Abstract
Coordinated variation has been reported for leaf structure, composition and function, across and within species, and theoretically should occur across populations of a species that span an extensive environmental range. We focused on Hawaiian keystone tree species Metrosideros polymorpha, specifically, 13-year old trees grown (2–4 m tall) in a common garden (approximately 1 ha field with 2–3 m between trees) from seeds collected from 14 populations along an altitude–soil age gradient. We determined the genetic component of relationships among specific leaf area (SLA), the concentrations of nitrogen (N) and pigments (chlorophylls, carotenoids, and anthocyanins), and photosynthetic light-use efficiency. These traits showed strong ecotypic variation; SLA declined 35% with increasing source elevation, and area-based concentrations of N, Chl a + b and Car increased by 50, 109 and 96%, respectively. Concentrations expressed on a mass basis were not well related to source elevation. Pigment ratios expressed covariation that suggested an increased capacity for light harvesting at higher source elevation; Chl/N and Car/Chl increased with source elevation, whereas Chl a/b declined; Chl a/b was higher for populations on younger soil, suggesting optimization for low N supply. Parallel trends were found for the photosynthetic reactions; light-saturated quantum yield of photosystem II (Φ PSII) and electron transport rate (ETR) increased with source elevation. Correlations of the concentrations of photosynthetic pigments, pigment ratios, and photosynthetic function across the ecotypes indicated a stoichiometric coordination of the components of the light-harvesting antennae and reaction centers. The constellation of coordinated morphological, biochemical and physiological properties was expressed in the leaf reflectance and transmittance properties in the visible and near-infrared wavelength region (400–950 nm), providing an integrated metric of leaf status among and between plant phenotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Leaf morphology and biochemistry often vary in predictable ways across environments, apparently to optimize carbon balance over leaf lifetimes in given conditions. In principle, coordinated variation in leaf features (structure, composition and function) is typically strongest across species, while within-species variation is generally constrained, hence limiting species to particular environmental settings (Field and Mooney 1986; Evans 1989b; Reich et al. 1997; Niinemets 2001; Wright et al. 2004). Hypothetically, this coordination of leaf traits should also occur across populations of a species that span an extensive environmental range to maximize net photosynthetic capacity and overall fitness. Metrosideros polymorpha Gaud. (Ohia; Myrtaceae), the dominant tree species in Hawaiian ecosystems, grows across a wide range of environmental conditions, from warm and wet to cold and dry climates on young, nutrient-poor lava flows as well as on older, nutrient-rich volcanic substrates, making it ideal for studies of within-species variation across environments (Mueller-Dombois 1987; Kitayama and Mueller-Dombois 1995; Vitousek 2004). The aim of this work was to determine the extent of genetic variation in leaf traits, across populations of M. polymorpha, and the degree to which in leaf traits is intercorrelated, through the use of a common garden.
One major locus of variation for leaf traits is specific leaf area (SLA = leaf area/dry mass). In particular, foliar concentrations of nitrogen (N) and photosynthetic pigments should be related to SLA, which is inversely related to leaf thickness and density. At a given cellular composition, leaves of lower SLA typically have higher N and pigment concentrations per area due to the additional thickness of mesophyll tissue; on the other hand, leaves of higher SLA tend to be less dense, with thinner cell walls, and commonly have higher N and pigment concentrations per mass (reviewed by Evans and Poorter 2001; Wright et al. 2004; Niinemets and Sack 2006). Thus, when sampled across environments, a constellation of leaf traits may vary in concert with SLA.
In addition to SLA-related traits, the composition and function of the photosynthetic apparatus often varies across environments (Poorter and Evans 1998; Niinemets 2001; Walters 2005). Species vary in the allocation of N within chloroplasts: on average across species, about half of foliage N is allocated to photosynthetic components, with about a third of this pool in light harvesting components dominated by Chl a and b and photophosphorylation complexes, and two thirds in Calvin cycle components, especially Rubisco (Evans 1989b; Evans and Seemann 1989; Warren and Adams 2004). Other pigments that do not contain N are also important potential determinants of realized photosynthetic capacity; carotenoids (Car) aid in light capture or dissipate excess energy, thus limiting damage to the photosynthetic apparatus (Björkman and Demmig-Adams 1995; Demmig-Adams and Adams 1996), and anthocyanins shield leaves from excess light (Chalker-Scott 1999). Functional variation in the photosynthetic apparatus can be assessed with pigment ratios (e.g., Young and Britton 1990; Anderson et al. 1995; Evans and Poorter 2001). The ratio of Chl/N indicates the proportion of N allocated to light harvesting, whereas the Chl a/b ratio reflects the proportion of chlorophyll bound by photosystem II cores relative to light-harvesting complexes. Additionally, Car/Chl indicates acclimation and adaptation to different environmental conditions, either towards increased light capture (low value) or excess energy dissipation (high value). Across species or within a canopy, variation in pigment concentration and light-harvesting capacity has been linked with differences in overall photosynthetic capacity (Hikosaka 2004; Warren and Adams 2004, and many others).
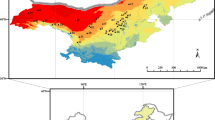
We determined the extent to which variation in leaf traits across populations of M. polymorpha were governed by genetic differences, rather than by environmental forcing, by studying plants in a 13-year-old common garden experiment located in Volcano, HI, developed from seeds collected from populations across an elevation–soil age gradient on the windward slopes of Mauna Loa volcano (Fig. 1). Previous studies at the common garden and source sites found strong genetic variation across the populations in leaf size, pubescence, SLA, foliar N and photosynthetic carbon assimilation rates (Kitayama et al. 1997; Cordell et al. 1998). Leaf size decreased with source elevation, the degree of pubescence increased, SLA declined, and area-based foliar N concentration and carbon assimilation rate increased. Variation across these populations in photosynthetic pigment concentrations and light reactions has not been previously characterized. To build upon previous work, we determined the genetic variation across source populations in the concentrations of pigments, Φ PSII and ETR in relation to the variation in SLA and N. We also sought linkages between leaf biochemical and physiological characteristics and their spectral reflectance and absorption properties, the latter being two integrative measures of overall leaf functional status. Previous work has shown that the amount and quality of visible light reflected from leaves is linked to leaf structure, N and pigment concentrations (Gates et al. 1965; Curran 1989); indices derived from narrowband reflectance and transmittance are often correlated with foliar concentrations of N and Chl (Markwell et al. 1995; Richardson et al. 2002; Sims and Gamon 2002; Gitelson et al. 2003; le Maire et al. 2004), Car (including specific xanthophyll pigments) and anthocynanin, as well as with the light-use efficiency of photosynthetic carbon gain (Gitelson et al. 2001, 2002; Merzlyak et al. 2003). Our study thus included leaf reflectance and absorption measurements to evaluate if spectral patterns of light use correlate with leaf biochemical constituents in a way that is indicative of photosynthetic capacity across distinct phenotypes of M. polymorpha.
Materials and methods
Study site
Our analysis focused on trees from 14 source populations of M. polymorpha in a common garden at the Hawai’i Volcano Experimental Station located in Volcano, HI (1,190 m above sea level). The mean annual precipitation was 4,300 mm year−1, and the photosynthetic photon flux density (PFD) varied between 2,100 μmol m−2 s−1 with full sun to 700 μmol m−2 s−1 with moderate cloud cover (Cordell et al. 1998). Plants were grown from seeds collected in 1991 along the east slope of Mauna Loa volcano from five elevations and two substrate ages, and from two varieties with distinct leaf types, pubescent and glabrous (Fig. 1). Five trees were selected from each population, as defined by the original sites’ elevation (meters above sea level), substrate age (young: range 114–140 years; or old: range 2,000–3,200 years), and the leaf type of the parent tree (pubescent or glabrous; Table 1).
Leaf nutrients and morphology
Small branches were collected from sunlit-exposed regions of the canopy and placed in plastic bags on ice for transport to the lab. Samples (35–75 leaves) were scanned for leaf area determination and dried at 70 °C for at least 48 h before weighing for dry mass and SLA determination (leaf area/dry mass). Dried leaves were ground in a 20-mesh Wiley mill, and subsets were analyzed for N concentration using a standard Kjeldahl sulfuric acid/cupric sulfate digest (using an Alpkem autoanalyzer; O-I Analytical, College Station, TX, USA).
Leaf pigments and physiology
Leaf discs were collected for determination of pigment concentrations. Three discs (0.8–1.1 cm2) were sampled from each of three leaves from each tree, and were immediately frozen on dry ice in the field, and then stored at −80 °C until analysis. Frozen discs were ground in 100% acetone with a small amount of quartz sand and MgCO3 to prevent acidification in a chilled mortar. Following centrifugation for 3 min at 3,000 rpm, the absorbance of the supernatant was measured using a dual-beam scanning UV–vis spectrophotometer (Lambda 25, PerkinElmer Ltd., Beaconsfield, UK). Chl a and b and total carotenoid concentrations were determined using a multiwavelength analysis at 470, 645, 662 and 710 nm (Lichtenthaler and Buschmann 2001). The absorption at 529 nm was used for the determination of anthocyanin concentration (Sims and Gamon 2002).
To determine the effective quantum yield of photosystem II and the light-saturated rate of photosynthetic electron transport, Chl a fluorescence (at 690 nm) was measured on one or two fully expanded, sunlit leaves per tree on two consecutive sunny days (PFD > 2,000 μmol m−2 s−1; Mini-PAM, Walz, Effeltrich, Germany). Leaves were chosen from a similar canopy location to that used for the other measurements. Measurements were taken between 10:00 and 12:00 (morning) and repeated between 13:00 and 15:00 (afternoon) on similar leaves of the same plants. PFD was measured inside the measuring field using the quantum sensor of the Mini-PAM. The effective quantum yield of PSII ( \( \Phi _{{{\text{PSII}}}} = \Delta F/{F}\ifmmode{'}\else$'$\fi_{{\text{m}}} \)) was calculated as \( ({F}\ifmmode{'}\else$'$\fi_{{\text{m}}} - F)/{F}\ifmmode{'}\else$'$\fi_{{\text{m}}} \), where F is the fluorescence yield of the light-adapted sample, and F′m is the maximum light-adapted fluorescence yield when a saturating light pulse (intensity ∼4,000 μmol m−2 s−1, 0.8 s duration) is superimposed on the current ambient irradiance (Genty et al. 1989; Schreiber and Bilger 1993). The apparent rate of photosynthetic electron transport of PSII was calculated as \( {\text{ETR}} = \Delta F/{F}\ifmmode{'}\else$'$\fi_{{\text{m}}} \times{\text{PFD}} \times 0.5 \times {\text{APAR}} \), where the factor 0.5 assumes equal excitation of both PSI and PSII, and APAR is the fraction of solar radiation absorbed by the photosystems. APAR was measured on each leaf using a spectroradiometer and integrating sphere (see following section).
Leaf optical properties
Full-spectrum reflectance measurements were used to calculate indices that relate to leaf chlorophyll and carotenoid concentrations. Leaf optical properties were measured on three leaves per tree from 400 to 800 nm using a field spectrometer with 1.4 nm sampling (Analytical Spectra Devices Inc., Boulder, CO, USA) and an integrating sphere (LI-COR LI-1800, Lincoln, NE, USA). Hemispherical reflectance and transmittance of each sample were measured within 5 min of collection from each tree. The spectra were calibrated for stray light, and were referenced to a calibration block in the integrating sphere (Asner et al. 1998). The spectra were used to calculate three spectral indices, the simple ratio (SR; R 800/R 680; Blackburn et al. 1998), the red–green ratio (R:G; ∑(R 600–R 699)/∑(R 500–R 599); Gamon and Surfus 1999), and the photochemical reflectance index [PRI; (R 531 − R 570)/(R 531 + R 570); Gamon et al. 1990], where R is the reflectance value at the respective wavelength. APAR was calculated as the leaf absorption in the 400–700 nm range (1 − reflectance − transmittance). Total Chl (a + b) concentration was also assessed in situ using a SPAD-502 chlorophyll meter, using the transmission of light through a leaf at 650 and 920 nm (Minolta Camera Co., Osaka, Japan; Marquard and Tipton 1987). For each sampled tree, one leaf was measured; three SPAD measurements were averaged for the right side of each leaf held adaxial side up.
Statistical analysis
Morphological, biochemical and biophysical measurements were averaged by population. One-way analyses of variance (ANOVA; Zar 1999) were used to test for differences in leaf traits between pubescent and glabrous forms from the same source elevation (sites included in this analysis: young substrate, 700 and 1,280 m; old substrate 100 and 1,280 m), and to test for differences between pubescent populations from lower (100–1,280 m) versus higher elevations (1,980 and 2,470 m). For populations with the pubescent form, which were available from sources across the full matrix of elevations and substrate ages, an ANOVA was used for each measured trait to test for differences between source elevations and substrate ages, and any interaction. This analysis was not performed for the glabrous populations, as these were not available for higher source elevations (Table 1). Relationships between leaf traits were evaluated using Pearson correlations and Spearman rank correlations (Abuzar and Al-Ghunaim 1997) and regression analyses.
Results
Leaf composition and chlorophyll fluorescence
Leaf pigment concentrations and SLA differed substantially between pubescent and glabrous leaf types; the pubescent leaves had a 31% lower SLA, and 48% higher Chl and 43% higher Car concentration per area (p < 0.05; Fig. 2; Table 2). Mass-based pigment concentrations showed similar trends, but varied less strongly (Table 2). Pigment ratios also varied between leaf types: pubescent leaves had 31% higher Chl/N, 8% lower Car/Chl and 9% lower Chl a/b ratios than glabrous leaves (p < 0.05; Fig. 3; Table 2). In contrast, mass- and area-based N and anthocyanin concentrations, and Φ PSII and ETR were similar between pubescent and glabrous leaves (Table 2).
Genetic variation across M. polymorpha populations from sources varying in elevation and soil substrate ages: in A specific leaf area, B foliar N concentration per area (and inset per mass), C total chlorophyll (a + b) concentration per area (and inset per mass), and D morning electron transport rate (ETR; inset morning effective quantum yield of PSII (Φ PSII)). Symbols represent mean values ± standard error for pubescent (closed symbols) and glabrous (open symbols) leaf-types, from young (triangles) and old (circles) source substrates
Genetic variation across M. polymorpha populations from sources varying in elevation and soil substrate ages in A Chl/N ratio, B Car/Chl ratio, and C Chl a/b ratio. Symbols represent mean values ± standard errors for pubescent (closed symbols) and glabrous (open symbols) leaf types, from young (triangles) and old (circles) source substrates
Area-based foliar concentrations of N and pigments increased with increasing source elevation, indicating increasing allocation to photosynthetic capacity per area at higher source elevations, concurrently with the decrease in SLA (Fig. 2A–C). Populations with pubescent leaves displayed the most variation in all traits, and could be analyzed in detail for differences across the full range of source elevations and substrate ages. For pubescent populations, SLA decreased from the lowest to highest elevation (100–2,470 m) by 35% on average, from 44.2 ± 1.8 to 28.7 ± 1.2 cm2 g−1 (mean ± SE), while area-based N and pigment concentrations doubled, increasing from 1.8 to 2.7 g m−2 for N; and from 33.4 ± 2.1 to 65.1 ± 3.7 μg cm−2 and 7.5 ± 0.4 to 13.9 ± 0.8 μg cm−2 for Chl and Car respectively (Fig. 2d; Table 2). Similarly, for pubescent leaves, Φ PSII and ETR increased with source elevation by 55 and 46%, respectively (Fig. 2D and inset, Table 2). Notably, the elevational differences in pigment concentrations were linked with the strong differences observed in SLA; concentrations of N and pigments expressed on a mass basis did not vary systematically with elevation (Fig. 2, insets B and C; Table 3; data for anthocyanins not shown). The variation in SLA and SLA-related traits may be partially attributable to the previously documented increase in degree of pubescence with source elevation (Geeske et al. 1994) as well as to the increase in leaf thickness for trees of higher source elevations (Cordell et al. 1998). In addition to these compositional differences linked with SLA, pigment ratios also changed with elevation; Chl/N increased by 39%, and Car/Chl and Chl a/b decreased by 8 and 9%, respectively (p < 0.05; Fig. 3, Table 2).
Populations from contrasting source substrates were similar in most leaf traits, including SLA, N and pigment concentrations, and most pigment ratios and chlorophyll fluorescence parameters, except for Chl a/b ratio, which was generally higher in populations from younger than older source substrates (p < 0.05). However, for area-based N, source substrate age interacted with source elevation, and this was often the case for pigments considered on a mass basis. Populations from younger substrate displayed greater variation than those from older substrate (sensu Raich et al. 1997), possibly due to adaptation to source nutrient limitation (Table 3).
Leaf composition and chlorophyll fluorescence correlations
Correlations were found across the 14 populations of M. polymorpha among SLA, concentrations and ratios of N and pigments, on both an area and mass basis, and chlorophyll fluorescence parameters (Table 4). Some correlations were extremely strong: on an area basis, N explained 90% of the variation in Chl (p < 0.001). Many traits were tightly correlated with SLA, and relationships among compositional traits were stronger when quantified on an area than on a mass basis (Fig. 2; Table 2). Relationships were also stronger when M. polymorpha populations with pubescent leaves were considered separately.
The effective quantum yield of photosystem II (Φ PSII) was measured to assess the function of the photosynthetic light reactions, as it typically correlates with the light-saturated rate of photosynthetic electron transport (ETR), and, at high light intensities, with carbon assimilation rate (Niinemets and Kull 2001; Walters 2005). The Φ PSII and ETR measured in the morning were correlated with leaf composition traits (Fig. 2c, d). Φ PSII and ETR values in the afternoon were significantly depressed relative to values in the morning (on average, by 35%, p < 0.05; paired t-test; Table 2). Morning values of Φ PSII and ETR were correlated with area-based concentrations of N, Chl, and Car, with r 2 values ranging from 0.43 to 0.44 (Table 4). Φ PSII and ETR were also correlated with mass-based Chl and Car concentrations, but not with mass-based N concentration. Neither Φ PSII nor ETR showed a relationship with anthocyanin concentration (data not shown). Chl/N, Chl a/b, Car/Chl were also correlated with Φ PSII and/or with ETR (Table 4).
Leaf optical properties
Leaf biophysical and biochemical traits were correlated with spectral properties measured in the visible to near-infrared wavelength region (400–950 nm), calculated as reflectance or transmission indices (SR, R/G, PRI, SPAD) and APAR. Spectral indices related primarily to Chl and N (SR, R/G, SPAD) were generally intercorrelated (r 2 range 0.54–0.88) and significantly correlated to SLA (r 2 range 0.49–0.90; Table 4). Spectral indices were better correlated with concentrations of N and photosynthetic pigments on an area than on a mass basis (r 2 range 0.30–0.90 and 0.05–0.74, respectively). Additionally, indices were correlated with Φ PSII and ETR (r 2 range 0.37–0.67). Leaf optical properties did not relate well to anthocyanin concentration (r 2 = 0.07–0.36). We did not have the ability to measure individual xanthophyll pigments in our study, and the PRI is thought to be best correlated with transformations among these pigments (Gamon et al. 1990). However, we found that the PRI was inversely correlated with Car/Chl ratio (r = −0.80 to −0.83), which may be a close proxy to xanthophyll pigment ratios (Gamon and Surfus 1999, Sims and Gamon 2002). Overall, correlations between leaf optical, biochemical and physiological properties were slightly weaker when considering pubescent leaf type alone and were not significant for most traits within the glabrous leaf type alone, probably as a result of low power due to the narrower ranges of values for the measured traits (analyses not shown).
Discussion
Leaf morphology, composition and function are often coordinated to maximize efficiency of resource capture and retention (e.g., Chapin et al. 1987; Wright et al. 2004). In particular, relationships among SLA, N concentration and net photosynthetic carbon assimilation rates have been observed across species globally, suggesting general functional relationships within plants. Typically, mass-based N concentration and mass-based photosynthetic rate tend to correlate positively with SLA, while area-based N concentration and area-based photosynthetic rate tend to correlate negatively with SLA (Field and Mooney 1986; Reich and Walters 1994; Reich et al. 1997; Niinemets 2001). This study demonstrates strong genetically based variation in biochemical, physiological, morphological, and optical traits across populations of M. polymorpha. Previous studies have shown genetically based variation in common garden M. polymorpha populations across elevations and soil ages for SLA, N-concentration and photosynthetic carbon assimilation rate (Kitayama et al. 1997; Cordell et al. 1998). This study extends those findings to pigment concentrations, pigment ratios, chlorophyll fluorescence parameters, and optical properties, and demonstrates the coordination of these traits across populations of this extremely variable species. The variation in leaf traits across the common garden trees represents genetic differences apparently related to adaptation of source populations to different climatic conditions, including differences in elevation, temperature, precipitation, substrate age, and nutrient supply.
The variation observed in leaf structure and photosynthetic capacity across the 14 populations of M. polymorpha was linked with differences in leaf type, source elevation and substrate age. Further, much of the variation was linked with differences in SLA values. Pubescent leaves had a lower SLA than glabrous leaves due to greater leaf thickness and the added mass of pubescence (Geeske et al. 1994; Kitayama et al. 1997), and they also had lower concentrations of N and pigments per area. Plants at elevation had lower SLA and associated pigment concentrations per area. The trends with elevation were not apparent for mass-based N and pigment concentrations, indicating that the area-based trends were primarily linked with differences in mesophyll thickness, with leaves of lower SLA at higher elevation having more or larger layers of cells containing more chloroplasts per leaf area (Cordell et al. 1998; Evans et al. 2004). Thicker leaves with lower SLA are adapted to high-irradiance conditions by having more mesophyll layers, a typically larger mesophyll surface area for CO2 absorption, and greater N and pigment concentrations per area for light capture and carbon assimilation (Evans and Poorter 2001). Lower SLA leaves may also be adapted to lower temperatures and lower soil resource availability by having greater photochemical and mechanical protection as well as longer lifespans (Field and Mooney 1986; Reich et al. 1997; Wright et al. 2004). We note that the elevational trends across populations of M. polymorpha in SLA and area-based concentrations, but not in mass-based concentrations, indicates that changes in SLA and SLA-linked composition traits drive adaptation across ecotypes, as previously reported to be the case within species sets and from given lineages (e.g., across 11 species of Hawaiian lobeliads, Givnish et al. 2004); and across species globally (Wright et al. 2004, 2005).
A novel finding of this study is the correlation between N and pigment concentrations with Φ PSII and ETR across the 14 M. polymorpha populations. Notably, pigment concentrations were correlated with Φ PSII and ETR only during peak daily function. Indeed, Φ PSII and ETR were higher in the morning, indicating an afternoon depression in photosynthetic function in M. polymorpha similar to the depression commonly found for carbon assimilation rates (Farquhar and von Caemmerer 1982), corroborating trends found in mangrove trees (Nichol et al. 2006), and grape leaves (Dobrowski et al. 2005), possibly due to an increase in nonphotochemical quenching. The correlations among leaf traits were stronger on an area basis and weaker on a mass basis (Fig. 2, Table 4) (Wright et al. 2004). For example, Chl and N concentrations were well correlated (r 2 = 0.90) on an area but not on a mass basis. As for the trends with source elevation described above, these findings for the intercorrelation of traits highlight the fact that much of the adaptation of leaf features across populations of M. polymorpha is in SLA-linked traits, suggesting that coordination is likely driven by differences in mesophyll layers and a higher number of chloroplasts per surface area in thicker leaves (Fig. 2, Table 4). We note that the correlations of N and pigment concentrations with Φ PSII and ETR are not likely to be causal. The increased Chl concentration in leaves of high source elevation, and the greater allocation of N to Chl and lower Car/Chl and Chl a/b indicates the association of high Chl with light harvesting rather than processing center reactions. A higher investment of Chl in light harvesting would not drive higher electron transport at saturating irradiance, because in fact a large proportion of the Chl in the light harvesting antennae is superfluous for driving the light reactions (Evans 1989a; Anderson et al. 1995; Baker et al. 2004). We propose that the correlations across M. polymorpha populations between N, pigment concentrations, Φ PSII and ETR suggests stoichiometric relationships that hold between the concentration of Chl in the light harvesting antenna and the much lower concentration of Chl that determines the reaction center function; these structured relationships are evident from the narrow variation in pigment ratios described above. These inter-relationships among SLA, N, pigment concentrations, Φ PSII, and ETR highlights the existence of a constellation of functional leaf traits that shifts in a coordinated way during the adaptation of M. polymorpha populations to diverse environmental conditions.
Not all of the observed variation in leaf traits was linked with SLA. Pubescent plants had higher leaf pigment and N concentrations compared to glabrous plants (Fig. 2, Table 2), and had higher Chl/N, and lower Chl a/b and Car/Chl ratios (Figure 3). These differences may arise because the pubescence, present on both the abaxial and adaxial leaf surfaces, may serve to change the spectral quality of incoming radiation as well as reflecting a proportion of it, thereby minimizing harm to the photosynthetic apparatus (Ehleringer 1984; Karabourniotis and Bornman 1999), and, in effect, producing a benefit for these leaves to invest in light harvesting. Indeed, APAR was higher in pubescent than in glabrous leaves, demonstrating increased light capture despite higher reflectance from pubescence (G. Asner, unpublished data). The relative contribution of higher biochemical concentration or greater pubescence to APAR was not evaluated in this study.
Elevational trends were also found for Chl/N and pigment ratios, and for chlorophyll fluorescence parameters. As described above, leaves at higher elevation had higher allocation of pigments and N to light harvesting (higher Chl/N, lower Chl a/b, lower Car/Chl). These trends, independent of SLA or pigment concentrations alone, indicate that adaptation to a range of elevations is associated with differences in light capture and use. A decrease in the Chl a/b ratio indicates an increase in photosystem II light harvesting complexes (LHCII) relative to processing centers, because LHCII contains more Chl b than the PSII chlorophyll-binding proteins (Evans 1989a; Evans and Seemann 1989; Terashima and Hikosaka 1995). The adaptation of pigment ratios toward light harvesting at high elevation was surprising, given the expectation of higher typical irradiances at higher elevation (Raich et al. 1997). In fact, biochemical adaptation to high irradiance for a given species generally results in the opposite patterns—a decreased Chl/N ratio and an increased Chl a/b ratio as plants shift resources from light harvesting to photochemical processing (Terashima and Evans 1988; Hikosaka 2004; Walters 2005). For M. polymorpha populations at higher elevation, the high capacity for light harvesting suggested by their higher Chl/N and lower Chl a/b may allow these plants to capture and utilize high irradiance. Indeed, the lower Car/Chl for plants of higher source elevation suggests that adaptation for increased light energy dissipation is not necessary for this species at higher elevations. We note that our studies in the common garden represent only baseline genetic differences as expressed at the mid-elevation common garden site. Further studies need to be made with common gardens across elevations to determine whether the different populations show different plasticities, and whether the trends found here would differ across elevations.
A significant difference was also found across source substrates in the Chl a/b ratio. The highest Chl a/b ratios were for populations from younger substrates, which have lower N and water availability than the older, more developed soils (Vitousek et al. 1995; Harrington et al. 2001). This indicates adaptation toward an optimized photosynthetic apparatus in high light environments, because N limitation, such as found at high-elevation, nutrient-poor sites, would favor allocation away from PSII light harvesting and toward Rubisco and other carbon assimilation reaction components (Field and Mooney 1986; Terashima and Evans 1988; Evans 1989a; Hikosaka and Terashima 1996; Kitajima and Hogan 2003). Our findings for M. polymorpha populations are the first evidence to our knowledge of this optimization taking place across naturally adapted populations of a given species, and support other findings for the acclimation of a species grown at high irradiance but under differing N supply (Kitajima and Hogan 2003).
Leaf optical properties
Across the populations sampled, variations in leaf physical, biochemical and physiological properties were linearly correlated with basic reflectance (SR, R/G, PRI), transmittance (SPAD) indices and APAR in the visible near-infrared wavelength region (400–950 nm). This finding builds off a rich history of studies focused on the estimation of leaf biochemical and physiological properties utilizing spectral indices (see reviews by Sims and Gamon 2002; Ustin et al. 2004). The R/G ratio was initially developed as an index of anthocyanin content (Gamon and Surfus 1999), and integrates leaf spectral reflectance over broad red (600–699 nm) and green (500–599 nm) wavelength regions. In contrast, the SR utilizes narrow red and near-IR bands at 680 and 800 nm, respectively (Blackburn 1998). Similar to the SR index, the SPAD measurements utilized two narrow spectral regions, one near the Chl a optimum (650 nm) and in the near-IR region (920 nm, Marquard and Tipton 1987). Here, transmission through the leaf was measured, thereby integrating internal interactions between light and biochemical constituents within the leaf. The PRI is tuned to capture variations in particular xanthophyll pigments, but this index is also generally sensitive to the relative amounts of all carotenoids versus chlorophylls (Sims and Gamon 2002). Although measured in several different ways, leaf spectral properties were generally well correlated with one another.
All spectral indices of M. polymorpha were best correlated with photosynthetic pigments and N concentration on an area basis, and were especially strong for the R/G ratio and the SPAD measurements. The low correlation between SR and N and pigment concentration is not surprising because, although 680 nm reflectance and transmittance, used in this study for direct comparison to the SPAD wavelengths, is sensitive to Chl a concentration, its low effectiveness is caused by saturation at low Chl concentrations (Jacquemoud et al. 1996). Sims and Gamon (2002) found the use of 705 nm greatly improved the sensitivity of this index. These very generic spectral indices of M. polymorpha were correlated not only with absolute pigment concentrations, but also with the Φ PSII and ETR indices of leaf photosynthetic capacity. Carotenoid pigment (including xanthophyll pigment subgroup) and concomitant reflectance measurements have provided indirect estimates of light-use efficiency in foliage (e.g., Gamon et al. 1990; Sims and Gamon 2002), canopies (Gamon et al. 1997) and forest stands (Asner et al. 2005; Asner et al. 2006).
Many leaf physiology studies have indicated the importance of constituent ratios as key parameters for diagnosing the physiological state of plants during acclimation and adaptation to different environments (reviewed by Hikosaka 2004; Takashima et al. 2004; Walters 2005). The spectral indices we measured were correlated with Chl/N, Car/Chl and Chl a/b ratios, demonstrating a linkage to a variety of stoichiometric adaptations in the leaf photosystem components of this species. In particular, the PRI was best correlated with Car/Chl ratio, which in turn is thought to be a generic indicator of photoprotection and indirectly light-use efficiency (Gamon et al. 1990; 1997). However, in our study the PRI was poorly correlated with Φ PSII and ETR, so further study should focus on the connections between pigments, physiology and reflectance indices in M. polymorpha. Importantly, the optical properties of M. polymorpha showed that its pattern of light capture and loss is convolved with the structural configuration of its leaves as well as the portfolio of biochemical properties expressed between phenotypes.
Conclusions
Genetic adaptation in M. polymorpha has led to a strong variation in leaf structure, composition and function, expressed in a common garden in the absence of environmental forcing. Our study shows that genetic modifications in M. polymorpha led to decreased SLA at higher elevations, with simultaneous modification of SLA-linked traits, including N and pigment concentrations per area, Φ PSII and ETR. Additional adaptation to higher irradiance included increased allocation to light harvesting expressed as increased Chl/N and decreased Car/Chl and Chl a/b ratios. Leaf-level variations in N, pigments, Φ PSII and ETR were clearly linked to spectral properties in the visible and near-IR wavelength regions, demonstrating that the ensemble of biochemical and physiological traits within and among phenotypes are directly expressed in the way the species interacts with its light environment.
The extreme polymorphism in M. polymorpha contributes to this species’ exceptional ability to populate, and often dominate, a wide range of natural environments. The findings of this study extend previous work on this species, highlighting the strong and coordinated adaptability in leaf structure, composition and function across populations. Successful integration of spectral indices with pigment and chlorophyll fluorescence parameters may allow us to remotely sense genetic as well as plastic variation in leaf traits at the canopy scale across a wide range of ecological and environmental conditions. Future studies will further investigate this possibility.
References
Abuzar M, Al-Ghunaim I (1997) Spatial disaggregation of spectral data for haze assessment in an arid environment. Int J Remote Sensing 18:1693–1702
Anderson JM, Chow WS, Park YI (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139
Asner GP, Carlson KM, Martin RE (2005) Substrate and precipitation effects in Hawaiian forest canopies from spaceborne imaging spectroscopy. Remote Sensing Environ 96:497–508
Asner GP, Martin RE, Carlson KM, Rascher U, Vitousek PM (2006) Vegetation–climate interactions among native and invasive species in Hawaiian rainforest. Ecosystems 9:1041–1054
Asner GP, Wessman CA, Archer S (1998) Scale dependence of absorption of photosynthetically active radiation in terrestrial ecosystems. Ecol Appl 8:1003–1021
Baker NR, Ort DR, Harbinson J, Whitmarsh J (2004) Chloroplast to leaf. In: Smith W, Vogelmann TC, Critchley C (eds) Photosynthetic adaptation: chloroplast to landscape, vol 178. Springer, Berlin Heidelberg New York, pp 89–104
Björkman O, Demmig-Adams B (1995) Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In: Schultze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin Heidelberg New York, pp 17–47
Blackburn GA (1998) Quantifying chlorophylls and carotenoids at leaf and canopy scales: an evaluation of some hyperspectral approaches. Remote Sensing Environ 66:273–285
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress. Photochem Photobiol 70:1–9
Chapin FS III, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37:49–57
Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM (1998) Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia (Berlin) 113:188–196
Curran PJ (1989) Remote sensing of foliar chemistry. Remote Sensing Environ 30:271–278
Demmig-Adams B, Adams WWI (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Dobrowski SZ, Pushnik JC, Zarco-Tejado PJ, Ustin SL (2005) Simple reflectance indices track heat and water stress-induced changes in steady-state chlorophyll fluorescence at the canopy scale. Remote Sensing Environ 97:403–414
Ehleringer JR (1984) Ecology and ecophysiology of leaf pubescence in North American plants. In: Rodriguez E, Healey PL, Mehta I (eds) Biology and chemistry of plant trichomes. Plenum, New York, pp 113–132
Evans JR (1989a) Partitioning of nitrogen between and within leaves grown under different irradiances. Aust J Plant Physiol 16:533–548
Evans JR (1989b) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In: Briggs WR (ed) Photosynthesis. Allan R. Liss Inc., New York, pp 183–205
Evans JR, Vogelmann TC, Williams WE, Gorton HL (2004) Chloroplast to leaf. In: Smith W, Vogelmann TC, Critchley C (eds) Photosynthetic adaptation: chloroplast to landscape, vol 178. Springer, Berlin Heidelberg New York, pp 15–41
Farquhar GD, von Caemmerer S (1982) Modeling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology (NS), vol 12B. Springer, Berlin Heidelberg New York, pp 549–587
Field C, Mooney HA (1986) The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Gamon JA, Field CB, Bilger W, Björkman A, Fredeen AL, Peñuelas J (1990) Remote sensing of the xanthophyll cycle and chlorophyll fluorescence in sunflower leaves and canopies. Oecologia 85:1–7
Gamon JA, Serrano L, Surfus JS (1997) The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia (Berlin) 112:492–501
Gamon JA, Surfus JS (1999) Assessing leaf pigment content and activity with a reflectometer. New Phytol 143:105–117
Gates DM, Keegan HJ, Schleter JC, Weidner VR (1965) Spectral properties of plants. Appl Opt 4:11–20
Geeske J, Aplet G, Vitousek PM (1994) Leaf morphology along environmental gradients in Hawaiian Metrosideros polymorpha. Biotropica 26:17–22
Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gitelson AA, Gritz Y, Merzlyak MN (2003) Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J Plant Physiol 160:271–282
Gitelson AA, Merzlyak MN, Chivkunova OB (2001) Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem Photobiol 74:38–45
Gitelson AA, Zur Y, Chivkunova OB, Merzlyak MN (2002) Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem Photobiol 75:272–281
Givnish TJ, Montgomery RA, Goldstein G (2004) Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. Am J Bot 91:228–246
Harrington RA, Fownes JH, Vitousek PM (2001) Production and resource use efficiencies in N- and P-limited topical forests: a comparison of responses to long-term fertilization. Ecosystems 4:646–657
Hikosaka K (2004) Interspecific difference in the photosynthesis–nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hikosaka K, Terashima I (1996) Nitrogen partitioning among photosynthetic components and its consequence in sun and shade plants. Funct Ecol 10:335–344
Jacquemoud S, Ustin SL, Verdebout J, Schmuck G, Andreoli G, Hosgood B (1996) Estimating leaf biochemistry using the PROSPECT leaf optical properties model. Remote Sensing Environ 56:194–202
Karabourniotis G, Bornman JF (1999) Penetration of UV-A and UV-B and blue light through the leaf trichome layers of two xeromophic plants, olive and oak, measured by optical fibre probes. Physiol Plant 105:655–661
Kitajima K, Hogan KP (2003) Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ 26:857–865
Kitayama K, Mueller-Dombois D (1995) Vegetation changes along gradients of long-term soil development in the Hawaiian montane rainforest zone. Vegetatio 120:1–20
Kitayama K, Pattison R, Cordell S, Webb D, Mueller-Dombois D (1997) Ecological and genetic implications of foliar polymorphism in Metrosideros polymorpha Guad. (Myrtaceae) in a habitat matrix on Mauna Loa, Hawaii. Ann Bot 80:491–497
le Maire G, Francois C, Dufrene E (2004) Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sensing Environ 89:1–28
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. In: Current protocols in food analytical chemistry. Wiley, New York, pp F4.3.1–F4.3.8
Markwell J, Osterman JC, Mitchell JL (1995) Calibration of the minolta SPAD-502 leaf chlorophyll meter. Photosynth Res 46:467–472
Marquard RD, Tipton JL (1987) Relationship between extractable chlorophyll and an in situ method to estimate leaf greenness. Hortscience 22:1327–1327
Merzlyak MN, Solovchenko AE, Gitelson AA (2003) Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol Technol 27:197–211
Mueller-Dombois D (1987) Forest dynamics in Hawaii. Trends Ecol Evol 2:216–220
Nichol C, Rascher U, Matsubara S, Osmond B (2006) Assessing photosynthetic efficiency in an experimental mangrove canopy using remote sensing and chlorophyll fluorescence. Trees 20:9–15
Niinemets U (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Niinemets U, Kull O (2001) Sensitivity of photosynthetic electron transport to photoinhibition in a temperate deciduous forest canopy: photosystem II openness, non-radiative energy dissipation and excess irradiance under field conditions. Tree Physiol 21:899–914
Niinemets U, Sack L (2006) Structural determinants of leaf light harvesting capacity and photosynthetic potentials. Prog Bot 67:385–419
Penuelas J, Filella I, Gamon JA (1995) Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol 131:291–296
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia (Berlin) 116:26–37
Raich JW, Russell AE, Vitousek PM (1997) Primary productivity and ecosystem development along an elevational gradient on Mauna Loa, Hawaii. Ecology 78:707–722
Reich PB, Walters MB (1994) Photosynthesis–nitrogen relations in Amazonian tree species. Oecologia 97:73–81
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Richardson AD, Dulgan SP, Berlyn GP (2002) An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol 153:185–194
Schreiber U, Bilger W (1993) Progress in chlorophyll fluorescence research: major developments during the past years in retrospect. Prog Bot 54:151–157
Sims DA, Gamon JA (2002) Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sensing Environ 81:337–354
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Terashima I, Evans JR (1988) Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol 29:143–155
Terashima I, Hikosaka K (1995) Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ 18:1111–1129
Ustin SL, Roberts DA, Gamon JA, Asner GP, Green RO (2004) Using imaging spectroscopy to study ecosystem processes and properties. Bioscience 54:523–534
Vitousek PM (2004) Nutrient cycling and limitation: Hawai’i as a model system. Princeton University Press, Princeton, NJ
Vitousek PM, Turner DR, Kitayama K (1995) Foliar nutrients during long-term soil development in Hawaiian montane rain forest. Ecology 76:712–720
Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56:435–447
Warren CR, Adams MA (2004) What determines rates of photosynthesis per unit nitrogen in Eucalyptus seedlings? Funct Plant Biol 31:1169–1178
Wright IJ et al. (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Wright IJ et al. (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Young A, Britton G (1990) Carotenoids and stress. In: Alscher RG, Cumming JR (eds) Stress responses in plants: adaptation and acclimation mechanisms. Wiley-Liss, New York, pp 87–112
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River, NJ
Acknowledgements
We thank K. Lo and C. Lunch for assistance in the field, D. Turner for assistance with leaf nitrogen determinations, and J. Berry for his important insights and interpretations. This work was supported by the National Science Foundation (DEB-0136957 and IOB-0546784), NASA Terrestrial Ecology and Biodiversity Program Grant (NNG-06-GI-87G), and The Carnegie Institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Robert Pearcy.
Rights and permissions
About this article
Cite this article
Martin, R.E., Asner, G.P. & Sack, L. Genetic variation in leaf pigment, optical and photosynthetic function among diverse phenotypes of Metrosideros polymorpha grown in a common garden. Oecologia 151, 387–400 (2007). https://doi.org/10.1007/s00442-006-0604-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0604-z