Abstract
To test the relative importance of long-term and working spatial memories in short-range foraging in bumblebees, we compared the performance of two groups of bees. One group foraged in a stable array of six flowers for 40 foraging bouts, thereby enabling it to establish a long-term memory of the array, and adjust its spatial movements accordingly. The other group was faced with an array that changed between (but not within) foraging bouts, and thus had only access to a working memory of the flowers that had been visited. Bees in the stable array started out sampling a variety of routes, but their tendency to visit flowers in a repeatable, stable order (“traplining”) increased drastically with experience. These bees used shorter routes and converged on four popular paths. However, these routes were mainly formed through linking pairs of flowers by near-neighbour movements, rather than attempting to minimize overall travel distance. Individuals had variations to a primary sequence, where some bees used a major sequence most often, followed by a minor less used route, and others used two different routes with equal frequency. Even though bees foraging in the spatially randomized array had access to both spatial working memory and scent marks, this manipulation greatly disrupted foraging efficiency, mainly via an increase in revisitation to previously emptied flowers and substantially longer search times. Hence, a stable reference frame greatly improves foraging even for bees in relatively small arrays of flowers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traplining is a foraging strategy that involves visiting food sources in a stable repeatable sequence. It has been reported in ten bumblebee species (Comba 1999; Makino and Sakai 2004; Manning 1956; Thomson et al. 1982; Williams and Thomson 1998), euglossine bees (Ackerman et al. 1982; Janzen 1971), honeybees (Ribbands 1949), hummingbirds (Garrison and Gass 1999; Gill 1988), tamarins (Garber 1988), rats (Reid and Reid 2005), pied wagtails (Davies and Houston 1981), long-nosed bats (Lemke 1984) and several species of Heliconius butterflies (Gilbert 1980). Despite the widespread nature of this strategy, the question of how traplines are formed remains relatively unexplored.
Once established, traplines can remain stable for extended time periods (Comba 1999; Thomson 1996). However, very little is known about how animals decide on suitable routes to link a set of known locations, i.e. whether they try out multiple routes and ultimately settle on the optimal solution. Janzen (1971) suggested that bees link plants in the order they encounter them, but there is no empirical evidence for this. More importantly, as Janzen points out, such a strategy might produce suboptimal results, because it would not serve to minimize travel paths. Research on honeybees has shown that bees can travel novel shortcuts between familiar locations (Menzel et al. 1998, 2005), indicating that they might be able to connect multiple locations in a different sequence from that in which they were encountered. One of the goals of this study was to quantify how searching among flowers of a stable array gradually turns into a trapline. Bees may do this by experiencing multiple possible paths and finally settling onto a near-optimal solution.
Bees have several ways of improving their foraging efficiency in an unfamiliar environment. They can use spatial working memory (Brown et al. 1997), which can help to keep track of recently visited flowers or plants. They also have hard-wired strategies, such as near-far-search, where animals foraging in patchy environments make short movements when encountering high rewards (maximizing the probability of staying in a rich patch), but travel longer distances after receiving poor rewards (Chittka et al. 1997; Pyke 1984). However, bees also have a keen long-term spatial memory, which they use when foraging from multiple food sources (Chittka et al. 1995; Collett 1993; Menzel et al. 1998). We use a simple, yet efficient procedure to identify the relative benefits of long-term spatial memory: we randomize flower positions between subsequent foraging bouts, thus preventing bees from using long-term spatial memory of flower position between bouts, but retaining the possibility to resort to classic foraging algorithms as well as working memory of visited flowers within bouts. The performance of these bees is compared to that of bees allowed access to long-term spatial memory, and thereby the ability to form traplines.
There are obvious potential advantages to traplining. Trapliners might learn to link resources in a more direct path, cutting down on travel distance. This can be seen in rats repeatedly visiting food sources (Reid and Reid 2005). Because traplining bees know the food source locations, they can spend less time searching for them, potentially even identifying individual, rewarding flowers with higher probability than naïve bees (Williams and Thomson 1998). Thus search times should be lower than for animals that do not, or in our case cannot, use this information. A traplining animal should also make fewer revisits to recently depleted food sources because it can circuit through them, making it less likely to back track on its path. This is especially important as flowers take time to replenish so revisiting at an incorrect schedule would waste time and energy (Williams and Thomson 1998).
This study uses bees as a model to determine: (1) the ontogeny and characteristics of stable traplining routes within arrays of multiple feeding sites; (2) whether access to long-term spatial memory results in stable routes that reduce flight time in search of food, revisits to the food source and travel distance between food sources.
Materials and methods
A bumblebee colony (Bombus impatiens) obtained from Biobest (Leamington, Canada) was housed in a nest box and connected to a flight arena [105 (length) × 75 (width) × 30 (height) cm] via a clear Plexiglas tunnel. Shutters in the tunnel allowed single bees to be tested by restricting access of other bees. Approximately 8 g of pollen were fed directly into the nest on a daily basis. Tests were conducted at temperatures of ≈ 20°C and a light–dark cycle of 10 h:14 h.
Flowers were 1 cm in diameter, made of blue cardboard and attached to the arena floor. Each bee foraged on six rewarding flowers [30% (w/w) sucrose solution] for 40 continuous bouts. A bout consists of the bee exiting the hive, feeding on flowers and returning to empty its honey crop. We adjusted the nectar rewards of each bee to its honey crop capacity by allowing it to forage on 15 flowers with 10 μl of 30% sucrose solution for three pre-experimental bouts. The average amount of sucrose ingested in all three bouts was divided by 6 and placed on each test flower. The flowers were replaced after each bout, so there were no scent marks from previous bouts available in any given foraging circuit (Saleh et al. 2006; Saleh and Chittka, 2006). Individual bees were placed in one of two treatments. In the stable flower treatment flowers remained in fixed positions throughout the 40 bouts, allowing use of spatial memory (and the formation of traplines) within and between bouts (n = 7 bees). In the random flower treatment, spatial positions were randomly allocated (using a computer algorithm) onto a 7 × 5-points square grid with 15 cm between points, in each new foraging bout. This protocol allowed the use of spatial working memory within bouts but did not allow use of long-term spatial memory between bouts (n = 5 bees). Bees in this treatment could not form traplines and their results were used only to test possible adaptive advantages to traplines. Trials were video taped and analysed using Behavior Tracker software (version 1.5). Each individual bee was tested once and bees with inter-bout times of less than 5 min were chosen to ensure that only highly motivated bees were used in the tests (very few bees failed this criterion).
Based on our knowledge of the visual system of a different bumblebee species, Bombus terrestris (Spaethe and Chittka 2003), it is unlikely that bees in the stable flower array could see the nearest flower from any one of the flower positions. In that species, large workers can detect a target that subtends 3.5°, corresponding to a distance of 16 cm in a target with a diameter of 1 cm. Since B. impatiens workers are smaller, their visual spatial resolution is likely to be less fine-grained, so that data from B. terrestris provide a conservative estimate. The smallest interfloral distance was 21 cm for the stable array, therefore bees foraging on the stable array could not detect the next nearest flower. However, bees foraging on the random arrays may have been able to detect at least one nearest flower in 58% of the spatial arrangements. Thus, if bees employed a simple visually mediated near-neighbour search strategy, the random floral arrays should, on average, have been the easier task of the two.
Data analysis
We terminated data analysis once the bee finished feeding on the sixth rewarding flower. We tested for normality of data where necessary and used appropriate tests utilizing Minitab version 12. Individual distances between each flower and the other five in every bout were not significantly different amongst the two treatments (Mann–Whitney test, n = 870, W = 7122.0, P = 0.5415; median ±1 SD, stable = 47.5 ± 19.4, random = 46.0 ± 21.8 cm). Thus, flower distances between the groups were comparable and observed differences are not due to this factor.
Ontogeny of traplines
We wanted to explore the similarity of individual bees’ spatial visitation sequences from one bout to the next, to see whether the tendency to repeat such sequences increased with experience. In comparing the sequences, the crucial question was how many substitutions, insertions and deletions of flowers are necessary to make two sequences identical. This is essentially the same as in DNA alignment, where DNA sequences are compared with one another to assess similarity between them (Waterman and Jones 1990). We calculated similarity indices using a computer program (courtesy of K. Ohashi and J .D. Thomson) formulated on a technique similar to DNA alignment where the endpoints of the sequences were fixed (Thomson et al. 1997). This technique takes into account insertions, deletions and substitutions to any primary sequence. We defined the two fixed end points as the bee’s nest entrance, where each foraging bout originates and eventually terminates. Similarity indices of 0 mean the visitation sequences are completely different and of 1 mean they are identical. We calculated similarity indices between each bout and its subsequent bout. We then averaged these values to give a mean value representing a moving average bin. The similarity indices were analysed by using a moving average of five bouts. For example, we had an average for bouts 1–5, 2–6, 3–7 etc; this averaging removed the effect of variation between pairs of bouts and revealed gradual changes in the bees’ tendency to trapline (Kenney 1967). For statistical analyses of the bees’ similarity indices, we only compared independent moving average bins with no overlapping bouts, so for example bin 1–5 to bin 6–10. Henceforth, when discussing the bees’ progress as a function of experience, we only refer to the midpoints of each bin.
To determine whether bees showed a higher tendency to trapline than expected by chance, we compared similarity indices based on observed sequences with those generated by a null model. We generated 5,000 random sequences of ten flower visits (the mean length of the bees’ sequences). Then we took sequences 1–5, 6–10, until 4,996–5,000, and calculated similarity indices for each set of five, so that we produced a distribution of 1,000 randomly generated similarity indices in total. A frequency histogram of these indices is shown below; 95% of the randomly determined indices fall below a threshold of 0.269. We defined an individual bee’s similarity index as non-random (at the 5% level) if it exceeded this threshold. To examine the characteristics of the bees’ traplines, we re-examined all visitation sequences this time excluding any revisits to determine trends in the basic visitation sequence. We also compared the distances of these basic visitation sequences to see if bees used shorter ones with experience. To do this, we divided the 40 bouts into early, middle and late bouts, which corresponded to bouts 1–13, 14–26 and 27–40, respectively.
Advantages of long-term spatial memory for foraging
We investigated how experience affected the number of revisits, and flight time per bout. In this case we compared all the revisits performed per bout by the bees in each treatment. Flight time is a function of search time as well as travel time between flowers, given that the distances between flowers within the two treatments are comparable, we expect any differences in flight time to be due to differences in time spent searching for the flowers. The mean number of revisits or flight time in the first bout was compared to the mean of the last 20 bouts for each group to determine changes within the group with experience. We chose to analyse 20 bouts because by then bees had stabilized in their use of traplines (see below). To evaluate differences between groups, only the last 20 bouts were examined so that comparisons were made after performance saturated. Next, we wanted to investigate if in the stable treatment bees performed fewer revisits when using their two favoured routes than the other less used routes. To this end, we compared the total revisits performed per bout when bees used their two favoured routes with the total revisits performed when using all other routes.
We were also interested in a detailed investigation of the revisits bees performed after visiting a rewarding flower to determine if there was a difference between the two bee treatments. We divided the revisits into two types: same flower (revisit to the same flower immediately after the bee finished feeding from it) and different flower (revisit to a different previously visited flower). We compared the stable and random flower treatments to investigate how they may differ. We also compared the number of each type of revisit performed per bout when bees in the stable array treatment used their two favoured routes compared to all other routes.
Flight distances were determined through frame-by-frame playback (using a JVC DV video cassette recorder, BR-DV3000E) of the first and last three bouts, when the bees had the least and the most experience with the setup. We arbitrarily chose to analyse three bouts before we began data analysis. A piece of clear cellophane was taped onto the television screen and the bee's path was marked. A string was used to determine the distance travelled by connecting these marks. We had complete video tapes for nine bees.
We needed to standardize the routes, because longer flight distances may be due to larger interfloral distances. We divided the total travel distance performed by each bee in each bout by the summed value of individual interfloral distances for that bout. We then averaged these standardized values to generate one value for the first three bouts and one for the last three bouts for each bee. The performance of the two groups of bees in the first and last three bouts was compared using a two sample t-test with bee as the unit of replication. We verified that interfloral distances were comparable between the two bee treatments for the first and last three bouts. To do this, we performed two sample t-tests comparing the interfloral distances of the stable arrangement with each of the random arrangements. Twenty-three out of 26 comparisons were not significant (P > 0.05). In the three cases where they were significant, the random array interfloral distances were much smaller than the stable distances (mean ± 1 SD, 29.7 ± 15.9, 28.5 ± 11.9 and 37.8 ± 16.9 cm for the random arrangements compared to 52.9 ± 19.6 cm for the stable arrangement). Henceforth, average values are reported as mean ± 1 SD throughout and sample sizes are number of bees used.
Results
Changes in sequence similarity with experience
Visitation sequences became more similar with experience until approximately bout 20, where repeatability saturated (see Figs. 1, 2). There was a significant difference in the route similarity indices of bees foraging in the stable spatial array of flowers from the start level to bout 10 (paired t-test, t = 2.76, P < 0.033, n = 7). This significance was consistent between the first and all subsequent bins. Comparing the randomly generated sequences to bees in the stable flower array, we find that bees, as a group, had a significantly higher similarity index by bout 5 (see Fig. 2). Bees C and F reached significance by bout 3, bees B, E and G by bout 5 and bees A and D by bouts 12 and 11, respectively.
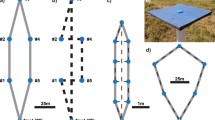
a, b Examples of routes taken by bees foraging on stable flower array of six artificial flowers. Numbers represent flower positions. The width of the arrow corresponds to the frequency each trajectory was taken throughout the 40 bouts. The arena was enclosed by four walls [105 (length) × 75 (width) × 30 (height) cm], illustrated in the figure. Flowers were 1 cm in diameter
Moving average for mean similarity indices of traplining bees. The higher the value of the similarity index the greater the similarity between bouts (i.e. tendency to trapline). Similarity indices were calculated using a technique similar to DNA sequence alignment. The bees’ similarity indices were compared to 1,000 indices produced from randomly generated sequences; 95% of the indices from randomly generated sequences fall below the threshold of 0.269, indicated by the dashed line. The bees’ similarity index was considered significantly different (at the 5% level) if it exceeded this threshold. Bees show increased similarity in visitation sequences with experience. Error bars represent SD
When revisits were removed from the analyses, bees foraging on stable spatial arrays had one of two strategies for traplining. They either relied heavily on one visitation sequence and less on another (bees B, D, E and F), or had two sequences that they equally used (bees A, C and G) (see Table 1). None of these preferred visitation sequences were performed in the first three bouts. The earliest was at bout 4 (bee A sequence 2–3–4–5–6–1) and the latest by bout 11 (bee C sequence 2–4–5–6–3–1). Four main sequences were used as traplines by five out of seven bees (henceforth called the four popular sequences). Of the remaining two bees, bee E used none of the four popular routes and bee G used only one. These sequences are 1–2–4–5–6–3, 1–2–3–4–5–6, 2–4–5–6–3–1 and 2–3–4–5–6–1. These routes are similar in some components, for example they all contain the sequence 4–5–6. The main differences between these routes are the start flower position and whether the bees visited flower 3 or 4 first after feeding from flower 2.
Investigating these routes in detail suggests that bees are minimising distance between flowers generally, except in the case of flowers 3 and 4, which were located at very similar distances, 33.5 and 33.75 cm respectively, from flower 2. In addition, when deciding on the start flower, bees did not always minimize distance travelled from the hive to the first flower. Flower 2 was about 7 cm farther away from the hive entrance than flower 1 (32 and 39.25 cm beeline distance from the hive entrance for flowers 1 and 2, respectively). Bees started at flower 2 in half of the four most popular routes.
Bees generally used longer routes in the early bouts compared to the middle and late bouts (see Fig. 3). There is also an obvious increase in the use of the four popular routes with experience, which is detectable from the early bouts. The number of routes used by the bees as a whole decreased with experience from 29 in the early bouts to 18 in the late bouts.
Percentage of times bees used each visitation sequence listed in Table 1 as a function of experience. These distances are for sequences that exclude revisits. The numbers on the x-axis represent the length of each route. Each sequence had its own unique distance and the length of every other sequence is marked on the x-axis. Bees followed longer routes in the early bouts, which were not used in the middle and late bouts. They also start to show a preference for the subsequent four popular routes in these early stages
Revisits
Bees in both treatments reduced their revisitation from the first bout (stable array, 11.14 ± 7.63 first bout, 2.36 ± 0.48 last 20 bouts; random array, 13.40 ± 5.85 first bout, 4.9 ± 1.6 last 20 bouts) (paired t-test, stable array, t = 3.04, P < 0.023, n = 7; random array, t = 3.22, P < 0.032, n = 5; see Fig. 4a). In the last 20 bouts, traplining bees that had access to long-term spatial memory performed fewer revisits than those unable to use this memory (two sample t-test, t = −3.45, P < 0.026, n = 12). The bees foraging on a stable spatial array of flowers made fewer revisits in 34 out of 40 bouts (85%). Traplining bees performed, on average, fewer revisits when using their favoured routes compared to other routes (3.13 ± 0.66 and 4.07 ± 1.36 revisits per bout for favoured and all other routes, respectively). However, this difference was not significant (Mann–Whitney U-test, W = 44.0, P < 0.31, n = 7).
a Mean number of revisits per bout as a function of experience. Traplining bees consistently had lower revisitation rates. b Mean time spent in flight per bout as a function of experience. The non-traplining bees performed better than the stable bees in only five of 40 bouts. Error bars represent SD. The continuous line with open circles represents data for bees foraging on random arrays and the dashed lined with filled circles represents data for bees foraging on the stable arrays. Vertical bars indicate SDs; where the grey bars below the data point are for stable array treatment bees and black bars above the data point are for random array treatments bees
Bees foraging on the stable array were more likely to revisit the same flower in the first few bouts (see Fig. 5). For example, they performed 3.42 ± 1.81 same-flower revisits compared to 0.86 ± 1.3 different-flower revisits in the first bout. This quickly reversed to 0.71 ± 0.98 and 1.43 ± 1.27 by bout 4 for same- and different-flower revisits, respectively. Interestingly, bees foraging on the random array, without access to long-term spatial memory, do not seem to change their use of same- and different-flower revisits. These bees used fewer same-flower revisits in only 25 out of 40 (62.5%) bouts. However, there appears to be more individual variation per bout in the number of revisits to different flowers compared to revisits to the same flower for these bees.
Mean number of revisits to same and different flowers for bees foraging on stable (top) and random (bottom) foraging arrays. The continuous line with open circles represents same flower revisits and the dashed lined with filled circles represents different flower revisits. Vertical bars indicate SDs. Grey bars pointing down are for same flower revisits and black bars pointing up are for different flower revisits
After traplining bees finished feeding on a flower, they made similar numbers of same (Mann–Whitney test, W = 50, P = 0.79) and different (Mann–Whitney test, W = 49, P = 0.70) flower revisits when using her favoured routes compared to other routes. Bees performed 0.90 ± 0.40 revisits to the same flower and 1.22 ± 0.52 revisits to a different flower when using their favoured routes. They performed 1.03 ± 0.48 revisits to the same flower and 1.3 ± 0.37 revisits to a different flower when using the other routes.
Flight time
Bees in both treatments reduced their flight time from the first bout; however, only the improvements for bees foraging on stable arrays are significant (stable array, 63.9 ± 33.1 s first bout, 22.5 ± 4.03 s last 20 bouts; random array, 71.0 ± 34.60 s first bout, 32.3 ± 5.29 s last 20 bouts) (paired t-test, stable array, t = 5.04, P < 0.002, n = 7; random array, t = 15.66, P < 0.072, n = 5; see Fig. 4b). Overall, bees foraging from stable spatial arrays with access to long-term spatial memory spent less time flying per bout (22.5 ± 4.03 s for stable, 32.3 ± 5.29 s for random spatial array of flowers; two sample t-test, t = −3.48, P < 0.01, n = 12). Bees foraging on stable spatial arrays did better than those on random arrays in 30 out of 40 bouts (75%). There was no significant difference in interfloral flight times per bout (2.55 ± 0.52 s for stable and 2.95 ± 0.18 s for random spatial arrays; two sample t-test, t = −1.86, P < 0.11, n = 12).
Differences in flight distance
Bees in both treatments travelled similar distances in the first three bouts (919.9 ± 276.93 cm for stable and 1,007.9 ± 282.42 cm for random spatial array) (two sample t-test, t = −0.87, P < 0.65, n = 9). Although there is strong suggestive evidence that bees foraging on stable arrays travel less distance than those on random arrays in the last three bouts (526.3 ± 55.51 cm for stable, 795.4 ± 216.67 cm for random spatial array), these results are not significant (two sample t-test, t = −2.63, P < 0.062, n = 9). Distance travelled was correlated with revisitations (Pearson correlation, r = 0.894, P < 0.001, n = 9), and bees foraging on random spatial arrays revisit more than those on the stable array. Thus, it is likely that with more bees, the difference in travel distance between the two treatments would become significant.
Discussion
Previous reports on traplining in bees and other animals have recorded this behaviour in field conditions, where either reward levels, plant visitation patterns and the bees’ previous experience were not controlled, or in the case of more controlled studies, the visitation sequences were entrained by an experimenter. This study investigates traplining behaviour in a controlled laboratory study where bees are able to freely optimize routes as they accumulate experience. It is the first to look at how spatial foraging strategies develop in naïve bees.
Bees form traplines with experience
To assess if the bees’ visitation sequences became more similar with experience, we produced a null model based on similarity indices from randomly generated sequences and compared it to similarity indices generated by the bees’ visitation sequences. While complete randomness of movements is perhaps a simplistic assumption of how bees might move in the absence of traplining, such a random null model has the virtue of being free of ad hoc assumptions about alternative strategies that bees might use.
Traplining bees began stereotyping their routes between bouts 8 and 12 (see Fig. 2), and continued to strengthen this repeatability until approximately bout 20, showing that bees develop their traplines after experiencing different routes. Indeed, the earliest one of the later preferred visitation sequences was performed at bout 4 (bee A) and the latest at bout 11 (bee C).
Thus, bees foraging on small spatial scales do not form traplines simply by following the visitation sequence in which they originally encountered the flowers (Janzen 1971). In order for a trapline to form, bees first need to locate the rewarding plants, and experience several subsequent rewarding revisits before linking them in a repeatable sequence. They also need to learn the locations of these flowers relative to each other. We expect that naïve bees with little foraging experience or experienced bees naïve to the area may sample different plants a few times before deciding to return to specific plants on a regular basis. In doing this, they may experience different routes within these plants until they find preferred ones. Bees that have experience foraging in an area may also sample different routes as resources change.
Preferred visitation sequences and minor variations to primary sequence
The two most used visitation sequences of each bee were used for about half of the foraging bouts. Of these, bees either had one preferred sequence and a second less used one, or they had two that they used with equal frequency. The differences between these two most used visitation sequences for each bee are very small, involving a change in one flower visit in the sequence. Thus, bees use variations to one primary visitation sequence when they trapline. The variations in the top two preferred visitation sequences where the first visited flower was moved to the last arise from starting at a different flower. For example, the two preferred visitation sequences used by bees A, B and E started with flower 1 instead of 2 and vice versa. Thus, in order to complete the circuit, they needed to visit the flower they skipped at the start. The three cases where replacements within the sequence took place (bees C, D and F), the start flower remained the same. In these cases the bees reversed their visitation of flowers 3 and 4. Variation in traplining routes have been reported previously (Comba 1999; Thomson et al. 1982, 1987). Although changes between days have been attributed to changes in plant status (Thomson et al. 1982; Williams and Thomson 1998), we show here that some variations can still occur, because traplines varied even though our flowers were entirely constant in position and reward status.
Optimal routes, and similarities in traplines used by individual bees
Most bees converged on two of the four most popular sequences as their favoured routes, indicating that they were using a common foraging strategy. A bee feeding from a flower in the stable array could not visually detect the next nearest flower, thus near-neighbour movements by direct detection of nearest flowers were not possible. Further evidence against such near-neighbour movements is that bees developed their traplines with experience, suggesting that they needed to become familiar with the spatial layout.
Analysis of the four most popular sequences suggests that bees learn to minimize travel distance between flowers, rather than minimizing overall travel distance. The four popular routes mainly involve movements between nearest flowers with two main differences: the start flower and the order with which flowers 3 and 4 were visited after feeding from flower 2. Flowers 3 and 4 were located 33.5 and 33.75 cm away from flower 2. Thus the switch in the visitation order is most likely due to the inability of the bees to differentiate between such small differences in distance. It is interesting that they did not choose one of the two variants but rather used both even though one of the routes always provided a longer overall travel path. Flower 2 was about 7 cm farther away from the hive entrance than flower 1, thus it seems bees did not always minimize distance travelled from the hive to the first flower when deciding on the start flower. The bees often followed a straight trajectory from the hive entrance into the foraging arena, where they encountered flower 2 and probably chose to feed there rather than change their path to find flower 1. However, the fact that half of the favoured routes began with a visit to flower 1 indicates that some bees were assessing distance of flowers from the hive entrance. It is interesting to note that bee E had her own unique routes, indicating that not all bees are minimizing flight paths by minimizing flight distance between flowers. Her routes resulted in longer flight distances (352.3 and 361.5 cm) than the four popular routes.
The bees as a group used 38 different visitation sequences throughout the 40 foraging bouts. In the early bouts they used 29 different routes. However, with experience, the number of routes used, especially those involving the longest visitation sequences, were reduced by about half, while usage of the subsequent four popular routes increased.
If the bees were trying to link the flowers optimally, we would expect their favoured visitation sequences to be very close to the optimal sequence. In addition to the optimal route, bees chose the 8th, 14th and 19th shortest routes. However, all these choices can be explained by attempts to reduce travel distance between pairs of flowers as opposed to minimizing total travel distance.
Thus, bees can reduce their travel distance with experience, but, at least for small foraging scales, they use simple rules that do not necessarily produce the global optimal solution. Rats (Reid and Reid 2005) also reduced their distance travelled between point sources with experience. Although that study was not analysing traplining behaviour, the rats’ behaviour can be explained by attempts to minimize travel distance between food sources. These rats never reached the optimal distance. It would be interesting to see if bees foraging on larger spatial arrays also reduce their travel distance by minimizing flight time between plants.
Revisits
Both groups reduced their revisitations from the initial bout (see Fig. 4a). Bees can use tactics such as working spatial memory (Brown et al. 1997) and scent marks (Saleh et al. 2006; Saleh and Chittka 2006) within bouts to reduce their revisitation rate. However, bees that were allowed to use long-term spatial memory, and thereby form traplines, reduced their revisits to a greater extent than bees that could not form traplines. The bees on the stable spatial array revisited less than those on random arrays in 85% of bouts. Therefore, the ability to use long-term spatial memory to locate and circuit through food sources in a repeatable order reduces the likelihood of revisiting recently visited food sources. We expect this to be especially important when the food sources are spatially aggregated and therefore revisits more likely.
There is suggestive evidence that bees foraging on the stable array revisited less when using their preferred sequences compared to the other sequences. However, these results were not significant.
We investigated the types of revisits performed by bees in the two treatments. We find that when bees have access to long-term spatial memory they reduce their same-flower revisits and use more different-flower revisits within the first few bouts. However, bees foraging on the random array did not change their performance of the two types of revisits. Both groups of bees had the opportunity to learn that flowers do not refill once emptied. Bees have been shown to reduce their flight angle upon encountering rewarding flowers, helping the bee stay in a rewarding patch (Chittka et al. 1997; Pyke 1984). It is possible that the immediate revisits to the same flower are similar to area-restricted searching. In our case the flowers were not spatially aggregated and often the bees could not see the next nearest flower, which may have resulted in the bees revisiting the same flower they just fed from in an attempt to perform area-restricted searching. The bees in the stable array learned not to use this behaviour while those without access to long-term spatial memory continued to employ it as a search strategy. The fact that bees foraging on the stable flower array increased their performance of different-flower revisits provides further evidence against the idea that bees are optimising a global route, because if this was the case we would expect a reduction in any kind of revisitation not just same-flower revisits.
Flight time and distance
We also find an advantage to traplining in terms of the time spent searching for flowers. Bees foraging on the stable spatial array had lower flight times and they reduced their flight time with experience, but those on random spatial arrays did so less. Bees having access to long-term spatial memory had lower flight times in 75% of the 40 bouts. This is found in other animals as well. Rats also took less time to move between food sources as they gained experience with the location of these resources (Reid and Reid 2005).
Both bee treatments had similar travel distances for their first three foraging bouts, and bees in both treatments reduced their travel distance with experience. There is strong suggestive evidence that traplining bees, with access to long-term spatial memory, have shorter flight distances than bees unable to form traplines, but our results are not statistically significant.
Conclusion
After sampling multiple routes, bees with access to long-term spatial memory arrive at traplining routes that help reduce revisitations and search times. Our study found large differences in performance between traplining bees, and those not allowed to trapline, despite its small spatial scale. Bees can forage in patches located several kilometres apart (Goulson 2000; Janzen 1971; Osborne et al. 1999; Walther-Hellwig and Frankl 2000) and their traplines can cover an area of at least 300 m2 (Comba 1999). Therefore, we expect the advantages of traplining to be greatly magnified in field conditions, where bees often cannot easily detect one flower (or patch) from another, and therefore a continuous search or relying solely on short-term memory may be a highly inefficient strategy. Thus, experiencing different routes and subsequently linking distant foraging locations by memorized vectors, while minimizing travel distance, should greatly enhance a bee’s foraging efficiency. We used a small spatial scale, which means we were investigating within-patch behaviour. It is important to identify if bees behave in a similar manner when foraging between patches and on food sources with varying levels of reward.
Traplining behaviour has been reported in over 20 animal species. This study provides clues to some of the advantages of this behaviour. Further research in this area should attempt to identify if the same advantages are present in other traplining animals. This will help clarify why this foraging strategy is so popular.
References
Ackerman JD, Mesler MR, Lu KL, Montalvo AM (1982) Food-foraging behavior of male Euglossini (Hymenoptera: Apidae): vagabonds or trapliners? Biotropica 14:241–248
Brown MF, Moore JA, Brown CH, Langheld KD (1997) The existence and extent of spatial working memory ability in honeybees. Anim Learn Behav 25:473–484
Chittka L, Kunze J, Shipman C, Buchmann SL (1995) The significance of landmarks for path integration of homing honey bee foragers. Naturwissenschaften 82:341–343
Chittka L, Gumbert A, Kunze J (1997) Foraging dynamics of bumle bees: correlates of movements within and between plant species. Behav Ecol 8:239–249
Collett TS (1993) Route following and the retrieval of memories in insects. Comp Biochem Physiol 104A:709–716
Comba L (1999) Patch use by bumblebees (Hymenoptera Apidae): temperature, wind, flower density and traplining. Ethol Ecol Evol 11:243–264
Davies NB, Houston AI (1981) Owners and satellites—the economics of territory defense in the pied wagtail, Motacilla alba. J Anim Ecol 50:157–180
Garber PA (1988) Foraging decisions during nectar feeding by tamarin monkeys (Saguinus mystax and Saguinus fuscicollis, Callitrichidae, Primates) in Amazonian Peru. Biotropica 20:100–106
Garrison JSE, Gass CL (1999) Response of a traplining hummingbird to changes in nectar availability. Behav Ecol 10:714–725
Gilbert L (1980) Ecological consequences of a coevolved mutualism between butterflies and plants. In: Gilbert L, Raven P (eds) Coevolution of animals and plants. University of Texas Press, Austin, Tex., pp 210–231
Gill FB (1988) Trapline foraging by hermit hummingbirds—competition for an undefended, renewable resource. Ecology 69:1933–1942
Goulson D (2000) Why do pollinators visit proportionally fewer flowers in large patches? Oikos 91:485–492
Janzen DH (1971) Euglossine bees as long-distance pollinators of tropical plants. Science 171:203–205
Kenney JF (1967) Mathematics of statistics. Van Nostrand, Princeton, N.J.
Lemke TO (1984) Foraging ecology of the long-nosed bat, Glossophaga soricina, with respect to resource availability. Ecology 65:538–548
Makino TT, Sakai S (2004) Findings on spatial foraging patterns of bumblebees (Bombus ignitus) from a bee-tracking experiment in a net cage. Behav Ecol Sociobiol 56:155–163
Manning A (1956) Some aspects of the foraging behavior of bumble-bees. Behaviour 9:164–201
Menzel R, Geiger K, Joerges J, Müller U, Chittka L (1998) Bees travel novel homeward routes by integrating separately acquired vector memories. Anim Behav 55:139–152
Menzel R et al (2005) Honey bees navigate according to a map-like spatial memory. Proc Natl Acad Sci USA 102:3040–3045
Osborne JL et al (1999) A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J Appl Ecol 36:519–533
Pyke GH (1984) Optimal foraging theory: a critical review. Annu Rev Ecol Syst 15:523–575
Reid RA, Reid AK (2005) Route finding by rats in an open arena. Behav Process 68:51–67
Ribbands CR (1949) The foraging method of individual honey-bees. J Anim Ecol 18:47–66
Saleh N, Ohashi K, Thomson J, Chittka L (2006) Facultative use of repellent scent mark in foraging bumblebees: complex Vs simple flowers. Anim Behav 71:847–854
Saleh N, Chittka L. The importance of experiance in the interpretation of conspecific chemical signals. Behav Ecol Sociobiol (in press)
Spaethe J, Chittka L (2003) Interindividual variation of eye optics and single object resolution in bumblebees. J Exp Biol 206:3447–3453
Thomson JD (1996) Trapline foraging by bumblebees. I. Persistence of flight-path geometry. Behav Ecol 7:158–164
Thomson JD, Maddison WP, Plowright RC (1982) Behavior of bumble bee pollinators on Aralia hispida Vent. (Araliaceae). Oecologia 54:326–336
Thomson JD, Peterson SC, Harder LD (1987) Response of traplining bumble bees to competition experiments: shifts in feeding location and efficiency. Oecologia 71:295–300
Thomson JD, Slatkin M, Thomson BA (1997) Trapline foraging by bumble bees. II. Definition and detection from sequence data. Behav Ecol 8:199–210
Walther-Hellwig K, Frankl R (2000) Foraging distances of Bombus muscorum, Bombus lapidarius, and Bombus terrestris (Hymenoptera, Apidae). J Insect Behav 13:239–246
Waterman MS, Jones R (1990) Consensus methods for DNA and protein-sequence alignment. Methods Enzymol 183:221–237
Williams NM, Thomson JD (1998) Trapline foraging by bumble bees. III. Temporal patterns of visitation and foraging success at single plants. Behav Ecol 9:612–621
Acknowledgements
We wish to thank K. Ohashi, J. D. Thomson for providing software, J. Gurnell, S. Le Comber, N. E. Raine, T. C. Ings and Elli Leadbeater for comments on the manuscript and advice on statistics. This study was funded by a Central Research Fund (University of London) to N. S. We would also like to thank two anonymous referees whose input greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ingolf Steffan-Dewenter.
Rights and permissions
About this article
Cite this article
Saleh, N., Chittka, L. Traplining in bumblebees (Bombus impatiens): a foraging strategy’s ontogeny and the importance of spatial reference memory in short-range foraging. Oecologia 151, 719–730 (2007). https://doi.org/10.1007/s00442-006-0607-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0607-9