. 1997; Kay et al. 2004; Denno and Kaplan 2007). Therefore, the type of induction (phytochemical or nutritional) may partly determine whether or not herbivore-induced resistance affects the strength of top-down control. For instance, inducible allelochemicals (e.g., alkaloids and glucosinolates) often cascade with toxic consequences for predators or parasitoids (Turlings and Benrey 1998; Hare 2002; Ode 2006), whereas induced nutritional changes are more likely to enhance prey suppression by slowing herbivore growth without poisoning secondary consumers (Moran and Hamilton 1980; Price et al. 1980; Loader and Damman 1991; Benrey and Denno 1997). Notably, sap-feeders were involved in the only other study demonstrating that induced plant responses facilitate top-down impacts on heterospecific herbivores (Kessler and Baldwin 2004). Specifically, prior herbivory by sap-feeding mirid bugs on wild tobacco delayed growth and enhanced predation of hornworm caterpillars that fed later in the season.
An additional factor that may explain why our study found synergism rather than interference between induction and prey suppression by predators is our choice of natural enemy. The dominant consumers of Colorado potato beetle larvae are invertebrate predators (Biever and Chauvin 1992; Cloutier and Bauduin 1995; Hough-Goldstein 1998), whereas most studies investigating the tri-trophic consequences of induced resistance have used parasitic wasps (Faeth 1994; Paré et al. 1999; Havill and Raffa 2000; Thaler 2002). Current evidence indicates that predators may be better adapted for tolerating plant defenses than parasitoids. Based on studies that examined the compatibility of host-plant resistance and biological control in agricultural crops, parasitoids were more likely to be negatively affected by plant resistance traits than predators (Hare 2002). Similarly, studies testing the slow-growth-high-mortality hypothesis report that prolonged development time is more likely to result in enhanced mortality when herbivores are attacked by predators than parasitoids (Williams 1999). Thus, despite their well-documented ability to exploit plant-based volatile signals, parasitoids may be more sensitive than predators to variation in plant chemistry because of their intimate relationship with host physiology (Turlings and Benrey 1998; Paré et al. 1999; Ode 2006). An additional possibility is that induced resistance alters herbivore size, an effect that may strongly constrain the performance of parasitoids developing on a single host. Predators, on other hand, often engage in compensatory feeding, increase their predation rate, and therefore are less likely to be adversely affected by small prey size (Erickson and Morse 1997).
In conclusion, our study demonstrates that induced resistance from early-season herbivory enhances the top-down suppression of later-feeding herbivores by delaying development in susceptible larval stages and increasing exposure to predators. It is important to consider such higher trophic level dynamics in future studies of plant-mediated herbivore interactions, as interactive effects between induced changes in plant quality and top-down forces are likely to be common.
References
Agrawal AA, Tuzun S, Bent E (1999) Inducible plant defenses against pathogens and herbivores: biochemistry, ecology, and agriculture. American Phytopathological Society Press, St Paul, Minn.
Backus EA, Serrano MS, Ranger CM (2005) Mechanisms of hopperburn: an overview of insect taxonomy, behavior, and physiology. Annu Rev Entomol 50:125–151
Benrey B, Denno RF (1997) The slow-growth-high-mortality hypothesis: a test using the cabbage butterfly. Ecology 78:987–999
Bergelson JM, Lawton JH (1988) Does foliage damage influence predation on the insect herbivores of birch? Ecology 69:434–445
Biever KD, Chauvin RL (1992) Suppression of the Colorado potato beetle (Coleoptera: Chrysomelidae) with augmentative releases of predaceous stinkbugs (Hemiptera: Pentatomidae). J Econ Entomol 85:720–726
Boege K (2004) Induced responses in three tropical dry forest plant species—direct and indirect effects on herbivory. Oikos 107:541–548
Cañas LA, O’Neil RJ, Gibb TJ (2002) Population ecology of Leptinotarsa decemlineata Stål (Coleoptera: Chrysomelidae): population dynamics, mortality factors, and potential natural enemies for biological control of the Colorado potato beetle. Biol Control 24:50–64
Cappaert DL, Drummond FA, Logan PA (1991) Population dynamics of the Colorado potato beetle (Coleoptera: Chrysomelidae) on a native host in Mexico. Environ Entomol 20:1549–1555
Cloutier C, Bauduin F (1995) Biological control of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) in Quebec by augmentative releases of the two-spotted stinkbug Perillus bioculatus (Hemiptera: Pentatomidae). Can Entomol 127:195–212
Denno RF, Kaplan I (2007) Plant-mediated interactions in herbivorous insects: mechanisms, symmetry, and challenging the paradigms of competition past. In: Ohgushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge, pp 19–50
Denno RF, McClure MS, Ott JR (1995) Interspecific interactions in phytophagous insects: competition revisited and resurrected. Annu Rev Entomol 40:297–331
Denno RF, Gratton C, Peterson MA, Langellotto GA, Finke DL, Huberty AF (2002) Bottom-up forces mediate natural-enemy impact in a phytophagous insect community. Ecology 83:1443–1458
Edwards PJ, Wratten SD, Gibberd RM (1991) The impact of inducible phytochemicals on food selection by insect herbivores and its consequences for the distribution of grazing damage. In: Tallamy DW, Raupp MJ (eds) Phytochemical induction by herbivores. Wiley, New York, pp 205–221
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci 101:1781–1785
Erickson KS, Morse DH (1997) Predator size and the suitability of a common prey. Oecologia 109:608–614
Faeth SH (1994) Induced plant responses: effects on parasitoids and other natural enemies of phytophagous insects. In: Hawkins BA, Sheehan W (eds) Parasitoid community ecology. Oxford University Press, New York, pp 245–260
Forkner RE, Hunter MD (2000) What goes up must come down? Nutrient addition and predation pressure on oak herbivores. Ecology 81:1588–1600
Häggström H, Larsson S (1995) Slow larval growth on a suboptimal willow results in high predation mortality in the leaf beetle Galerucella lineola. Oecologia 104:308–315
Hare JD (1990) Ecology and management of the Colorado potato beetle. Annu Rev Entomol 35:81–100
Hare JD (1992) Effects of plant variation on herbivore–natural enemy interactions. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens: ecology, evolution and genetics. University of Chicago Press, Chicago, Ill., pp 278–298
Hare JD (2002) Plant genetic variation in tritrophic interactions. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, New York, pp 8–43
Havill NP, Raffa KF (2000) Compound effects of induced plant responses on insect herbivores and parasitoids: implications for tritrophic interactions. Ecol Entomol 25:171–179
Heidel AJ, Baldwin IT (2004) Microarray analysis of salicylic acid- and jasmonic acid-signaling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant Cell Environ 27:1362–1373
Heil M, Kost C (2006) Priming of indirect defences. Ecol Lett 9:813–817
Hough-Goldstein JA (1998) Use of predatory pentatomids in integrated management of the Colorado potato beetle (Coleoptera: Chrysomelidae). In: Coll M, Ruberson JR (eds) Predatory Heteroptera: their ecology and use in biological control. Say, Lanham, pp 209–223
Hunter MD (1992) Interactions within herbivore communities mediated by the host plant: the keystone herbivore concept. In: Hunter MD, Ohgushi T, Price PW (eds) Effect of resource distribution on plant–animal interactions. Academic Press, San Diego, Calif., pp 287–325
Hunter MD, Price PW (1992) Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology 73:724–732
Hunter MD, Schultz JC (1993) Induced plant defenses breached? Phytochemical induction protects an herbivore from disease. Oecologia 94:195–203
Hunter MD, Varley GC, Gradwell GR (1997) Estimating the relative roles of top-down and bottom-up forces on insect herbivore populations: a classic study revisited. Proc Natl Acad Sci USA 94:9176–9181
Kaitaniemi P, Vehviläinen H, Ruohomäki K (2004) Movement and disappearance of mountain birch defoliators are influenced by the interactive effects of plant architecture and induced resistance. Ecol Entomol 29:437–446
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago, Ill.
Kay AD, Scott SE, Schade JD, Hobbie SE (2004) Stoichiometric relations in an ant–treehopper mutualism. Ecol Lett 7:1024–1028
Kessler A, Baldwin IT (2004) Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant J 38:639–649
Lill JT, Marquis RJ (2001) The effects of leaf quality on herbivore performance and attack from natural enemies. Oecologia 126:418–428
Lill JT, Marquis RJ, Ricklefs RE (2002) Host plants influence parasitism of forest caterpillars. Nature 417:170–173
Loader C, Damman H (1991) Nitrogen content of food plants and vulnerability of Pieris rapae to natural enemies. Ecology 72:1586–1590
Lynch ME, Kaplan I, Dively GP, Denno RF (2006) Host-plant-mediated competition via induced resistance: interactions between pest herbivores on potatoes. Ecol Appl 16:855–864
Marquis RJ, Whelan CJ (1996) Plant morphology and recruitment of the third trophic level: subtle and little-recognized defenses? Oikos 75:330–333
Moran N, Hamilton WD (1980) Low nutritive quality as defense against herbivores. J Theor Biol 86:247–254
Ode PJ (2006) Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy interactions. Annu Rev Entomol 51:163–185
Ohgushi T (2005) Indirect interaction webs: herbivore-induced effects through trait change in plants. Annu Rev Ecol Evol Syst 36:81–105
Olmstead KL, Denno RF, Morton TC, Romeo JT (1997) Influence of Prokelisia planthoppers on the amino acid composition and growth of Spartina alterniflora. J Chem Ecol 23:303–321
Paré PW, Lewis WJ, Tumlinson JH (1999) Induced plant volatiles: biochemistry and effects on parasitoids. In: Agrawal AA, Tuzun S, Bent E (eds) Inducible plant defenses against pathogens and herbivores: biochemistry, ecology, and agriculture. American Phytopathological Society Press, St Paul, Minn., pp 167–180
Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65
Raven JA (1983) Phytophages of xylem and phloem: a comparison of animal and plant sap-feeders. Adv Ecol Res 13:135–234
Rodriguez-Saona C, Thaler JS (2005) The jasmonate pathway alters herbivore feeding behaviour: consequences for plant defences. Entomol Exp Appl 115:125–134
Schultz JC (1983) Habitat selection and foraging tactics of caterpillars in heterogeneous trees. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 61–90
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Stiling P, Rossi AM (1997) Experimental manipulations of top-down and bottom-up factors in a tri-trophic system. Ecology 78:1602–1606
Takabayashi J, Dicke M (1996) Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci 1:109–113
Thaler JS (2002) Effect of jasmonate-induced plant responses on the natural enemies of herbivores. J Anim Ecol 71:141–150
Tomlin ES, Sears MK (1992) Indirect competition between the Colorado potato beetle (Coleoptera: Chrysomelidae) and the potato leafhopper (Homoptera: Cicadellidae) on potato: laboratory study. Environ Entomol 21:787–792
Turlings TCJ, Benrey B (1998) Effects of plant metabolites on the behavior and development of parasitic wasps. Ecoscience 5:321–333
Van der Meijden E, Klinkhamer GL (2000) Conflicting interests of plants and the natural enemies of herbivores. Oikos 89:202–208
Van Loon JJA, de Vos EW, Dicke M (2000) Orientation behaviour of the predatory hemipteran Perillus bioculatus to plant and prey odours. Entomol Exp Appl 96:51–58
Van Zandt PA, Agrawal AA (2004) Community-wide impacts of herbivore-induced plant responses in milkweed (Asclepias syriaca). Ecology 85:2616–2629
Viswanathan DV, Narwani AJT, Thaler JS (2005) Specificity in induced plant responses shapes patterns of herbivore occurrence on Solanum dulcamara. Ecology 86:886–896
Voelckel C, Weisser WW, Baldwin IT (2004) An analysis of plant-aphid interactions by different microarray hybridization strategies. Mol Ecol 13:3187–3195
Walgenbach JF, Wyman JA (1985) Potato leafhopper (Homoptera: Cicadellidae) feeding damage at various potato growth stages. J Econ Entomol 78:671–675
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Weissbecker B, Van Loon JJA, Dicke M (1999) Electroantennogram responses of a predator, Perillus bioculatus, and its prey, Leptinotarsa decemlineata, to plant volatiles. J Chem Ecol 25:2313–2325
Weissbecker B, Van Loon JJA, Posthumus MA, Bouwmeester HJ, Dicke M (2000) Identification of volatile potato sesquiterpenoids and their olfactory detection by the two-spotted stinkbug Perillus bioculatus. J Chem Ecol 26:1433–1445
Williams IS (1999) Slow-growth, high-mortality—a general hypothesis, or is it? Ecol Entomol 24:490–495
Acknowledgements
We thank Heather Harmon, Amy Miller, and Terry Patton for their assistance with field experiments. Later drafts of the manuscript were greatly improved by comments from Andre Kessler, Shannon Murphy, and Gina Wimp. Funding for this research was provided by a USDA Competitive Research Grant (NRICGP, Entomology and Nematology, 00-35302-9334) to R. F. Denno and G. P. Dively. These experiments comply with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Richard Karban.
Rights and permissions
About this article
Cite this article
Kaplan, I., Lynch, M.E., Dively, G.P. et al. Leafhopper-induced plant resistance enhances predation risk in a phytophagous beetle. Oecologia 152, 665–675 (2007). https://doi.org/10.1007/s00442-007-0692-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0692-4