Abstract
Global climate models predict that in the next century precipitation in desert regions of the USA will increase, which is anticipated to affect biosphere/atmosphere exchanges of both CO2 and H2O. In a sotol grassland ecosystem in the Chihuahuan Desert at Big Bend National Park, we measured the response of leaf-level fluxes of CO2 and H2O 1 day before and up to 7 days after three supplemental precipitation pulses in the summer (June, July, and August 2004). In addition, the responses of leaf, soil, and ecosystem fluxes of CO2 and H2O to these precipitation pulses were also evaluated in September, 1 month after the final seasonal supplemental watering event. We found that plant carbon fixation responded positively to supplemental precipitation throughout the summer. Both shrubs and grasses in watered plots had increased rates of photosynthesis following pulses in June and July. In September, only grasses in watered plots had higher rates of photosynthesis than plants in the control plots. Soil respiration decreased in supplementally watered plots at the end of the summer. Due to these increased rates of photosynthesis in grasses and decreased rates of daytime soil respiration, watered ecosystems were a sink for carbon in September, assimilating on average 31 mmol CO2 m−2 s−1 ground area day−1. As a result of a 25% increase in summer precipitation, watered plots fixed eightfold more CO2 during a 24-h period than control plots. In June and July, there were greater rates of transpiration for both grasses and shrubs in the watered plots. In September, similar rates of transpiration and soil water evaporation led to no observed treatment differences in ecosystem evapotranspiration, even though grasses transpired significantly more than shrubs. In summary, greater amounts of summer precipitation may lead to short-term increased carbon uptake by this sotol grassland ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changes in the global climate system due to increased levels of CO2 and other greenhouse gases are predicted to significantly impact the Earth’s terrestrial ecosystems. Anthropogenic emissions have been linked to an increase in both air and soil temperatures, thereby affecting patterns of global air circulation and hydrologic cycling, including regional precipitation regimes (Easterling et al. 2000; NAST 2000; Houghton et al. 2001). The most widely accepted general circulation models (GCMs) have predicted a rise in the mean temperature of the Earth’s surface from 1.4 to 5.8°C during this century, which could trigger a subsequent increase in mean global precipitation of up to 7% (Houghton et al. 2001). In particular, both the HadCM2 and HadCM3 GCMs predict a 3°C increase in air temperature by 2100, which is expected to increase summer and winter precipitation by 25–100% in the southwestern United States (Johns et al. 1997; Gordon et al. 2000; Pope et al. 2000). Precipitation events are predicted to become more intense with large precipitation events becoming more frequent (Houghton 2004; Dore 2005; Groisman et al. 2005). Indeed, Groisman et al. (2004) have shown an increase in the magnitude of precipitation in the south-central region of the USA, primarily in the summer when the most intense rainfall occurs.
In arid and semi-arid environments, changes in precipitation may have an even greater impact on ecosystem dynamics than the singular effects of rising [CO2] or temperature (Weltzin et al. 2003) because the spatio-temporal availability of water will have direct impacts on plant recruitment, growth and reproduction, nutrient cycling, and net ecosystem productivity (Noy-Meir 1979; Smith et al. 1997; Knapp et al. 2002; Whitford 2002; Weltzin and McPherson 2003). Therefore, alterations in precipitation regimes could significantly affect the functions of desert plant and soil communities through their effects on overall ecosystem carbon and water balance (Huxman et al. 2004a).
Ecosystem carbon fluxes are linked to precipitation patterns through precipitation amount, infiltration depth, distribution of soil microbes, soil microfauna and plant roots, and differences in the response time of microbes and plants to wetting events (Huxman et al. 2004b; Loik et al. 2004). A conceptual model developed by Huxman et al. (2004b) proposes three activity states for deserts that affect the autotrophic and heterotrophic balance of ecosystem CO2 fluxes: (1) a low activity state of plant and soil CO2 fluxes that reflects the availability of water only in ecosystem reserve pools, (2) a high activity state for soil CO2 efflux that is triggered by small rainfall events (<5 mm), and (3) a high activity state for plant CO2 flux that is triggered by large rainfall events (>5 mm). Long-term measurements of CO2 fluxes in the Chihuahuan Desert support this model, as rainfall increments in a low rainfall season had less of an impact on ecosystem CO2 flux than an increment of the same size in a high rainfall season (Mielnick et al. 2005). Daily ecosystem fluxes of CO2 were near zero during periods of low precipitation when the ecosystem was in a low activity state, while precipitation events generated brief periods of large positive CO2 fluxes due to high activity states for plant and soil fluxes. Other studies in semiarid ecosystems have also suggested that precipitation may drive seasonal variability in ecosystem CO2 fluxes (Emmerich 2003; Hastings et al. 2005) that may ultimately affect the resilience and resistance of the ecosystem's functional response to precipitation (Potts et al. 2006).
Ecosystem fluxes of H2O are determined by rates of soil evaporation and plant transpiration and, like CO2, are tightly coupled to precipitation events. In the Chihuahuan Desert, evapotranspiration over a 6-year period followed an expected seasonal trend of lower rates in the winter months due to low precipitation and evaporative demand, and greater rates in the summer when precipitation and solar forcing were higher (Mielnick et al. 2005). In arid ecosystems where radiation is high and leaf area is small, high soil temperature drives evaporation and reduces infiltration following rain events (Scott et al. 2000; Law et al. 2002). In the Sonoran Desert, evaporative water loss from the soil exhibited a faster peak response time following a precipitation event than did transpiration (Huxman et al. 2004a). This temporal separation of plant and soil responses occurred because soil water evaporated before reaching the plant rooting zone, thereby precluding transpiration. The delay in plant responses to precipitation were later shown to contribute to a limitation in net ecosystem CO2 uptake (Huxman et al. 2004a).
Although components of ecosystem CO2 and H2O exchanges have been shown to exhibit different response times to precipitation pulses, thus influencing overall flux responses, it is important to partition the relative contribution of each component (i.e., photosynthesis, respiration, transpiration, soil respiration and evaporation) to biosphere/atmosphere CO2 and H2O exchanges. Arid ecosystem processes are not a direct consequence of rainfall events but, rather, are closely linked to the soil water system (Ogle and Reynolds 2004; Reynolds et al. 2004; Schwinning et al. 2004). The relative contribution of soil respiration to CO2 efflux and soil evaporation to soil H2O flux will depend on how soil moisture is affected by the seasonality of rainfall, temperature, storm intensity-duration relationships, frequency of precipitation events, frequency-event size, soil characteristics, soil surface microorganisms, and microclimate (Whitford 2002; Loik et al. 2004; Reynolds et al. 2004). In arid and semiarid regions, small precipitation events that only wet surface soil layers are more common than larger precipitation events that may reach deeper soil layers (Sala and Laurenroth 1982; Dougherty et al. 1996). The amount of water near the soil surface is therefore less reliable than water in deeper soil layers in terms of plant uptake (Noy-Meir 1973; Monson and Smith 1982; Schlesinger et al. 1987), and this can lead to more highly variable responses of soil microbial respiration and soil evaporation to precipitation.
The exchange of whole plant CO2 and H2O in desert plants is influenced by environmental factors and plant activity during short periods of erratic precipitation (Larcher 2003). The degree of change in leaf gas exchange rates following a rain event will depend on the length of the interpulse period (i.e., dry days between wet days) as well as the magnitude of the precipitation pulse received (Yan et al. 2000; Schwinning et al. 2002). For example, as interpulse length increases and soil water becomes increasingly scarce, stomata may remain closed for longer periods of time, thereby increasing physical, enzymatic, and hormonal constraints on photosynthesis (Kaiser 1987; Mansfield et al. 1990; Lambers et al. 1998). Photosynthetic responses to precipitation are also species-specific. Following a 5-mm precipitation event, Bouteloua gracilis showed an increased leaf water potential and leaf conductance in less than 12 h, which lasted for up to 2 days (Sala and Lauenroth 1982), whereas a pulse representing a 25% increase in summer precipitation had no effect on gas exchange for seedlings of the Great Basin Desert shrub Purshia tridentata (Gillespie and Loik 2004). The increase in plant respiration rates immediately following a rainfall event can also significantly contribute to overall ecosystem CO2 flux (Huxman et al. 2004a). In subalpine coniferous forests, the temperature response of ecosystem respiration – not photosynthesis – controlled overall carbon dynamics throughout the growing season (Huxman et al. 2003). Ecosystem carbon flux may also change after a water pulse due to respiration from plant roots. For Abies balsamea, root respiration was found to be more sensitive than microbial respiration to spring water stress (Lavigne et al. 2004).
Increases in leaf-level transpiration may also result from increased soil moisture (Gillespie and Loik 2004). Following a water pulse application in the Sonoran Desert, both native and invasive grasses exhibited rates of stomatal conductance approximately threefold higher than pre-pulse values, demonstrating that increased plant transpiration significantly affected overall ecosystem H2O balance (Huxman et al. 2004a). For a managed olive plantation, transpiration accounted for 100% of the total ecosystem H2O flux prior to a 100-mm irrigation event, but for only 69–86% of evapotranspiration during peak midday fluxes over the 5-day period following the irrigation event (Williams et al. 2004).
The goals of this study were to determine if changes in seasonal precipitation affect ecosystem fluxes of CO2 and H2O in a Chihuahuan Desert mid-elevation sotol grassland and to determine how plant and soil flux components contribute to ecosystem responses. While previous studies have quantified ecosystem responses to elevated CO2 in the Mojave Desert (Jasoni et al. 2005), to increased precipitation with respect to invasive species in the Sonoran Desert (Huxman et al. 2004a), and to natural conditions in a native community in the Great Basin Desert (Arnone and Obrist 2003), there are few published studies of the responses of ecosystem fluxes to global change factors and, in particular, to precipitation, in a Chihuahuan Desert ecosystem (but see Mielnick et al. 2005).
Materials and methods
Study site
Our study site is located in a sotol grassland ecosystem within the Pine Canyon Watershed in Big Bend National Park (29°5′N, 103°10′W, 1526 m a.s.l.) in the Chihuahuan Desert (Hermann et al. 2000). The site is dominated by woody perennials (e.g., Dasylirion leiophyllum, Nolina texana), annual native grasses (e.g., Bouteloua curtipendula, Aristida purpurea), and succulents (e.g., Opuntia phaeacantha, Echinocereus chloranthus). The soil is an unconsolidated rocky loam with no horizon development. Average gravimetric soil moisture is 1% in the spring and 17% in the late summer. Average soil bulk density for the site is 1.46 g cm−3 (Ziehr 1997). The site receives 370 mm of precipitation annually, but this varied from 148 to 578 mm for the period 1976–2004 (National Park Service, USA). Annual precipitation in both 2003 and 2004 was above average due to large amounts of precipitation in the summer and fall (Fig. 1a). About 45% of the annual rainfall occurs during the summer months (June, July, and August) and arrives in the form of monsoonal rains (MacMahon 1997). Winter precipitation (December, January, and February) accounts for 11% of the annual rainfall. From 1986 to 2004, 43% of all rainfall events were ≤2 mm; a larger number of both small and large precipitation events occurred in 2004 than in previous years, and annual precipitation was overall higher in 2004 than average (Fig. 1b). In 2004, the average interpulse length was 16 days in the winter and 3 days in the summer. From 1986 to 2004, maximum average daily summer air temperatures ranged from 32 to 36°C, while minimum average daily summer air temperatures ranged from 18 to 22°C. Maximum average daily winter temperatures over the same time period ranged from 14 to 20°C, and minimum average daily temperatures ranged from 1 to 6°C.
Precipitation manipulation
Since January 2002, the following water treatments have been seasonally applied to 3 × 3-m plots (three plots per treatment) in order to simulate a HadCM2 scenario of a 25% increase in ambient precipitation in the area: (1) no water addition (C = control), (2) summer addition (in June, July, and August; S = summer addition), (3) winter addition (in February; W = winter addition), and (4) both summer and winter addition (SW = summer and winter addition). The amount of water added for each simulated storm was determined based on the natural amount of rainfall received in the C plots prior to the water addition treatment. In the summer of 2004, we added a total of 48 mm of precipitation in three distinct precipitation pulses (June: 3 mm, July: 18 mm, August: 27 mm) to the S and SW plots. Water was added manually and evenly within the plots over both plants and soil so that the rate of application was similar to the rate of infiltration into the soil. Measurements of plant and soil responses (described below) were made in C plots (hereafter referred to as control plots) and S plots (hereafter referred to as watered plots) at the following times during 2004: (1) in June, the day before and for 7 consecutive days following the watering event; (2) in July, 1 day before the watering event and for 4 consecutive days following the watering event; (3) in August, 1 day before the watering event and every other day for 6 days following the event; (4) in September, for 1 day, approximately 1 month after the last supplemental summer watering event.
To determine if supplemental water pulses were effective in increasing soil water, volumetric soil moisture content was measured throughout the experimental period using one ECH2O-10 dielectric aquameter probe (Decagon Devices, Pullman, Wash.) placed horizontally in each plot at a soil depth of 15 cm. Measurements were logged every 2 h on Em5 dataloggers and then averaged for the 24-h period to determine mean volumetric water content. To determine if plant available water increased with watering treatment, plant water potential (Ψ) was measured in June, July, and September (but not August due to technical difficulties) using a Scholander-type pressure chamber (3000 Series; Soilmoisture Equipment, Santa Barbara, Calif.). These same individual plants were used for photosynthetic gas exchange measurements. Measurements of Ψ were made pre-dawn (Ψpre) in June and July and both pre-dawn and midday (Ψmid) in September. Leaves were initially sampled using a scissors and then re-cut immediately prior to insertion into the pressure chamber. The order of plant sampling alternated between treatments to avoid time-of-day bias.
Ecosystem CO2 and H2O fluxes
We measured the time course of whole-plot CO2 and H2O exchange with an open path infrared gas analyzer (IRGA; model LI-7500, LI-COR, Lincoln, Neb.) located inside a static, closed gas exchange system, as described by Huxman et al. (2004a). Briefly, this system consists of a static chamber (1.5 m wide × 1.8 m long × 1.8 m tall) constructed of a PVC pipe frame covered by a clear polyethylene film. Similar systems have been successfully used previously to measure whole ecosystem CO2 and H2O fluxes in arid environments (Arnone and Obrist 2003; Jasoni et al. 2005). During each ecosystem flux measurement, the IRGA was interfaced with a laptop computer and placed in the plot adjacent to a tripod mounted with two 15-cm-diameter fans to maximize chamber mixing. The chamber was then placed on top of the 3 × 3-m rainfall-treatment or control plot in a designated location to encompass vegetation representative of the whole plot. Data collection began approximately 30 s after placement of the chamber to allow for adequate mixing within the chamber, and whole-plot CO2 and H2O fluxes were recorded for 90 s (thermocouples showed no appreciable warming inside the chamber). A 24-h time course of ecosystem flux measurements was conducted from 1530 hours on September 14, 2004 to 1400 hours on September 15, 2004.
In order to calculate CO2 or H2O fluxes in the chamber, all measurements during the 90-s measurement period were plotted over time, and the slope (mg m−3 s−1) was computed. The following equation was then used to convert the change in concentration (mg m−3 s−1) measured by the LI-7500 gas analyzer to a flux (μmol m−2 ground area s−1 flux):
where the chamber volume was 4.86 m3, the soil surface area covered by the chamber was 2.7 m2, and the molecular weights of CO2 and H2O are 44 and 18 g mol−1, respectively. Daytime net ecosystem exchange (NEEday) of CO2 and evapotranspiration (ETday) were measured eight times from dawn to dusk (0730, 0900, 1030, 1200, 1400, 1530, 1700 and 2000 hours). Nighttime net ecosystem exchange of CO2 (NEEnight) and evapotranspiration (ETnight) were measured twice from dusk to dawn (1930 and 0730 hours). Measurements of NEEnight and ETnight at 0430 hours were estimated by averaging data measured at 1230 and 0730 hours. Ecosystem CO2 and H2O flux measurements throughout the day and night were used to estimate integrated total daily fluxes of CO2 (NEEtotal) and H2O (ETtotal) for each plot. To do this, the integrating function in SigmaPlot ver. 8.02 (SPSS, Chicago, Ill.) was used to evaluate the area under a spline-fit curve for each flux during a 24-h period. We define ecosystem carbon gain as the difference in CO2 uptake by the plot and release from the soil (i.e., photosynthesis > respiration), while ecosystem carbon loss indicates CO2 released from the terrestrial components to the atmosphere (i.e., respiration > photosynthesis).
Plant CO2 and H2O fluxes
June, July, and August 2004
We measured leaf photosynthetic gas exchange [net assimilation rate (A), transpiration during the day (E day), and stomatal conductance (g s)] with a portable open-flow gas exchange system (model LI−6400, LI-COR) on newly mature leaves on one plant per functional type per plot. Plants were chosen to represent two functional types: grasses (B. curtipendula) and shrubs (D. leiophyllum). The same leaves on each plant were repeatedly measured throughout the experimental period. Measurements were taken in the morning when gas exchange was at its maximum rate (0700–0930 hours, based on preliminary measurements). Leaf-to-air vapor pressure deficit (VPD), air temperature, and CO2 concentration (370 μmol mol−1) of the cuvette were set to ambient environmental values for each measurement period and maintained constant for all measurements across plots. Irradiance (Q) was set to saturating light conditions (2000 μmol m−2 s−1). Data were logged five times for each leaf and then averaged for each plant to be used as a statistical unit.
September 2004
We measured leaf photosynthetic gas exchange (A), net respiration rate (R p ), transpiration during day and night (E day, E night), and g s with the portable open-flow gas exchange system on recently mature leaves on three dominant plants per plot. Measurements were taken immediately following ecosystem flux measurements over the course of 24 h. Gas exchange methods were identical to those stated above for June, July, and August. Plants were chosen to represent two functional types: grasses (Bouteloua curtipendula, B. hirsuta) and shrubs (Dasylirion leiophyllum, Nolina texana, Artemisia ludoviciana, and Gutierrezia microcephala). For leaf areas that could not be accurately measured in the field (A. ludoviciana, G. microcephala), leaves were harvested at the end of the experiment. To determine specific leaf area, leaves were pressed flat, colored black, and scanned using a CI-202 Area Meter (CID, Camas, Wash.). Gas exchange values were corrected for leaf area using the recomputation function in LI6400SIM (LI-COR). In order to make sure that plant leaf area of all plots was similar in September for flux measurements, leaf area index (LAI) and canopy areas were calculated for each plot. LAI was calculated with a portable PAR/LAI ceptometer (AccuPar LP-80, Decagon Devices). LAI was measured at the center of each plot and perpendicular to each edge and then averaged for a plot mean. Canopy areas were determined using digital photographs taken 2 m above the canopy in September. Digital images were processed with PAX-It imaging software (MIS, Franklin Park, Ill.) using color recognition schemes to distinguish between shrubs (represented by D. leiophyllum plus other minor species), grasses (B. curtipedula and B. hirsuta), and bare soil.
Soil CO2 fluxes
June and August 2004
Measurements of daytime soil CO2 efflux (R sday) were made with an EGM-4 Environmental Gas Monitor connected to a USASRC-1 Soil Respiration Chamber (PP Systems, Amesbury, Mass.). Within this closed system, the CO2 concentration was measured every 8 s for a period of 1 min, and a quadratic equation was fitted to the relationship between the increasing CO2 concentration and elapsed time to calculate a rate of soil CO2 efflux. In June, measurements were made 1 day before watering and three consecutive days after watering. In August, measurements were made 1 day before watering and 2 consecutive days after watering. All measurements were made between 0900 and 1100 hours.
September 2004
Measurements of R sday were made in September with a closed loop static chamber system immediately following ecosystem measurements throughout the daytime period. A 3_L PVC lid was fitted tightly upon soil collars 10.2 cm in diameter. The chamber formed a closed loop system with a LI-820 IRGA (LI-COR), where air was drawn with a pump (0.8 L/min flow rate) from the chamber through the LI-820 and then returned to the chamber. Measurements were logged every second for 2 min on a laptop computer interfaced with the IRGA. Soil CO2 flux rates were corrected for chamber volume, collar area, and chamber air temperature. Measurements of chamber air temperature and soil temperature were also logged at two depths (2 and 10 cm; DiGi-Sense, Eutech Instruments, Vernon Hills, Ill.). Soil temperature was also recorded every 36 min using HOBO temperature probes (Onset Computer, Pocasset, Mass.) which were permanently in place at a soil depth of 15 cm in each plot. In order to determine the rate of nighttime CO2 flux (R snight), soil CO2 flux was calculated with temperature data using the Fang and Moncrieff (2001) equation with a Q 10 of 1.15.
Statistical analyses
Repeated measures ANOVA was used to test the significance of treatment, time of day (or day), and their interactions for the extent of the experiment, using instantaneous measurements of NEEday, NEEnight, ETday, ETnight, R sday, and R snight from September and A, E, and R sday from June, July and August as response variables (SPSS ver. 11.5; SPSS). Repeated measures ANOVA was also used to test for differences in A, R p , A total, g s, E day, E night, and E total from September with functional type included in the model. Average soil moisture values in June, July, August, and September were compared with a paired t-test. Integrated NEE values in September were evaluated using an independent t-test. Leaf water potential differences in June, July, and September were tested using a one-way ANOVA or independent t-test. Correlations of A/g s and NEE/ET were compared using an analysis of variance approach to regression (Neter et al. 1985). While an alpha-level of 0.05 was primarily used for statistical tests, a statistical significance level of 0.10 was also adopted to accommodate for the high variability in the ecosystem.
Results
Soil moisture and plant water status
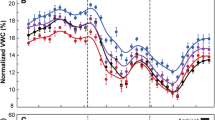
Prior to the measurement period in September 2004, watered plots received three supplemental water pulses which contributed to increased average soil moisture in the watered plots compared to control plots during June, July, and August (t = 1.66, P < 0.001; Fig. 2). The impact of supplemental summer precipitation pulses on soil moisture was dependent on environmental factors such as pulse magnitude and the amount and timing of natural rainfall (Fig. 2). In June, soil moisture in the watered plots did not significantly increase relative to control plots after supplemental watering due to the small pulse size and high amount of natural rainfall. In July, natural precipitation received before the supplemental water addition increased soil moisture in the plots such that the supplemental pulse, while small, penetrated to a soil depth of approximately 15 cm. One day after supplemental watering in July, average soil moisture in watered plots increased by 8%, while average soil moisture in control plots decreased by 5%. Additionally, average soil moisture remained significantly greater in watered plots than in control plots for approximately 3 weeks due to a larger experimental pulse size and a lower amount of recent natural precipitation (t = 1.68, P < 0.001; Fig. 2). While the final supplemental water pulse in August was the largest, soil moisture did not significantly increase in watered plots after the event, and the soil moisture of these was similar to that of the control plots due to multiple large (>10 mm) natural rainfall events in July and high evaporative demand. For 16 days before measurements in September 2004, there was less than 1 mm of natural precipitation received at the site. Since watered plots had access to more water throughout the summer, average soil water content was greater in watered plots than control plots during the week of our measurement period in September (t = 1.72, P < 0.001; Fig. 2).
Summer precipitation (vertical bars, mm) received in Big Bend National Park from June to September 2004. Supplemental pulse events, representing a 25% increase over prior ambient precipitation events, are indicated by arrows. The September measurement period is indicated by the asterisk. Soil moisture (volumetric water content, %) is shown for supplementally summer-watered (dashed line) and control plots (solid line) throughout the same time period
The difference in soil moisture between control and watered plots during the summer was reflected in the xylem water potential responses by the plants. In June, after the small watering event, there were no significant treatment differences in Ψpre (−2.1 ± 0.1 MPa; mean ± SEM for all plants combined). In July, however, plants in watered plots had significantly greater Ψpre (−1.3 ± 0.1 MPa) than control plots (−1.6 ± 0.1 MPa) the day after watering, (ANOVA, F 1,22 = 2.47, P = 0.1). In September, neither Ψpre nor Ψmid were significantly different for shrubs and grasses by treatment (−2.0 ± 0.1 MPa, for all plants combined); however, the Ψmid of B. curtipendula was significantly higher in watered plots (−2.3 ± 0.3 MPa) than in control plots (−3.0 ± 0.1 MPa; t = 3.55, P = 0.02).
LAI and canopy leaf area
Leaf area index was not significantly different by treatment in September; watered plots had an average LAI of 1.5 ± 0.3 m2 m−2, while control plots averaged 1.8 ± 0.3 m2 m−2. Control and watered plots had similar portions of each plot’s canopy area dominated by shrubs and grasses. For watered plots, there was an average of 70% shrub cover, 14% grass cover, and 16% bare ground. The majority of the control plot area was also dominated by shrubs (68%), with bare ground (23%) and grasses (9%) covering a smaller area. While D. leiophyllum and B. curtipendula represented the species of shrubs and grasses with the greatest percentage cover (42 and 2%, respectively), other species were found to be abundant in individual plots (e.g., O. phaeacantha, Gymnosperma glutinosum, N. texana; 5–17% cover each).
Ecosystem CO2 and H2O fluxes
In September, NEEday was significantly greater in watered plots than control plots (Table 1; Fig. 3a). Whereas control plots were a greater CO2 sink than watered plots early in the day, NEEday quickly decreased after 0900 hours in control plots and remained close to zero until late afternoon. This response is similar to that expected from leaf photosynthetic responses. In watered plots, NEEday increased (plots became a greater CO2 sink) from the early morning to the late afternoon. Even though there were differences in NEEday by treatment, there were no differences between treatments for nighttime ecosystem CO2 fluxes. Overall, when ecosystem CO2 fluxes were integrated over the 24-h period, watered plots had significantly greater NEEtotal than control plots (t = −2.26, P = 0.08; Table 2). Notably, values for NEEnight were similar by treatment, yet differences in NEEday led control plots to be a source of CO2 to the atmosphere, and watered plots were a sink for CO2.
Average ecosystem fluxes of: a CO2 during the day (NEEday; μmol CO2 m−2 s−1 ground area) and b H2O during the day (ETday; mmol H2O m−2 s−1 ground area) in September 2004. Positive values indicate a net flux to the atmosphere, filled symbols control plots, open symbols watered plots. Data are means ± standard error for n = 3 plots
Ecosystem fluxes of H2O throughout the day and night were similar for control and watered plots in September (Table 1, Fig. 3b). ETday varied significantly over time in all plots, as rates were low in the early morning and then increased until mid-afternoon. In September, higher rates of ETday were correlated with increased ecosystem CO2 assimilation (Fig. 4; P = 0.01).
The relationship between CO2 flux during the day (NEEday; μmol CO2 m−2 s−1 ground area) and H2O during the day (ETday; mmol H2O m−2 s−1 ground area). The linear regression equation for control plots (dashed line) is y = −2.4x + 0.81, with r 2 = 0.32; for watered plots (solid line), it is y = −3.6x + 1.1, with r 2 = 0.49
Plant CO2 fluxes
After the first summer watering event in June (Fig. 5a), A was greater in both shrubs (Fig. 5b) and grasses (Fig. 5c) in watered plots than in control plots (Table 3). In D. leiophyllum (dominant shrub), A was fivefold greater in watered plots than in control plots 3 days after watering (Fig. 5b). In B. curtipendula (dominant grass), A was approximately 50% greater in watered plots than control plots 7 days after the watering event (Fig. 5c). In July, A in watered plots was greater 1 day after watering in shrubs (Fig. 5b) and 3 days after watering in grasses (Fig. 5c). In August, after the largest supplemental watering event of the summer season (Fig. 5a), there was no significant treatment difference in A for shrubs (Fig. 5b).
a Total precipitation (mm) received at the study site in Big Bend National Park during June, July, and August 2004. Supplemental events, representing a 25% increase in summer precipitation, are indicated by filled bars. Also shown are rates of leaf-level photosynthesis (A; μmol CO2 m−2 s−1 leaf area) for Dasylirion leiophyllum (b) and Bouteloua curtipendula (c) as well as rates of transpiration (E; mmol H2O m−2 s−1 leaf area) for D. leiophyllum (d) and B. curtipendula (e) in supplemental summer watered (W) and control (C) plots during the same time period. Values are expressed as a ratio of W to C, such that values above the line at 1 indicate greater fluxes in W compared to C plots. Ratios are presented for 1 day prior and up to 7 days after supplemental watering
In September, plant fluxes of CO2 during the day were not significantly different by watering treatment, plant functional type, or their interactions (Table 1; Fig. 6a, b). Overall, grasses in watered plots trended towards greater A than grasses in control plots throughout the day, with a short decrease at mid-day (Fig. 6a). For shrubs in both treatments, A exhibited similar increases and decreases throughout the day (Fig. 6b). R p was not significantly different for time, treatment, functional type, or their interactions (Table 1).
Daily values of photosynthesis (A; μmol CO2 m−2 s−1 leaf area) in B. curtipendula (a) and D. leiophyllum (b), and rates of transpiration (E; mmol H2O m−2 s−1 leaf area) in B. curtipendula (c) and D. leiophyllum (d) in supplemental summer watered (W) and control (C) plots in September 2004. Data are means ± standard error for n = 3 plots
Plant H2O fluxes
In June, fluxes of H2O were significantly greater in watered plot grasses than control plot grasses throughout the 7 day measurement period (Table 3, Fig. 5d). For shrubs in June, E increased in watered plots 2 days after watering, but then returned to control plot values after natural rainfall on day six (Fig. 5e). In July, E was significantly greater in grasses in watered plots than control plots, with rates increasing almost twofold in watered plots 3 days after watering (Fig. 5d). E was greater in shrubs in watered plots the day after watering, but this effect was lost after all plots received a large amount of natural rainfall on day two (Fig. 5e). In August, there was no significant effect of water treatment on E by shrubs (Fig. 5e). There was a strong relationship between A and g s in June, July, and August 2004 (Fig. 7a). During this time, g s explained 60% of the variation in A for B. curtipendula and D. leiophyllum.
a The relationship between leaf-level photosynthesis (A; μmol CO2 m−2 s−1) and stomatal conductance (g s; mmol CO2 m−2 s−1) in D. leiophyllum and B. curtipendula in supplemental summer watered (W) and control (C) plots in June, July, and August 2004. There was a positive relationship between A and g s in B. curtipendula (r 2 = 0.52, P = 0.01) and D. leiophyllum (r 2 = 0.81, P = 0.01) with C and W plots combined. b The relationship between A and g s in B. curtipendula and D. leiophyllum in September 2004. There was no difference in the relationship between A and g s between treatments and/or plant functional types (r 2 = 0.08, P = 0.9)
In September, E day was not significantly different by treatment, but it was significantly different by plant functional type (repeated measures ANOVA F 1,14 = 4.12, P = 0.06; Fig. 6c, d). Grasses in the watered plots tended to release more H2O to the atmosphere than those in the control plots, while shrubs in both control and watered plots showed similar daily patterns of H2O loss. At night, E night was significantly higher for grasses than shrubs in the watered plots (repeated measures ANOVA F 1,14 = 4.02, P = 0.06). In September, g s only explained 8% of the variation in A (Fig. 7b).
Soil CO2 fluxes
In June, there was no significant difference in R s by treatment or plant functional type 1 day after the watering event (Table 3). Soil temperature was greater in control plots (25.6 ± 0.3°C) than watered plots (24.8 ± 0.4°C, Table 3), which may be due to evaporative cooling. In August, watered plots had significantly greater rates of R s (4.5 ± 0.1 μmol CO2 m−2 s−1) than control plots (3.4 ± 0.1 μmol CO2 m−2 s−1; Table 3). Soil temperature was similar in watered plots and control plots up to 2 days after watering (23.8 ± 0.2°C for all plots; Table 3) but was greater under shrubs (24.5 ± 0.4°C) than grasses (23.2 ± 0.2°C, P = 0.01). In September, R sday was not significantly different between plots throughout the day (1.6 ± 0.5 μmol CO2 m−1 s−1 for all plots; Fig. 8). R snight and soil temperature (25.7 ± 0.2°C) were not significantly different by treatment (Table 1).
Discussion
CO2 fluxes: ecosystem, plant, and soil
In September, control plots were a net ecosystem CO2 source, releasing an average of 3.6 mmol CO2 m−2 day−1 to the atmosphere, while watered plots were a net ecosystem sink of CO2, assimilating on average 31.3 mmol CO2 m−2 day−1. Other studies have also shown arid ecosystems to be sinks of carbon, especially following summer rain events, with interesting differences in time scale (Meyers 2001). In the Jornada Basin during a wet year, large amounts of CO2 were assimilated in association with precipitation during the growing season, with the assimilation period lasting for 70 days (Mielnick et al. 2005). In the Sonoran Desert, both invasive and native grasslands accumulated CO2 for up to 4 days following a large water pulse applied in June (Huxman et al. 2004a). In the Mojave Desert, the highest observed daytime and mean daily ecosystem CO2 uptake occurred during periods of high soil moisture in late October (Jasoni et al. 2005). Interestingly, Big Bend National Park control and watered plots differed in their directions of CO2 fluxes. Ham and Knapp (1998) observed mid-September to be the time of year when the grassland ecosystem at Konza Prairie made a seasonal transition from a CO2 sink to a CO2 source. It is possible that increased summer precipitation in watered plots in the sotol grassland delayed their seasonal transition to a CO2 source by enabling continued CO2 uptake by the ecosystem.
The increase in ecosystem CO2 assimilation in watered plots was due in part to an increased CO2 uptake by grasses during the day. Grasses, and not shrubs, exhibited a positive photosynthetic response to the 25% increase in summer precipitation 1 month after the last watering event. This suggests that grasses in watered plots still had access to water in September. Other studies have shown that precipitation events increase photosynthesis over time in grasses, with the results relating to pulse timing and magnitude. A small precipitation event (5 mm) increased leaf water potential and stomatal conductance in B. gracilis in less than 12 h, and this increase lasted for up to 2 days (Sala and Lauenroth 1982). After a summer precipitation pulse in the Sonoran Desert, A and g s increased in grasses for up to 7 days following the event (Huxman et al. 2004a). In the cold desert on the Colorado Plateau, Schwinning et al. (2002) showed that summer rain increased the rate of photosynthesis almost fourfold in the C4 grass, Hilaria jamesii. Data from our study in July showed increased photosynthesis in grasses for up to 7 days after the watering event. In September, watered plots had greater average soil moisture than control plots, and increased access to water by grasses was supported by a higher Ψmid in September in B. curtipendula in watered plots compared to control plots. Since grasses, such as B. curtipendula, have shallow root systems concentrated in the top 10 cm of soil, it is possible that higher water status might have been obtained through the hydraulic redistribution of water by neighboring shrubs (Hultine et al. 2004) or maintained over time through a high cavitation resistance of roots (Sperry and Hacke 2002).
While differences in CO2 fluxes were found for grasses 1 month after supplemental watering, there was no effect of the water treatment on CO2 assimilation in shrubs. Since desert shrubs tend to utilize deeper soil water in the summer (Schwinning et al. 2002), the question that arose was why would a treatment difference be observed in grasses and not shrubs? Immediately following pulse events in June and July, shrubs in watered plots did increase their uptake of CO2. During this time, photosynthetic responses of shrubs were dependent on rainfall magnitude; increased A was observed for 5 days after the small pulse in June and 1 day after the large pulse in July. The temporal difference in the response of A after watering in shrubs was a function of antecedent soil moisture conditions and the delay in the ability of roots to access water. As illustrated in the ‘threshold-delay’ model, plant responses to precipitation will differ across functional types based on soil moisture conditions, the timing and magnitude of the pulse, plant rooting depth, and plant phenology (Ogle and Reynolds 2004). In August, an increase in A in watered plots was not observed due to high natural precipitation. During periods of high natural precipitation in June, July, and August, there was a strong relationship between A and g s for shrubs, with g s explaining 81% of the variation in A.
In September, shrubs in watered and control plots had similar leaf water potentials and exhibited similar rates of CO2 fluxes. In pulse-driven arid ecosystems, the ability of shrubs to maximize access to deep soil water is essential to co-existence in the community (Donovan and Ehleringer 1994; Ehleringer et al. 2001). Studies in semiarid grasslands, however, have revealed that summer rains do not usually recharge soil layers >10 cm because soil water is quickly lost to evaporation before it can infiltrate deeply (Kurc and Small 2004). Because measurements in September were made during a long interpulse period, soil moisture was low and air temperatures were high enough to promote surface soil water evaporation. In Big Bend National Park, D. leiophyllum has fibrous roots in the shallow soil layer; however, the roots of most plants extend >10 cm, with some even reaching 1 m in depth (J. Sirotnak, personal communication). Over the course of the summer, evaporation made access to soil moisture by deep root systems, such as those of shrubs, more difficult. Additionally, the soil at the study site is extremely rocky, which increases infiltration, but precludes deep water storage.
Increased daily CO2 uptake by the ecosystem in September in watered plots could also be attributed to decreased CO2 efflux from the soil. Rates of carbon cycling may be slowed by the increased variability in soil water content (Harper et al. 2005). Soil CO2 efflux generally increases substantially after a precipitation event. Potential mechanisms include: the physical displacement of CO2 (Lee et al. 2004), the breakdown of inorganic carbonates (Emmerich 2003), and the activity of microbes (Austin et al. 2004). In the late summer, our data supported the latter, with rates of soil CO2 efflux increasing for up to 3 days after watering in August. This has been found in other studies, where Huxman et al. (2004a) observed that after a large summer precipitation event in the Sonoran Desert, soil CO2 efflux was substantial enough to influence ecosystem CO2 flux. Chimner and Welker (2005) showed that ecosystem respiration rates (primarily from the soil) were driven by precipitation, especially during the summer. If we assume that our ecosystem experienced increased soil CO2 efflux after each precipitation event, we would expect high rates of soil CO2 efflux throughout the rainy summer months. However, once the ecosystem entered an interpulse period and soil moisture decreased due to evaporation and plant water uptake, rates of soil CO2 efflux declined, as observed in September. Similarly, in a Kansas grassland, Ham and Knapp (1998) observed an autumnal decline in soil-surface CO2 flux which they attributed to the combined effect of decreased root and microbial respiration resulting from reduced available substrate (e.g., soil organic matter) and lower seasonal soil temperatures.
In general, our data support many aspects of the Huxman et al. (2004b) conceptual model describing the impact of precipitation on soil, plant, and ecosystem CO2 fluxes. For example, in June, the supplemental pulse size was small, but it occurred at a time of low natural precipitation, and thus generated increases in plant photosynthesis in watered plots. The ecosystem remained in a high activity state through July, where a larger supplemental pulse generated greater rates of photosynthesis in watered grasses and shrubs. In August, the ecosystem received large amounts of natural precipitation, which led to no treatment differences in plant CO2 fluxes. The supplemental pulse was large enough, however, to generate a high activity state through increased soil CO2 efflux in watered plots. While the immediate effects of water on soil CO2 efflux were lost by September, increased photosynthesis in grasses indicated that water in ecosystem reserve pools allowed these plots to maintain a higher activity state than control plots. Consequently, an increase in the autotrophic component of ecosystem CO2 exchange drove supplementally watered plots to an increase in terms of whole ecosystem CO2 uptake in September.
H2O fluxes: ecosystem, plant, and soil
The responses of ecosystem H2O fluxes were similar in both watered and control plots in September, with an average flux of 45.1 and 39.7 mmol H2O m−2 day−1, respectively. In desert ecosystems, rates of ET are greatest in the summer, when temperature, VPD, and ambient rainfall are often at their maximum. In an Oklahoma grassland, ET during the summer comprised roughly 50% of annual water loss (Meyers 2001). While there was significant variation in daytime and nighttime fluxes of H2O in the Jornada Basin, average ET during non-drought years was 253 ± 12 mm H2O (Meyers 2001). The lack of a treatment response for ET in September is not surprising since supplemental water is quickly lost to evaporation or utilized by plants and microbes. In a semiarid grassland in New Mexico, decreases in ecosystem water fluxes were observed for no more than 3 days following a rainfall event (Kurc and Small 2004). Following supplemental precipitation treatments in June, July, and August, E for both grasses and shrubs in the sotol grassland increased over the first 24 h. In September, the only residual effect of increased summer precipitation was greater E in grasses in watered plots, which corresponded to an increase in A. Greater average soil moisture in watered plots in mid-September could have led to increased transpiration in these grasses. Partitioning of supplemental water towards storage, rather than metabolism, might have led to the lack of treatment response in transpiration of shrubs. In June, July, August, and September, soil moisture patterns closely followed trends in natural precipitation. For most of the summer, average soil moisture remained greater in watered plots than control plots, and this difference was especially pronounced during periods of low natural rainfall. While soil water evaporation was not measured in September, we would not expect to see any significant treatment differences in this component of the ecosystem water balance at that time due to the low water content of the soil (4–5%) and utilization of available soil water by plants.
Conclusions
Changes in the global climate system due to increased levels of greenhouse gases are predicted to significantly impact the timing and magnitude of precipitation received by arid and semi-arid ecosystems. A thorough understanding of the environmental controls of ecosystem fluxes is essential to anticipating how global change will affect natural ecosystems (Law et al. 2002). We found that a 25% increase in precipitation during an above-average wet summer increased soil moisture, which was sufficient to generate CO2 release by soils and CO2 uptake by shrubs over the short term (1 week) and CO2 uptake in grasses over a longer term (up to 3 weeks). From June to August 2004, supplementally watered ecosystems transitioned from a low activity state of plant and soil CO2 fluxes to a high activity state for soil CO2 efflux and plant CO2 fluxes. Ecosystems maintained a high activity state for plant CO2 fluxes in September, which subsequently impacted whole ecosystem CO2 fluxes. One month after the final summer supplemental watering pulse, watered plots were greater CO2 sinks than control plots. Overall, plant and soil responses to increased precipitation throughout the early summer did not affect ecosystem H2O fluxes in September. Because GCM precipitation scenarios include a >25% increase for our arid study area, we predict the sotol grassland ecosystem in the Chihuahuan Desert may respond to increased summer precipitation by increasing CO2 fixation in late summer.
References
Arnone JA III, Obrist D (2003) A large daylight geodesic dome for quantification of whole-ecosystem CO2 and water vapor fluxes in arid shrublands. J Arid Environ 55:629–643
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–235
Chimner RA, Welker JM (2005) Ecosystem respiration responses to experimental manipulations of winter and summer precipitation in a mixed grass prairie, WY, USA. Biogeochemistry 73:257–270
Donovan LA, Ehleringer JR (1994) Contrasting water-use patterns among size and life-history classes of a semi-arid shrub. Funct Ecol 6:482–488
Dore MHI (2005) Climate change and changes in global precipitation patterns: what do we know? Environ Int 31:1167–1181
Dougherty RL, Lauenroth WK, Singh JS (1996) Response of a grassland cactus to frequency and size of rainfall events in a North American shortgrass steppe. J Ecol 84:177–183
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Means LO (2000) Climate extremes: observations, modeling, and impacts. Science 289:2068–2074
Ehleringer JR, Phillips SL, Schuster WSF, Sandquist DR (2001) Differential utilization of summer rains by desert plants. Oecologia 88:430–434
Emmerich WE (2003) Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agric For Meteorol 116:91–102
Fang C, Moncrieff JB (2001) The dependence of soil CO2 efflux on temperature. Soil Biol Biochem 33:155–165
Gillespie IG, Loik ME (2004) Pulse events in Great Basin Desert shrublands: physiological responses of Artemisia tridentata and Purshia tridentata seedlings to increased summer precipitation. J Arid Environ 59:41–57
Gordon C, Cooper C, Senior CA, Banks H, Gregory JM, Johns TC, Mitchell JFB, Wood RA (2000) The simulation of SST, sea ice extents and ocean heat transports in a version of the Hadley Centre coupled model without flux adjustments. Clim Dynam 16:147–168
Groisman PY, Knight RW, Karl TR, Eastering DR, Sun B, Lawrimore JM (2004) Contemporary changes of the hydrological cycle over the contiguous United States: trends derived from in situ observations. J Hydrometeorol 5:64–85
Groisman PY, Knight RW, Easterling DR, Karl TR, Hegerl GC, Razuvaev VN (2005) Trends in intense precipitation in the climate record. J Clim 18:1326–1350
Ham JM, Knapp AK (1998) Fluxes of CO2, water vapor, and energy for a prairie ecosystem during the seasonal transition from carbon sink to carbon source. Agric For Meteorol 89:1–14
Harper CW, Blair JM, Fay PA, Knapp AK, Carlisle JD (2005) Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Global Change Biol 11:322–334
Hastings SJ, Oechel WC, Muhlia-Melo A (2005) Diurnal, seasonal and annual variation in the net ecosystem CO2 exchange of a desert shrub community (Sarcocaulescent) in Baja California, Mexico. Global Change Biol 11:927–939
Hermann R, Stottlemyer R, Zak JC, Edmonds RL, Van Miegroet H (2000) Biogeochemical effects of global climate change on U.S. national parks. Am Water Res Assoc 36:337–346
Houghton JT (2004) Global warming: the complete briefing. Cambridge University Press, Cambridge
Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA (2001) Climate change 2001: the scientific basis. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Hultine KR, Scott RL, Cable WL, Goodrich DC, Williams DG (2004) Hydraulic redistribution by a dominant, warm-desert phreatophyte: seasonal patterns and response to precipitation pulses. Funct Ecol 18:530–538
Huxman TE, Turnipseed AA, Sparks JP, Harley PC, Monson RK (2003) Temperature as a control over ecosystem CO2 fluxes in a high-elevation subalpine forest. Oecologia 134:537–546
Huxman TE, Cable JM, Ignace DD, Eits JA, English NB, Weltzin J, Williams DG (2004a) Response of net ecosystem gas exchange to a simulated precipitation pulse in a semiarid grassland: the role of native versus non-native grasses and soil texture. Oecologia 141:295–305
Huxman TE, Snyder KA, Tissue DT, Leffler AJ, Ogle K, Pockman WT, Sandquist DR, Potts DL, Schwinning S (2004b) Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 141:254–268
Jasoni RL, Smith SD, Arnone JA (2005) Net ecosystem CO2 exchange in Mojave Desert shrublands during the eighth year of exposure to elevated CO2. Global Change Biol 11:749–756
Johns TC, Carnell RE, Crossley JF, Gregory JM, Mitchell JFB, Senior CA, Tett SFB, Wood RA (1997) The second Hadley Centre coupled ocean-atmosphere GCM: model description, spinup and validation. Clim Dynam 13:103–134
Kaiser WM (1987) Effect of water deficits on photosynthetic capacity. Physiol Plant 71:142–149
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, Harper CW, Danner BT, Lett MS, McCarron JK (2002) Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298:2202–2205
Kurc SA, Small EE (2004) Dynamics of evapotranspiration in semiarid grassland and shrubland ecosystems during the summer monsoon season, central New Mexico. Water Resour Res 40: W09305
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, Berlin Heidelberg New York
Larcher W (2003) Physiological plant ecology. Springer, Berlin Heidelberg New York
Lavigne MB, Foster RJ, Goodine G (2004) Seasonal and annual changes in soil respiration in relation to soil temperature, water potential, and trenching. Tree Physiol 24:415–424
Law BE, Falge E, Gu L, Baldocchi DD, Bakwin P, Berbigier P, Davis K, Dolman AJ, Falk M, Fuentes JD, Goldstein A, Granier A, Grelle A, Hollinger D, Janssens IA, Jarvis P, Jensen NO, Katul G, Mahli Y, Matteucci G, Meyers T, Monson R, Munger W, Oechel W, Olson R, Pilegaard K, Paw KT, Thorgeirsson H, Valentini R, Verma S, Vesala T, Wilson K, Wofsy S (2002) Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric For Meteorol 113:97–120
Lee X, Wu HJ, Sigler J, Oishi C, Siccama T (2004) Rapid and transient response of soil respiration to rain. Global Change Biol 10:1017–1026
Loik ME, Breshears DD, Lauenroth WK, Belnap J (2004) A multi-scale perspective of water pulses in dryland ecosystems: climatology and ecohydrology of the western USA. Oecologia 141:269–281
MacMahon J (1997) Deserts. Alfred Knopf, New York
Mansfield TA, Hetherington AM, Atkinson CJ (1990) Some current aspects of stomatal physiology. Annu Rev Plant Physiol Plant Mol Biol 41:55–75
Meyers TP (2001) A comparison of summertime water and CO2 fluxes over rangeland for well watered and drought conditions. Agric For Meteorol 106:205–214
Mielnick P, Dugas WA, Mitchell K, Havstad K (2005) Long-term measurements of CO2 flux and evapotranspiration in a Chihuahuan desert grassland. J Arid Environ 60:423–436
Monson RK, Smith SD (1982) Seasonal water potential components of Sonoran Desert plants. Ecology 63:113–123
National Assessment Synthesis Team (2000) Climate change impacts of the United States: the potential consequences of climate variability and change. Overview. U.S. Global Change Research Program. Cambridge University Press, Cambridge
Neter J, Wasserman W, Kutner MH (1985) Applied linear statistical models. Richard Irwin Press, Homewood, Ill.
Noy-Meir E (1979) Structure and function of desert ecosystems. Israel J Bot 28:1–19
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–41
Ogle K, Reynolds JF (2004) Plant responses to precipitation in desert ecosystems: integrating functional types, pulses, thresholds, and delays. Oecologia 141:282–294
Pope VD, Gallani ML, Rowntree PR, Stratton RA (2000) The impact of new physical parametrizations in the Hadley Centre climate model – HadAM3. Clim Dynam 16:123–146
Potts DL, Huxman TE, Enquist BJ, Weltzin JF, Williams DG (2006) Resilience and resistance of ecosystem functional response to a precipitation pulse in a semi-arid grassland. J Ecol 94:23–30
Reynolds JF, Kemp PR, Ogle K, Fernandez RJ (2004) Modifying the ‘pulse-reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141:194–210
Sala OE, Laurenroth WK (1982) Small rainfall events: an ecological role in semiarid regions. Oecologia 53:301–304
Schlesinger WH, Fonteyn PJ, Marion GM (1987) Soil moisture and plant transpiration in the Chihuahuan desert of New Mexico. J Arid Environ 12:119–126
Schwinning S, Davis K, Richardson L, Ehleringer JR (2002) Deuterium enriched irrigation indicates different forms of rain use in shrub/grass species of the Colorado Plateau. Oecologia 130:345–355
Schwinning S, Sala OE, Loik ME, Ehleringer JR (2004) Thresholds, memory and seasonality: understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 141:191–193
Scott RL, Shuttleworth WJ, Keefer TO, Warrick AW (2000) Modeling multiyear observations of soil moisture recharge in the semiarid American Southwest. Water Resour Res 36:2233–2247
Smith SD, Monson RK, Anderson JE (1997) Physiological ecology of North American Desert plants. Springer, Berlin Heidelberg New York
Sperry JS, Hacke UG (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Funct Ecol 16:367–378
Weltzin JF, McPherson GR (2003) Changing precipitation regimes and terrestrial ecosystems. University of Arizona Press, Tucson
Weltzin JF, Loik ME, Schwinning S, Williams DG, Fay P, Haddad B, Harte J, Huxman TE, Knapp AK, Lin G, Pockman WT, Shaw MR, Small EE, Smith MD, Smith SD, Tissue DT, Zak J (2003) Assessing the response of terrestrial ecosystems to potential changes in precipitation. BioScience 53:941–952
Whitford WG (2002) Ecology of desert systems. Academic, San Diego
Williams DG, Cable W, Hultine K, Hoedjes JCB, Yepez EA, Simonneaux V, Er-Raki S, Boulet G, de Bruin HAR, Chehbouni A, Hartogensis F, Timouk T (2004) Evapotranspiration components determined by stable isotope, sap flow and eddy covariance techniques. Agric For Meteorol 125:241–258
Yan S, Wan C, Sosebee RE, Wester DB, Fish EB, Zartman RE (2000) Responses of photosynthesis and water relations to rainfall in the desert shrub creosote bush (Larrea tridentata) as influenced by municipal biosolids. J Arid Environ 46:397–412
Ziehr LLH (1997) Microbial biodiversity along an arid watershed. Master’s thesis, Texas Tech University
Acknowledgements
The authors would like to acknowledge the support of a NPS grant (1434-01HQRU1570) to DT, JZ, and ML that allowed for the collaboration on this project. LP received support from a Texas Tech University Association of Biologists Summer Mini-Grant. We thank the staff at Big Bend National Park for their logistical and scientific support, especially Joe Sirotnak, John Forsythe, and Susan Simmons. We are indebted to the Big Bend Fire Crew for assistance with re-filling water tanks. Rich Strauss provided statistical advice. Field work could not have been completed without the help of Colin Bell, Heath Grizzle, Justin Jenkins, Ken Seal, Mindy Rice, and John McVay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jim Ehleringer.
Rights and permissions
About this article
Cite this article
Patrick, L., Cable, J., Potts, D. et al. Effects of an increase in summer precipitation on leaf, soil, and ecosystem fluxes of CO2 and H2O in a sotol grassland in Big Bend National Park, Texas. Oecologia 151, 704–718 (2007). https://doi.org/10.1007/s00442-006-0621-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0621-y