Abstract
The establishment pattern of monocultures of 61 species common to Central European semi-natural grasslands was analysed in a field experiment. The objectives were to identify key traits for successful establishment, defined in terms of above-ground biomass production, and to characterize the degree of niche overlap with respect to the use of above-ground resources, such as light and space. Four months after sowing, 15 species reached an above-ground biomass of more than 400 g m−2. Highly productive monocultures adopted extremely different strategies of space filling in terms of canopy height, biomass density and centre of gravity of vertical biomass distribution. Regression tree analysis identified (1) the number of seedlings and (2) a trade-off between the development of a large number of small-sized shoots of species with intensive clonal growth in contrast to the establishment of fewer large-sized shoots as the two most important traits for successful establishment. Further variables associated with high above-ground biomass production by individual species were traits known to be relevant to the relative growth rate of herbaceous species, such as specific leaf area, leaf nitrogen or allocation between shoots and roots. The principle finding of this study is that the success of the 15 most productive species was not based on a single pathway but on a variable combination of traits. There are clearly many possible combinations of morphological and physiological features that will result in a species becoming productive, and these combinations differ among species in a local species pool.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The threat to species diversity over the last decades has attracted growing attention to the consequences of this decline on ecosystem functioning (Loreau et al. 2001). A steadily increasing body of evidence indicates that an understanding of the changes in ecosystem properties requires an analysis of the functional characteristics of the species involved (Walker et al. 1999; Hooper et al. 2005).
All plant species compete for the same major resources – available light, water, carbon dioxide, minerals and space. Morphological, physiological and phenological differences result in plant species adapting various strategies to acquire these resources. The grouping of species into functional types is one widely accepted approach to making studies of the underlying complex mechanisms more comprehensible (Schulze 1982; Smith et al. 1997). Plant functional types are defined as groups of species that have comparable effects on major ecosystem processes (functional effect groups) or which respond similarly to environmental changes (functional response groups) (Díaz and Cabido 2001). The underlying logic of the approach is that similarities between species can be attributed to a set of key functional traits (e.g. Grime et al. 1997; Lavorel et al. 1997; Westoby 1998; Weiher et al. 1999) and that trait combinations result in discrete groups rather than a continuum. Another common approach to predict species effects on ecosystem functioning is to focus on single traits that are supposed to be most important for a given ecosystem process. Both the functional group approach and the focus on single traits are closely related as long as single traits tend to co-vary with other traits.
Functional types or single traits have been applied successfully in many cases to predict effects on ecosystem processes (functional effects). Experimental studies have demonstrated the existence of relationships between plant traits and soil properties such as microbial biomass, pH and nitrate concentrations (Groffman et al. 1996; Wardle et al. 1998), nitrogen cycling (Wedin and Tilman 1990; Wedin and Pastor 1993; Craine et al. 2002), decomposition (Cornelissen 1996; Cornelissen and Thompson 1997) or primary productivity (review in Lavorel and Garnier 2002). Many other studies have identified key processes and associated feedbacks which facilitate an understanding of the differences in response (see reviews in Weiher et al. 1999; Westoby et al. 2002). For example, the competitive ability of adult plant individuals under nutrient-rich conditions appears to be associated with fast growth and high relative growth rates coupled with a rapid transfer of captured resources into new leaves and roots, but also to a short life span of individual plant parts (e.g. Grime and Hodgson 1987; van der Werf et al. 1993; Ryser and Urbas 2000). Leaf nitrogen concentration, specific leaf area, low tissue density and high shoot-root ratios are positively related to the relative growth rate and the rate of photosynthesis (e.g. Hilbert 1990; Garnier 1991, 1992; Poorter and Remkes 1990; Hunt and Cornelissen 1997; Aerts and Chapin 2000).
Correlative relationships between traits have been derived from comparisons within and between ecosystems (e.g. Reich et al. 1997; Enquist and Niklas 2002; Westoby et al. 2002; Díaz et al. 2004; Reich and Oleksyn 2004). However, there are also some studies which show that traits which may co-vary on a large scale can, in fact, vary independently on a local scale (e.g. Fonseca et al. 2000; Lavorel and Garnier 2002; Eviner and Chapin 2003). Furthermore, a study on experimental monocultures has shown that the mechanisms by which plant species influence biogeochemical cycling, such as by biomass production, litter chemistry, carbon input from root turnover and exudation, soil moisture and temperature, differ among species (Eviner 2004). These findings emphasize the uniqueness of these species and their effects on ecosystem processes.
In the present study, we investigated the establishment of 61 grassland species in experimental monocultures in order to identify traits which are associated with the success of the species. We concentrated our analysis on above-ground productivity during the first growing season as the functional effect on the ecosystem level and investigated a number of traits at the plant individual and population level. Our main questions were: which plant traits determine a successful monoculture development? Do successful species coincide in combinations of certain traits, or are there several strategies? Additionally, we studied the spatial structure of biomass distribution to ask whether there are similar strategies to use such above-ground resources as space and light among the most successful species.
Materials and methods
Study site
The experimental site of the study was located at the floodplain of the Saale river in Jena (Thuringia, Germany; 50°55′N, 11°35′E, 130 m a.s.l.). The soil is a nutrient-rich riverside soil (Eutric Fluvisol) developed from up to 2 m-thick loamy fluvial sediments. The site was used as an arable field before the sowing of the experimental species and has been heavily fertilized over the last decades. Mean annual air temperature is 9.3°C, and the average annual precipitation amounts to 587 mm in the area around Jena (Kluge and Müller-Westermeier 2000). Central European semi-natural species-rich grasslands, which are traditionally mown twice a year (Arrhenatherion alliance according to the vegetation classification of Ellenberg 1988), served as the target plant community. A pool of 61 species common to these grasslands was chosen for the study (Roscher et al. 2004). A complete species list is given in Table 1. All species were grown in two monoculture plots of 3.5 × 3.5 m as part of a large biodiversity experiment. The experimental site was divided into four blocks following a gradient of soil characteristics. Each block contained 30 monocultures selected randomly from the species pool. The occurrence of identical species replicates in the same block was not allowed.
Plots were sown from 11 to 16 May 2002. Modifications in the number of seeds sown was based on laboratory germination tests; the objective was to obtain 1000 germination events (seedlings per square metre). Seeds of Ajuga reptans, Carum carvi, Cirsium oleraceum, Pastinaca sativa, Pimpinella major, Primula veris, Ranunculus acris, R. repens and Sanguisorba officinalis were pre-treated with gibberellic acid (500 mg l−1; 24 h) to break dormancy. Seeds of some legume species (Lathyrus pratensis, Lotus corniculatus, Medicago lupulina, Trifolium campestre, T. dubium, Vicia cracca, V. sepium) and Geranium pratense were scarified (scratched) to perforate the testa. All plots were weeded twice during the first growing season to remove unwanted species. Eight weeks after sowing (beginning of July), plots were mown to a height of 10 cm and the biomass removed. A second mowing was carried out in September 2002 (for detailed description see Roscher et al. 2004).
Data collection
Seed mass was determined by weighing five batches of 50 seeds for each species. Seedling emergence was controlled weekly in the field. Two weeks after the first appearance of the seedlings, but not later than 8 weeks after sowing, we counted established seedlings in two randomly selected quadrants (50 × 50 cm) per plot. The number of established individuals or shoots (in the case of species with vegetative reproduction) was determined at the end of the first growing season by counting the same subplots. This number is referred to as “plant shoots”. Total vegetative cover was estimated visually (using a 5% scale), and average canopy height of foliage was measured monthly.
Fifteen weeks after sowing (end of August 2002) (two–)five randomly selected shoots per plot were harvested above the soil surface. Shoots were put in sealed plastic bags immediately after harvesting, stored in a cool box and transported to the laboratory. All measurements were done as soon as possible (between 1 and 8 h after harvest). The harvested plant material was field fresh and did not show any signs of wilting. Shoots were separated into stems, leaves and reproductive (flowering or fruiting) parts, and the total leaf area per shoot was measured immediately as part of the biomass separation process (LAI-3100 Area Meter; LI-COR, Lincoln, Neb.). Petioles and the rachis of compound leaves were included in the leaf area measurements. Leaf area measurements comprised the entire shoot and were not restricted to the upper adult leaves of a shoot (Cornelissen et al. 2003) because our aim was to encompass the species-specific plastic adaptation of trait values to the growing conditions in monoculture stands of species-dependent densities. Dry weight per plant compartment was obtained after drying for 48 h (70°C). Leaf tissue was analysed for total nitrogen and carbon concentration with an elemental analyzer (Vario EL Element Analyzer; Elementar, Hanau, Germany). Based on these measurements the following variables were calculated for each species:
-
specific leaf area (SLA), which is the total leaf area per shoot divided by total leaf mass per shoot (mm2 leaf mg−1 leaf);
-
leaf mass fraction (LMF), which is the leaf dry weight per shoot dry weight (mgleaf mg−1 shoot);
-
stem mass fraction (SMF), which is stem dry weight per shoot dry weight (mgstem mg−1 shoot);
-
leaf area ratio (LAR), which is the product of specific leaf area and leaf mass fraction (mm2 leaf mg−1 shoot);
-
mass-based leaf nitrogen (leaf Nmass), which is nitrogen mass per leaf dry weight (mgN g−1 leaf);
-
area-based leaf nitrogen (leaf Narea), which is nitrogen mass per leaf area (gN m−2 leaf).
Leaf inclinations were estimated in the field using the classification scheme of Barkman (1979). Field data were summarized in three categories as the deviation from horizontal position (see Table 1).
Above-ground biomass was determined by harvesting plant material in strata of 10 cm in two randomly selected 20 × 50-cm sampling areas per plot (early in September 2002). The sample area was extended to 50 × 100 cm in plots with sparse vegetation cover. The area was clipped to 3 cm above ground. Only a sub-area of 10 × 20 cm was harvested to the ground surface in order to minimize destructive sampling. Clipped vegetation was sorted into living plant material and litter. The dry weights of the samples were used to calculate strata biomass per square metre and added together to make up the above-ground biomass production per square metre. For some species with very low abundances and cover, the number of shoots per square metre was counted, multiplied with the average dry mass per shoot (see above) and used as the estimate for above-ground biomass production. In order to obtain a measure of vertical biomass distribution in the canopy, we computed weighted mean height (WMH) – or centre of gravity – (cm) of above-ground biomass by multiplying the biomass of each layer (m i ) with the mean height of the layer (h i ) and dividing the sum of h i × m i by the total biomass in accordance with Gibson et al. (1987):
Biomass density (g m−3) was estimated as biomass (g m−2) divided by the height of the upper layer (h max) to characterize the three-dimensional space filling per stand.
Below-ground biomass was sampled by removing a soil core sample [5 (diameter) × 30 (depth)cm] centered at a randomly chosen plant individual per plot. Below-ground biomass was washed from the soil cores over a 2-mm and 200-μm-mesh sieve until it was free of soil and picked out with tweezers. Samples were dried at 70°C for 48 h before weighing. Below-ground biomass per square metre was estimated by multiplying the number of individuals per square metre by below-ground biomass per core. Only for species with an intensive vegetative growth and tightly packed shoots (e.g. grasses, Trifolium repens) was below-ground biomass per core area used directly to calculate below-ground biomass per square metre corrected for above-ground plant cover. Above-ground biomass (g m−2) was divided by below-ground biomass (g m−2) as estimate of the shoot-root ratio.
Data analysis
Data on plant individuals, counts of seedlings and shoots and biomass samples were averaged per plot. A logarithmic or square-root transformation was applied in the case of non-normality. Percentage cover and leaf and stem mass fractions were square-root arcsin-transformed. Analysis of variance (ANOVA) was used to test for effects of the experimental blocks on the measured variables. Because no significant block effect was detected, the means of all variables were calculated for identical replicates of each single species. In general, data analysis was performed per species.
Analysis focused on above-ground biomass as a functional effect. Traits supposedly being related to above-ground biomass production during the first growing season and used as predictors are listed in Table 2. The chosen traits comprise population characteristics as well as measurements on the individual plant level. The number of shoots counted at the end of the first growing season was divided by the number of seedlings and used as an estimate of the establishment success of seedlings (= relative change of shoot number). This measure combines the effects of seedling survivorship on the one hand and the capacity for vegetative growth of several species on the other. Stand characteristics necessarily linked with above-ground biomass, such as cover, canopy height, the derived variables biomass density and centre of gravity of biomass distribution, were not included in these analyses. Spearman rank correlations were calculated for all variables. A standardized principal component analysis (PCA) was performed with the matrix of plant traits considered to be predictors for above-ground biomass (see Table 2). Relative loadings of traits in an eigenvector represent their relevance to a given axis. The relative importance of axes in explaining the variation among species is indicated by the eigenvalues. A regression tree model was fitted to analyse the explanatory power of predictor traits for above-ground productivity over the first growing season as a functional effect. Tree models are a useful exploratory tool by which to analyse data with non-linear relationships and high-order interactions (De`ath and Fabricius 2000). Such trees are constructed by splitting the data repeatedly into two groups (nodes) according to a threshold value of the explanatory variable. The mean values of the possible response variables above and below this threshold as well as the deviance are calculated. The explanatory variable that accounts for the greatest decrease in deviance is selected for the partitioning (Crawley 2002). In theory, the regression tree can be grown until each terminal node (= leaf) represents a single species, but a complex tree is often too well adapted to the particular set of attributes and is hardly reproducible for new data. To decide upon a reasonable tree size, we therefore used cross-validation to assess the reliability of the partitioning splits. In the cross-validation procedure the data are randomly divided into a training set (used for constructing the regression tree) and a test set (used to evaluate the predictive power). There is typically an optimum intermediate size of the tree where the prediction error is minimized. The construction of a regression tree, therefore, proceeded in two steps. First, all functional attributes were included in a tree analysis based on a chosen minimal node size of 4 and a minimal node deviance of 0.01. In a second step, the subset of variables included in the maximum tree was used as the basis for the cross-validation procedure. Data analysis was performed using S-Plus ver. 6.1 (Insightful Corp., Seattle, Wash.). CANOCO (ter Braak and Šmilauer 1998) was applied for ordination analysis.
Results
Above-ground biomass and stand structure
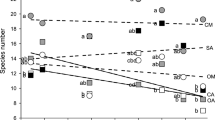
Seven weeks after sowing (end of June 2002) 14 species reached a cover of more than 50%. Only two species (Anthriscus sylvestris, Heracleum sphondylium) failed to germinate completely during the first growing season, and these were excluded from further analysis. In September 2002 (4 months after sowing), 39 species covered at least 50% and 20 species exceeded 95% cover. At this time the above-ground biomass varied between 1 g m−2 (Primula veris) and 580 g m−2 (Achillea millefolium). Fifteen species (25% of all species) produced more than 400 g m−2; of these highly productive monocultures, five were legumes, five were grasses and five were non-legume herbs. Canopy height ranged from 20 cm (Festuca rubra) to more than 60 cm (Centaurea jacea, Onobrychis viciifolia). There were highly significant positive correlations between above-ground biomass and stand characteristics such as cover, canopy height, weighted mean height of biomass distribution and biomass density (Table 2). The 15 most productive monocultures developed clearly different strategies of vertical biomass distribution (Fig. 1; Table 1). Weighted mean height (centre of gravity) of biomass distribution ranged from 9 cm (F. rubra) to 32 cm (O. viciifolia); biomass density varied between 447 g m−3 (Phleum pratense) and 1875 g m−3 (Crepis biennis) for these species. Species with a higher centre of gravity of biomass distribution tended to exhibit a lower biomass density, but medium values of centre of gravity were coupled with strongly different biomass densities.
Relationship between weighted mean height (cm) of biomass distribution and biomass density (g m−3). Strata of 10 cm were harvested in all monocultures in September 2002. Species were divided into three categories according to above-ground biomass production: filled circle (upper quartile) 15 most productive species – above-ground biomass >400 g m−2, open circle species of intermediate productivity (30 species – above-ground biomass between 120 and 400 g m−2, filled inverted triangle (lower quartile) 14 least productive species – above-ground biomass <120 g m−2. Dashed lines mark the upper and lower quartiles of data distribution for weighted mean height of biomass distribution (cm) and biomass density (g m−3)
Relationships between above-ground biomass and other growth variables
Principal components analysis
The two leading axes of the PCA of the matrix of growth variables explained more than 47% of the total variation. The first axis accounted for 30.5% of the variation and the second axis for 17.0%. The first axis was characterized by high loadings for variables such as the stem mass fraction (SMF) and the negatively correlated leaf mass fraction (LMF). Further variables with higher importance on this axis were mass-based nitrogen in leaf tissue, specific leaf area, shoot-root ratio and rooting depth. The number of seedlings and time to seedling emergence (being highly significantly correlated r s = −0.589, P < 0.001, n = 59) explained most of the variation on the second axis of PCA (Fig. 2a; Table 3). In Fig. 2b, species were divided into three groups defined by the lower (15 least productive species) and upper (15 most productive species) quartiles of values for above-ground biomass production. Species' groups were enveloped with polygons. The positions of the 15 most productive species in ordination space were denoted with symbols. Species with low biomass production during the year of establishment occupy the two upper quadrants of the two-dimensional ordination space, whereas the opposite quadrants comprise the polygons of species with intermediate and high above-ground biomass production. A long time to seedling emergence and a low number of seedlings were correlated with the division into high- and low-productive species, respectively. However, the extent of ordination space covered by the polygon of the 15 most productive species along the first ordination axis clearly indicates that several trait combinations were responsible for high biomass production.
Principal components analysis (first vs. second axis) of the trait matrix (Table 3) based on 59 species. a Biplot including the complete trait matrix. Open circles indicate species positions. b Biplot including traits selected in regression tree analysis (Fig. 3). The classification of species into three groups is in accordance with above-ground biomass production of the species. Polygons indicate the ordination space covered by the 15 most productive species (I), species with intermediate productivity (II) and the 14 least productive species (III). The 15 most productive species are denoted by closed circles. Abbreviations of species names: Am Achillea millefolium, Ae Arrhenatherum elatius, Cb Crepis biennis, Cj Centaurea jacea, Dg Dactylis glomerata, Fp Festuca pratensis, Fr Festuca rubra, Ka Knautia arvensis, Lv Leucanthemum vulgare, Mv Medicago × varia, Ov Onobrychis viciifolia, Pp Phleum pratense, Tf Trifolium fragiferum, Th Trifolium hybridum, Tr Trifolium repens
Regression tree analysis
Further insight into the traits which lead to high biomass production was extracted from the regression tree analysis (Fig. 3). Cross-validation indicated an optimal tree size of four leaves, thus making further sub-partitioning based on the chosen criteria questionable (Fig. 4). The first split of data (N1) was based on the number of seedlings and indicated that species with fewer than 168 seedlings per square metre (less than 20% of the aimed seedling density of 1000 seedlings per square metre) generally reached a low standing crop. The second split of the left branch (N2), which comprised species with few seedlings, was based on the time to seedling emergence. It separated five species that germinated within 2 weeks after sowing from 14 species that needed more than 2 weeks for germination. The early-germinating species were split with a low predictive power based on seed mass (N4). Species with a lower seed mass obtained higher biomass, but only one of these species (Festuca pratensis) was among the 15 most productive species at the end of the first growing season. Species separation on the opposite branch (N5) was based on the dry mass of individual shoots, but nearly all species of this branch (except for Ranunculus acris and Veronica chamaedrys) belonged to the species with the lowest biomass production.
Regression tree of above-ground biomass production (= functional effect) of all species in monoculture (n = 59). Functional traits (Table 2) were used as predictor variables. Each node (N) is numbered. The variables determining the split (= nodes) are listed in the legend. The thresholds for partitioning are given above the branches. The values of the variable on the left branch are lower than the value at the node, but they are higher on the right branch. All species are listed on terminal leaves of the tree. Species ranks according to their above-ground biomass production are given in parentheses. For the abbreviations of the species names, see Table 1
Prediction error for differently sized sub-trees of Fig. 3 based on cross-validation. The estimated prediction error is smallest for a tree size of four terminal leaves
In a continuation of the regression tree in terms of the right half, which comprises all species with a higher number of seedlings, the first splitting divided species with dry mass of single shoots above and below 1.5 g (N3). All subsequent divisions of the tree were not very reliable according to the results of cross-validation, but they were helpful in providing some insights into the strategies of individual species. The next split (N6) separated a group of five species and included species with a high germination success, but a low rate of survivorship of these seedlings. Species with a higher survivorship were redivided based on differences in shoot mass (N12). Five species with a shoot mass of more than 245 mg belonged to the 15 most successful species in terms of above-ground biomass. These species – three grasses (Arrhenatherum elatius, Dactylis glomerata, Phleum pratense) and two legumes with the capacity for vegetative growth (Trifolium fragiferum, T. repens) – were additionally characterized by a lower specific leaf area compared to a group of less productive species group (N14). Only one species (Festuca rubra) of those with a shoot mass of less than 245 mg reached a high biomass production during the first growing season.
Among the species with a high mass of individual shoots, those with a shoot-root ratio larger than 2.9 (N7, N8) comprised the most productive species, with the exception of Achillea millefolium and Medicago × varia that reached a high above-ground biomass production in combination with a higher below-ground biomass (shoot-root ratio <2.9). The highly productive legumes Trifolium hybridum and Onobrychis viciifolia and the non-legume herbs Centaurea jacea and Leucanthemum vulgare were characterized by high numbers of seedlings during establishment. The slightly more productive T. hybridum and L. vulgare had a lower seed mass compared to C. jacea and O. viciifolia.
The trade-off between the mass of individual shoots and the number of shoots developed during the first growing season is illustrated in Fig. 5. On the one hand, the 15 most productive monocultures included species with a large number of shoots caused by an intensive vegetative growth coupled with a low investment in the biomass of individual shoots. This strategy is typically performed by grasses, but the creeping legumes Trifolium fragiferum and T. repens showed a similar behaviour. On the other hand, successful species developed relatively few shoots with a high mass of individual shoots, such as the legumes T. hybridum, Onobrychis viciifolia and Medicago × varia and some highly productive non-legume herbs. Reduced major axis regression of the logarithms of shoot mass versus shoot density resulted in a slope of −1.02 for the 15 most productive species. Species of intermediate productivity were located close to this regression line, whereas the poorly established species were scattered below it.
Relationship between shoot mass (mg) and shoot density (m−2) plotted on a log-log-scale. The separation into three groups is based on the above-ground biomass production of the species. For explanation of the symbols, see Fig. 1. Reduced major axis regression (Quinn and Keough 2002) was fitted for the 15 most productive species (y = 5.67 − 1.02x). The null hypothesis (slope = −1) was not rejected (t = 0.019, P = 0.493, n = 15)
Discussion
Functional features (“traits”) and measures of species performance do not have a consistent definition because any specific categorization may depend on the function under consideration. Applied in the broadest sense, functional traits at the species level can be considered to be biochemical, physiological, morphological, developmental and/or behavioural mechanisms (Geber and Griffen 2003). The productivity of ecosystems has been related to a large number of traits of different plant species at several levels of organization. Such traits include measures at the whole-plant level (e.g. growth form, life span, maturation age, period of photosynthetic activity), at the whole-shoot level (e.g. shoot height, canopy architecture), at the leaf level (e.g. specific leaf area, dry matter content, nitrogen concentration, leaf life span, leaf phenology) and root characteristics (rooting depth, specific root length) (Lavorel and Garnier 2002). In addition to these traits, which mainly cover the adult stage of plant life cycle, regeneration and demographic traits may be important during establishment as well as for ecosystem stability.
Our analysis focusing on the establishment of 61 grassland species in a field experiment indicated that both traits directly related to seedling recruitment and traits describing growth and development at the individual and population level were key factors for above-ground biomass production during the first growing season. We found a number of significant correlations between biomass production, stand structure and measured functional traits, or between the functional traits themselves (Table 2). The majority of these relations, however, showed considerable scatter. While these findings support the fact that not a single trait but a combination of several traits is relevant to biomass production, thereby supporting the conclusions expressed in the review by Lavorel and Garnier (2002), they also emphasize the importance of certain particular traits that do not coincide among species. Thus, our results do not allow us to support any single one of a number of general strategies, as shown by Craine et al. (2002). Our analyses of the trait matrix by ordination and regression tree analyses revealed the uniqueness of trait combinations for individual species. Nevertheless, both the ordination and regression tree analyses indicated that a high germination rate was particularly crucial to reach a high above-ground biomass during the first growing season. However, even this “rule” has an exception: one species, Festuca pratensis, belonged to the 15 most productive species despite its inability to establish a high number of seedlings.
Several studies have reported that smaller seed size and/or early seed emergence are correlated with higher seedling relative growth rates (e.g. Fenner 1983; Gross 1984; Shipley and Parent 1991). Contrary observations do exist, however, and a survey, including several studies by Shipley and Peters (1990), failed to find such a relationship. Little information exists on the relationship between seedling establishment and the subsequent development of plant species. Fenner (1987) identified a variety of causes for seedling mortality that make survivorship unpredictable and context-dependent (Leishman 1999). In our study, we found a positive correlation between the number of emerged seedlings and a short time for seedling emergence. The 15 most productive species germinated within 2 weeks after sowing, whereas all species of the group with the lowest biomass production, except Vicia sepium, needed 3 weeks or longer for seedling emergence. Despite these results, fast germination was not a guarantee for a successful subsequent development and the highest biomass production.
Further analysis clearly demonstrated that a variety of combinations of species-dependent traits supported a high productivity in monocultures. The most productive species differed considerably in terms of shoot mass and the number of shoots grown during the first growing season. The plotting of shoot number and shoot mass on log-log-scale (Fig. 5) visualized a trade-off between shoot density and shoot size. Highly productive species were found at different positions of the regression line. Population biology studies with plant monocultures of different densities have demonstrated that the relationship between shoot mass and shoot number plotted on log-log-scale is represented by regression line with a slope of −1 at maximum yield (“law of constant final yield”; Kira et al. 1953 in Harper 1977). In our study of species with an above-ground biomass production of more than 400 g m−2 the regression of log shoot mass over log shoot number resulted in a slope of nearly −1, even though a uniform regression line was not the expected result in a comparison of species with different plant architecture. A comparison of the most productive species showed that species with small-sized shoots reached a high total biomass due to an increase in shoot number by vegetative growth during the first growing season. This characteristic was particularly strong in Festuca rubra and the poorly germinated F. pratensis (Table 1). In contrast, number of shoots of species with large shoots decreased as the number of counted seedlings increased. This reduction may be due to a low seedling survival, but it may also result from mortality at a later stage of stand development.
Species differences in terms of germination success and population development are expected to result in species-specific differences in the extent of intra-specific competition. Increasing intra-specific competition during stand development implies a change in environmental conditions, such as the light climate or nutrient availability for plant individuals. Such changes are known to induce phenotypic adaptation in plant species. Modular growth and indeterminate development enable plants to have a particularly flexible response to a changing environment (Sultan 1995; Geber and Griffen 2003). Competition for light may lead to increased biomass allocation to above-ground plant parts and induce stem elongation (Bloom et al. 1985). Taller growth favours individual plants in the interception of more light but necessitates increasing the allocation of resources to supporting tissue (Tilman 1988; Lambers and Poorter 1992; Hirose and Werger 1995). The specific leaf areas of leaves developing under high light conditions in the upper strata of a plant stand often differ within the same plant individual when compared to plant parts developed in the lower canopy (Poorter and de Jong 1999). Additionally, features such as leaf area ratio, nitrogen concentration in plant tissue or allocation to below-ground biomass have been shown to change during ontogeny in perennial herbs (Niinemets 2004, 2005). However, a comparative analysis of the leaf traits specific leaf area and leaf nitrogen concentrations also indicated that inter-specific variation exceeds changes within a species (e.g. Garnier et al. 2001, 2004). Nevertheless, the expression of phenotypic plasticity is an important characteristic of plant species that underlies species-specific genetically manifested morphological and physiological constraints. The potential for plastic adaptation is an often neglected aspect in the analysis of plant growth and their effects on ecosystem processes, mainly due to the difficulty in measuring plasticity (Weiher et al. 1999).
The wide spread of the most productive species in the ordination space (Fig. 2b) and among the leaves of the regression tree (Fig. 3) indicated that a variety of unique trait combinations assembled at the population level (number of shoots), at the plant individual level (shoot mass, shoot-root ratio) and/or at the leaf level (specific leaf area) enabled individual species to be highly productive in monocultures. The diverse stand architecture of the most productive monocultures showed that these species differ in the use of such above-ground resources as light and space. These findings agree with the argumentation of Reich et al. (2003) that the selection for traits resulting in different plant strategies may be equally strong within a site as across environmental gradients. The great variety of trait combinations leading to similar functional effects contributes to the resilience of ecosystems against stress (Walker et al. 1999). Obviously, our study refers to the behaviour of species in monocultures during their establishment, and an assessment of the relative importance of the trait plasticity induced by intra-specific competition and environmental conditions falls outside of the context of our analysis. As such, our analysis does not allow us to predict whether the importance of individual traits will change with the age of the monocultures. We used a wide range of grassland species for our experiment. Despite all of the these species being typical for semi-natural Central European grasslands of alluvial plains with nutrient-rich soil conditions, we cannot exclude the influence of different soil preferences of individual species on the outcome of our study.
Although there may be additional traits related to above-ground biomass production that were not included in our analysis, our results show that there are numerous ways of getting into the league of the most productive species and that it depends on the environmental conditions and competitive effects determining which species become dominant (= highly productive) in mixtures of species. The study confirms that the uniqueness of trait combinations in most species does not allow any prediction of functional effects from single or few co-varying traits (Eviner and Chapin 2003) or the use of categorical or continuous functional classifications.
References
Aerts R, Chapin FS III (2000) The mineral nutrition of wild plant species revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Barkman JJ (1979) The investigation of vegetation texture and structure. In: Werger MJA (ed) The study of vegetation. Dr. W. Junk, The Hague, pp 123–160
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants – an economic analogy. Annu Rev Ecol Syst 16:363–392
Cornelissen JHC (1996) An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol 84:573–582
Cornelissen JHC, Thompson K (1997) Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytol 135:109–114
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MAG, Pausas JG, Poorter H (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Craine JM, Tilman D, Wedin D, Reich P, Tjoelker M, Knops J (2002) Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct Ecol 16:563–574
Crawley MJ (2002) Statistical computing. An introduction to data analysis using S-Plus. Wiley, Chichester
De`ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Díaz S, Hodgson JG, Thompson K, Cabido M, Cornelissen JHC, Jalili A, Montserrat-Martí G, Grime JP, Zarrinkamar F, Asri Y, Band SR, Basconcelo S, Castro-Díez P, Funes G, Hamzehee B, Khoshnevi M, Pérez-Harguindeguy N, Pérez-Rontomé MC, Shirvany FA, Vendramini F, Yazdani S, Abbas-Azimi R, Bogaard A, Boustani S, Charles M, Dehghan M, de Torrres-Espuny L, Falczuk V, Guerrero-Campo J, Hynd A, Jones G, Kowsary E, Kazemi-Saeed F, Maestro-Martínez M, Romo-Díez A, Shaw S, Siavash B, Villar-Salvador P, Zak MR (2004) The plant traits that drive ecosystems: evidence from three continents. J Veg Sci 15:295–304
Ellenberg H (1988) Vegetation ecology of central Europe. Cambridge University Press, Cambridge
Enquist BJ, Niklas KJ (2002) Global allocation rules for patterns of biomass partitioning in seed plants. Science 295:1517–1520
Eviner VT (2004) Plant traits that influence ecosystem processes vary independently among species. Ecology 85:2215–2229
Eviner VT, Chapin FS III (2003) Functional matrix: a conceptual framework for predicting multiple plant effects on ecosystem processes. Annu Rev Ecol Syst 34:455–485
Fenner M (1983) Relationships between seed weight, ash content and seedling growth in twenty-four species of composite. New Phytol 95:697–706
Fenner M (1987) Seedlings. New Phytol 106[Suppl]:35–47
Fonseca CR, Overton JM, Collins B, Westoby M (2000) Shifts in trait-combinations along rainfall and phosphorus gradients. J Ecol 88:964–977
Garnier E (1991) Resource capture, biomass allocation and growth in herbaceous plants. Trends Ecol Evol 6:126–131
Garnier E (1992) Growth analysis of congeneric annual and perennial grass species. J Ecol 80:665–675
Garnier E, Laurent G, Bellmann A, Debain S, Berthelier P, Ducout B, Roumet C, Navas ML (2001) Consistency of species ranking based on functional leaf traits. New Phytol 152:69–83
Garnier E, Cortez J, Billès G, Navas M-L, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint JP (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Geber MA, Griffen LR (2003) Inheritance and natural selection on functional traits. Int J Plant Sci 164[Suppl 3]:21–42
Gibson CWD, Dawkins HC, Brown VK, Jepsen M (1987) Spring grazing by sheep: effects on seasonal changes during early old field succession. Vegetatio 70:33–43
Grime JP, Hodgson JG (1987) Botanical contributions to contemporary ecological theory. New Phytol [Suppl]106:283–295
Grime JP, Thompson K, Hunt R, Hodgson JG, Cornelissen JHC, Rorison IH, Hendry GAF, Ashenden TW, Askew AP, Band SR, Booth RE, Bossard CC, Campbell BD, Cooper JEL, Davison AW, Gupta PL, Hall W, Hand DW, Hannah MA, Hillier SH, Hodkinson DJ, Jalili A, Liu Z, Mackey JML, Matthews N, Mowforth MA, Neal AM, Reader RJ, Reiling K, Ross-Fraser W, Spencer RE, Sutton F, Tasker DE, Thorpe PC, Whitehouse J (1997) Integrated screening validates primary axes of specialisation in plants. Oikos 79:259–281
Groffman PM, Eagan P, Sullivan WM, Lemunyon JL (1996) Grass species and soil type effects on microbial biomass and activity. Plant Soil 183:61–67
Gross KL (1984) Effects of seed size and growth form on seedling establishment of six monocarpic perennial plants. J Ecol 72:369–387
Harper JL (1977) Population biology of plants. Academic, London
Hilbert DW (1990) Optimization of plant root-shoot ratios and internal nitrogen concentration. Ann Bot 66:91–99
Hirose T, Werger MJA (1995) Canopy structure and photon flux partitioning among species in a herbaceous plant community. Ecology 76:466–474
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Hunt R, Cornelissen JHC (1997) Components of relative growth rate and their interrelations in 59 temperate plant species. New Phytol 135:395–417
Kluge G, Müller-Westermeier G (2000) Das Klima ausgewählter Orte der Bundesrepublik Deutschland: Jena, Berichte des Deutschen Wetterdienstes 213
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 23:187–261
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Lavorel S, McIntyre S, Landsberg J, Forbes TDA (1997) Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends Ecol Evol 12:474–478
Leishman MR (1999) How well do plant traits correlate with establishment ability? Evidence from a study of 16 calcareous grassland species. New Phytol 141:487–496
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledges and future challenges. Science 294:804–808
Niinemets Ü (2004) Adaptive adjustment to light in foliage and whole-plant characteristics depend on relative age in the perennial herb Leontodon hispidus. New Phytol 162:683–696
Niinemets Ü (2005) Key plant structural and allocation traits depend on relative age in the perennial herb Pimpinella saxifraga. Ann Bot 96:323–330
Poorter H, de Jong R (1999) A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol 143:163–176
Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83:553–559
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Reich PB, Oleksyn J (2004) Global patterns of plant N and P in relation to temperature and latitude. Proc Natl Acad Sci USA 101:11001–11006
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reich PB, Wright IJ, Cavender-Bares J, Craine MJ, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164[Suppl 3]:143–164
Roscher C, Schumacher J, Baade J, Wilcke W, Gleixner G, Weisser WW, Schmid B, Schulze E-D (2004) The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl Ecol 5:107–121
Rothmaler R (2002) Exkursionsflora von Deutschland. Bd. 4. Kritischer Band. In: Jäger EJ, Werner K (eds) Exkursionsflora von Deutschland, 9th edn. Spektrum Akademischer Verlag, Heidelberg
Ryser P, Urbas P (2000) Ecological significance of leaf life span among Central European grassland species. Oikos 91:41–50
Schulze ED (1982) Plant life forms and their carbon, water and nutrient relations. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology. Physiological plant ecology II, vol 12B. Water relations and photosynthetic productivity. Springer, Berlin, pp 615–676
Shipley B, Peters RH (1990) The allometry of seed weight and seedling relative growth rate. Funct Ecol 4:523–529
Shipley B, Parent M (1991) Germination responses of 64 wetland species in relation to seed size, minimum time to reproduction and seedling relative growth rate. Funct Ecol 5:111–118
Smith TM, Shugart HH, Woodward FI (1997) Plant functional types and their relevance to ecosystem properties and global change. Cambridge University Press, Cambridge
Sultan SE (1995) Phenotypic plasticity and plant adaptation. Acta Bot Neer 44:363–383
ter Braak CJF, Šmilauer P (1998) CANOCO reference manual and user`s guide to CANOCO for Windows. PUDOC, Wageningen
Tilman D (1988) Dynamics and structure of plant communities. Princeton University Press, Princeton
van der Werf A, van Nuenen M, Visser AJ, Lambers H (1993) Contributions of physiological and morphological plant traits to a species’ competitive ability at high and low nitrogen supply. Oecologia 94:434–440
Walker B, Kinzig A, Langridge J (1999) Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2:95–113
Wardle DA, Barker GM, Bonner KI, Nicholson KS (1998) Can comparative approaches based on plant ecophysiological traits predict the nature of biotic interactions and individual plant species effects in ecosystems? J Ecol 86:405–420
Wedin DA, Tilman D (1990) Species effects on nitrogen cycling: a test with perennial grasses. Oecologia 84:433–441
Wedin DA, Pastor J (1993) Nitrogen mineralization dynamics in grass monocultures. Oecologia 96:186–192
Weiher E, van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O (1999) Challenging Theophrastus: a common core list of plant traits for functional ecology. J Veg Sci 10:609–620
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–227
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159
Acknowledgments
We thank B. Böhme, S. Eismann, I. Hilke, I. Kuhlmann, B. Lenk and H. Scheffler for technical assistance in the field and the laboratory. We also thank B. Schmid and two anonymous referees whose valuable comments helped to improve the manuscript. This study was supported by the Deutsche Forschungsgemeinschaft (DFG), Forschergruppe, “The role of biodiversity for element cycling and trophic interactions – an experimental approach in a grassland community” (FOR 456), with additional support from the Friedrich Schiller University of Jena and the Max Planck Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernhard Schmid.
Rights and permissions
About this article
Cite this article
Heisse, K., Roscher, C., Schumacher, J. et al. Establishment of grassland species in monocultures: different strategies lead to success. Oecologia 152, 435–447 (2007). https://doi.org/10.1007/s00442-007-0666-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0666-6