Abstract
A central point in life history theory is that parental investment in current reproduction should be balanced by the costs in terms of residual reproductive value. Long-lived seabirds are considered fixed investors, that is, parents fix a specific level of investment in their current reproduction independent to the breeding requirements. We tested this hypothesis analysing the consequences of an experimental increase in flying costs on the foraging ecology, body condition and chick condition in Cory’s shearwaters Calonectris diomedea. We treated 28 pairs by reducing the wing surface in one partner and compared them with 14 control pairs. We monitored mass changes and incubation shifts and tracked 19 foraging trips per group using geolocators. Furthermore, we took blood samples at laying, hatching and chick-rearing to analyse the nutritional condition, haematology, muscle damage and stable isotopes. Eighty-day-old chicks were measured, blood sampled and challenged with PHA immune assay. In addition, we analysed the effects of handicap on the adults at the subsequent breeding season. During incubation, handicapped birds showed a greater foraging effort than control birds, as indicated by greater foraging distances and longer periods of foraging, covering larger areas. Eighty-day-old chicks reared by treated pairs were smaller and lighter and showed a lower immunity than those reared by control pairs. However, oxygen demands, nutritional condition and stable isotopes did not differ between control and handicapped birds. Although handicapped birds had to increase their foraging effort, they maintained physical condition by reducing parental investment and transferred the experimentally increased costs to their partners and the chick. This result supports the fixed investment hypothesis and is consistent with life history theory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major argument of life history theory is that current investment in breeding should be balanced against the negative effects on future reproductive output (Williams 1966; Stearns 1992). For many bird species, the incubation and nestling rearing phases are critical periods for decisions influencing this balance (Drent and Daan 1980; Croxall 1982). Two main mechanisms have been proposed to explain how birds optimise this balance: (1) the ‘fixed investment hypothesis’ posits that parents have a fixed level of investment in their current reproduction to maximise their own survival, independently of breeding requirements (Ricklefs 1987; Mauck and Grubb 1995); and (2) the ‘flexible investment hypothesis’ suggests that parents can alter the level of investment in their current reproduction depending on the breeding requirements (Reid 1987; Johnsen et al. 1994; Weimerskirch et al. 1997). In short-lived passerines, the probability of survival to future reproduction is low, so if chick demands increase we would expect a parallel increase of parental effort at the expense of their survival (Linden and Møller 1989). In contrast, in long-lived species, parents should be more restrictive in increasing effort in current reproductive investment in order to moderate the impact on future reproduction attempts. Thus, seabirds are generally considered fixed investors (Croxall 1982; Chaurand and Weimerskirch 1994; Tveraa et al. 1997).
One possible approach to explore these hypotheses is through an experimental manipulation of the reproductive effort (Partridge and Harvey 1988). An increase in energetic costs may negatively affect life span or lifetime reproductive success, depending on how birds balance the additional costs with the reproductive investment. In seabirds, reproductive costs have often been manipulated by reducing wing areas, increasing wing loading or altering offspring demands (Table 1). To maintain their body condition seabirds can change foraging effort, principally adjusting the duration of the foraging trips (Table 1). Handicapped birds might find more difficulties in recovering body condition after an incubation bout, resulting in longer foraging trips (Tveraa et al. 1997). Similarly, during the chick rearing period, handicapped birds may increase the number and length of foraging trips to maintain their body condition (Weimerskirch et al. 1995, 1999; Duriez et al. 2000). As a result, chicks reared by handicapped adults can show poor nutritional condition and poor development (Mauck and Grubb 1995; Velando 2002). However, how these changes may affect access to food resources, body reserves, muscle condition and oxygen carrying capacity of handicapped adults or their chicks have not been so far addressed. Difficulties in obtaining enough quantity or good quality of food can deplete body reserves of handicapped birds as well as the immune system of their chicks (Alonso-Álvarez and Tella 2001; Silva et al. 2001; Forero et al. 2002). Furthermore, the increment in flight costs due to handicapping may alter haematological constants and inflict muscle damage. Haematocrit and haemoglobin levels increase associated with breeding activity and flight-induced muscle damage is reflected by increased levels of muscular enzymes (Davey et al. 2000; Guglielmo et al. 2001). Recent developments on the study of foraging ecology, diet, nutritional condition and health can elucidate life history trade-offs faced by long-lived species. We can currently investigate foraging strategies by using devices to track movements of birds (e.g. Birdlife International 2004). Changes in diet or foraging area can be investigated through the analysis of stable isotopes of C and N in blood (Forero and Hobson 2003). Furthermore, with the advent of small and powerful autoanalysers, ecophysiological studies are now easier to conduct.
Procellariiformes are particularly appropriate for manipulating breeding and foraging costs and investigating life history trade offs. All the species display specialised life history characteristics: they are long-lived and have delayed maturity, long incubation and chick-rearing periods, one-egg clutches and slow postnatal growth (Warham 1990). Moreover, the large size of many Procellariiform species allows the use of instruments (geolocators or platform terminal transmitters) to track their foraging movements as well as the extraction of enough blood to perform a wide variety of ecophysiological analyses. In the present study, we manipulated flight costs by reducing the wing area of some Cory’s shearwaters (Calonectris diomedea), a medium Procellariiform species. We tested the prediction of the ‘fixed investment hypothesis’ in relation to the trade offs between current and future reproduction with an integrative approach by comparing control and handicapped birds with regards to: (1) foraging strategies; (2) nutritional condition; (3) food resources exploitation; (4) haematology and muscular damage; and (5) return rates and breeding phenology in the subsequent breeding season. Moreover, we also compared the nutritional condition between chicks reared by treated pairs (one of the parents handicapped) and those reared by a control pair (Table 2). The main novelty of our study is the integration of ecological, ecophysiological and reproductive aspects examined in parents and in their chicks over the entire breeding season, as well as on adults at the subsequent breeding season. If the fixed investment hypothesis is true, we can expect a transfer of the breeding costs from the handicapped bird to the partner and their chick. In this case, parental investment of the partner should increase and condition of their chick may deteriorate (Table 2).
Materials and methods
Model species
Cory’s shearwater is a colonial pelagic Procellariiform breeding on the northeast Atlantic and Mediterranean islands. The species shows a high reproductive investment (8 months), long incubation (54 days) and chick-rearing (90 days) periods and a life span of over 30 years. Birds nests in rock crevices and burrows under rocks or soil. Incubation duties are shared by both sexes and when one partner is incubating the other one is foraging. Diet mainly comprises epipelagic and epimesopelagic fish and squid (see Thibault et al. 1997 for more details).
Manipulation of the flying cost
We conducted the study in Gran Canaria (15°47′18″N; 27°50′41″E, Canary Islands, Spain), from April to November 2004 and from May to June 2005 at a breeding colony of about 150 pairs of Cory’s shearwaters. To test the effects of about 5% increase in flying costs on parental investment, we handicapped some birds by clipping the tip of each primary feather, by 3 cm for males and 2.5 cm for females. This threshold is expected to minimise the damage caused by the cost increase while still eliciting a measurable response (Velando 2002; Velando and Alonso-Álvarez 2003). Clipping values for each sex were calculated according to the body size and the mean sexual size dimorphism for this species [body mass (kg): males=0.810±0.07 (n=55), females=0.716±0.05 (n=44), wingspan (m): males=1.26±0.03 (n=55), females=1.23±0.02 (n=44); see Pennycuick 1989 for calculations]. Only 2 of the initial 42 pairs abandoned the nest presumably as a consequence of the treatment.
Experimental procedure
We randomly assigned 14 and 28 breeding pairs to the control or the treated group, respectively. Pairs from the treated group included one handicapped bird and its unmanipulated partner. Birds were handicapped according to the sex until reaching parity. Figure 1 shows the monitoring and the measures carried out throughout the breeding season. We started the experiment at the onset of the laying period. Burrows were visited daily until an egg was laid and the first incubating bird found at the burrow was ringed, weighed, measured and scored for the number of fault bars in all primary and rectrice feathers and, finally, handicapped if appropriate. Bill depth, bill depth at nostril, tarsus, culmen and maximum head length were measured using a digital calliper to the nearest ±0.1 mm. Wing length was measured using a ruler to the nearest ±1 mm. We determined the sex of adult birds using a discriminant function based on morphological measures of 154 birds measured in previous years and sexed with molecular techniques at the same breeding colony (unpublished data, 95% effectiveness, D=1.044 × bill depth + 0.131 × maximum head length −35.803, Wilks Lambda 0.273, χ 2=158.535, P<0.001, positive is male).
Incubation shifts and foraging trips
During the incubation period, we studied the duration of the incubation shifts and foraging trips as well as changes in mass in all birds by daily visits to the nests. One member of each pair was randomly marked with picric acid on the head, allowing us to identify the incubating bird from a distance and thus to infer the number of incubating days without disturbing the bird at each visit. We measured changes in mass by weighing the birds every 3 days until foraging trip departure as well as at the arrival day (González-Solís et al. 2000). Birds were weighed using Pesola spring balances to the nearest ±10 g. We deployed 10 g Geolocators (GLS units) during the incubation phase on 19 control (8 males and 11 females) and 19 handicapped (7 males and 12 females) shearwaters to study their foraging trips. GLS units were equipped with an internal clock, a battery, a photoelectric cell and a microchip, and measured the light levels every 60 s, recording the maximum reading within each 10-min interval (full details in Afanasyev 2004). From this information, 2 positions per day (one corresponding to midday and the other to midnight) can be inferred with an average accuracy of 186 km (Phillips et al. 2004). We tracked only one foraging trip per bird to ensure independence among trips.
Haematology, plasma biochemistry and stable isotopes
Breeding adults were weighed and blood sampled (1 ml) from the braquial vein at three stages (Fig. 1): between 0 and 10 days after laying, between 0 and 3 days after hatching, and between 50 and 60 days after hatching. Eighty-day-old chicks were also blood sampled (1 ml) 24 h after PHA injection (see below). From the total blood volume extracted, 0.9 ml was transferred to a vial with lithium heparin for chemical analysis and 0.1 ml to a vial with 1 ml of absolute ethanol for determining isotopic signatures of N and C.
Blood with lithium heparin was stored at 2–4°C until haematological analyses within 5 h of extraction. We determined haematocrit in a 70-μl capillary after centrifugation for 8 min at 12,000 rpm. Haemoglobin was measured photometrically (Clima 3.01; RAL, Spain) after haemolysis in Drabkin’s solution (Drabkin and Austin 1936). The remaining blood (0.8 ml) was centrifuged at 5,500 rpm for 10 min and the plasma was frozen at –22°C up to 2–3 days until analysed (see Bustamante and Travaini 1994). Plasma triglyceride, cholesterol, total protein, urea and uric acid (Jenni-Eiermann and Jenni 1998; Totzke et al. 1999; Alonso-Álvarez et al. 2002) were determined using a spectrophotometer and commercial kits (Clima 3.01). We also analysed creatinine and alanin aminotransferase enzyme to detect possible muscular damage (Rosskopt et al. 1982).
Blood with absolute ethanol was refrigerated until analyses of stable isotope ratios of nitrogen (δ15N) and carbon (δ13C) to study trophic level and inshore versus offshore foraging (see Forero and Hobson 2003) in the month previous to sampling (Hobson 1993). Blood was dried at 60°C for 24 h to remove ethanol and 0.4 mg of homogenised blood was weighed to the nearest μg and placed into a Sn capsule. The samples were oxidised with CuO and CO3O4/Ag at about 900°C in a Flash EA 1112 Elemental Analyser coupled to a pirolizator TC-EA and a breath bench, through an interface Conflo III Finnigan MAT. NO2 was reduced with Cu at 680°C. The combustion products N2 and CO2 were dried using MgClO4 and transported to a Delta C Finnigan MAT mass spectrometer (Isotopic ratio mass spectrometry; Serveis Científico-Tècnics of University of Barcelona, Spain) which applies international standards, generally run for each of 12 samples; IAEA CH7 (87% of C), IAEA CH6 (42% of C) and USGS 24 (100% of C) for 13C and IAEA N1 and IAEA N2 (with 21% of N) and IAEA NO3 (13.8% of N) for 15N.
Chick condition
We sampled 28 80-day-old chicks; 10 reared by control and 18 by treated pairs. Chicks were ringed, weighed, measured and blood sampled as adults. In addition, chicks were also tested for cell mediated immune response (CMI) by the PHA assay. This test provides a measure of the proliferative response of circulating T lymphocytes to the injected mitogen phytoaemagglutinin (PHA). After injection, a prominent perivascular accumulation of T lymphocytes is followed by macrophage infiltration. CMI was measured as the amount of swelling after a period of 24 h from the injection (Smits et al. 1999). We measured CMI by injecting 0.05 ml of 2 mg/ml PHA (Sigma) in phosphate buffered saline into a marked site on the right external foot web. The thickness of the foot web was measured with a digital micrometer (Mitutoyo, ±0.001 mm) at the injection site just prior to and 24 h after injection. Each measure was repeated three times to reduce measurement error. Since the repeatability of the three measurements was high (intra-class correlation coefficient, r>0.9; Zar 1984), we calculated the PHA response as the mean change in thickness between the day of injection and the following day (Smits et al. 1999). PHA assay was performed 24 h before blood sampling to avoid potential interference of blood extraction on CMI response. Sex of the chicks was determined by molecular procedures (Ellegren 1996).
Effect in the subsequent breeding season
Between 20 May and 15 June 2005, we visited the colony daily and checked all burrows of the colony site to record return rates of adults, whether they were nesting or not, and their laying dates.
Variables definition and statistical analyses
To analyse the effects of the increased cost on the incubation routine, we compared the intra-pair incubation difference between control and treated pairs, i.e. the number of days incubated by the partner A (handicapped) minus partner B (unmanipulated). Intra-pair difference of control pairs was calculated by randomly assigning half of the males to be the partner A and the other half to be the partner B.
We measured the foraging efficiency as the rate of daily mass gain while foraging (foraging efficiency = mass gain foraging / total foraging duration). The total mass gain foraging is the sum of the mass gained in each foraging trip (mass at return – mass at departure). For those birds that we weighed 2 or 3 days before departure, we estimated the mass at departure using the last mass recorded and the proportional daily loss of mass for the appropriate sex (mean daily mass loss: males=15.38 g/day, females=14.25 g/day; calculated for incubating birds that were weighed more than once).
Data on the positions of birds tracked by geolocators were obtained according to BirdLife International (2004) recommendations. Positions were calculated using Multitrace-3/16 light (Jensen Software Systems) by inspecting the integrity of the light curve day-by-day and fitting dawn and dusk times. To filter unrealistic positions, we removed those positions with a velocity index (V i ) above 50 km/h, indicating an unlikely movement out and back from the normal track as defined by the preceding and following position. By this procedure we discarded 7% of 755 positions. The velocity index was calculated as the root of the square velocity average of the segments between the two preceding and the two following positions (see McConnell et al. 1992). Mean velocity for each trip was estimated by averaging the velocities between validated positions. Total trip distance and total trip duration was calculated as the sum of distances and durations between validated positions. We defined the main foraging area at three different levels as the areas encompassing 50, 75 and 95% of validated positions using the Kernel analysis (cylindrical-equivalent projection, cell size of 5,000 m and smoothing factor of 200 m).
To study the treatment effect on nutritional condition, haematological parameters and muscular damage, we calculated the percentage variation at the end of incubation and chick-rearing with respect to the onset incubation. We transformed logarithmically the biochemical, haematological and body mass variables to normalise them. To determine the body size of chicks we used a Principal Component Analysis combining three linear measurements (culmen, tarsus and wing length) and extracting the first principal component (PC1). This accounted for 57.7% of the variance. To evaluate the treatment effect on each variable we used the ANOVA test, including sex and treatment (control or handicapped) as fixed factors. In the variables where sex did not show significant differences, we removed this factor and repeated the analysis. Differences between sexes were not significant unless otherwise indicated. To study potential effects of the experiment on the chicks, we included the treatment of the parents (control or treated) and the sex of the chick as fixed factors. To study the effect of the treatment on the return rates and laying dates between control and treated, we used the χ 2 test (Yates correction; Zar 1984). We used SPSS 11.0 (SPSS for Windows 1999) and the Animal Movement Extension for ArcView 3.2 (Hooge and Eichenlaub 1997) to perform statistical and Kernel analyses, respectively. Results are shown as means±standard deviation except when otherwise indicated.
Results
Incubation routine and foraging efficiency
Intra-pair incubation in treated pairs difference was lower than zero and differed significantly from control pairs (control pairs=0.18±3.82 days, treated pairs=−3.71±5.28 days, F 1,37=4.93, P=0.03), indicating that handicapped birds incubated fewer and foraged more days than their partners as well as than the control birds.
Total mass gained while foraging over the entire incubation did not differ between control and handicapped animals (control birds=335.27±96.87 g, handicapped birds=339.06±70.57 g, F 1,46=0.03, P=0.87), and was significantly greater for females than for males (males=308.72±83.39 g, females=366.12±71.80 g, F 1,46=7.18, P=0.01). Foraging efficiency was 11.08% greater for control than for handicapped birds, although values did not differ significantly (control=14.73±3.54 g/day, handicapped=13.26±3.32 g/day, F 1,46=1.56, P=0.22). The relationship between total mass gain while foraging and total number of days foraging was larger in control than in handicapped birds, although correlation coefficients did not differ significantly between these two groups (Fig. 2; Z=2.11, df=2, P=0.13).
Foraging trips tracked by geolocators
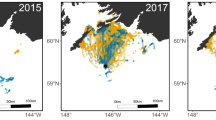
All geolocator positions were located along the Western Sahara coast of Africa (Fig. 3). Foraging areas encompassing 50, 75 and 95% of locations (Fig. 4), trip distance and trip duration were significantly greater for handicapped than for control birds (foraging area 50%, F 1,36=5.58, P=0.02; foraging area 75%, F 1,36=4.56, P=0.03; foraging area 95%, F 1,36=4.78, P=0.03; Fig. 4; trip distance (km): control=1,584±725, handicapped=2,310±877, F 1,36=7.72, P=0.009; trip duration (days): control=7.11±2.86, handicapped=10.19±2.45, F 1,36=12.65, P=0.001). Mean velocity and maximum foraging range did not differ between control and handicapped animals [mean velocity (km/h): control=9.41±2.82, handicapped=9.92±4.61, F 1,36=0.19, P=0.66; maximum foraging range (km): control=787±202, handicapped=858±302, F 1,36=0.72, P=0.42]. None of the variables derived from the tracked movements differed between males and females.
Positions of one foraging trip of 19 control (filled circle) and 19 handicapped (open circle) shearwaters during the incubation period, tracked with geolocators. The cross indicates the breeding site (Gran Canaria, Canary Island, Spain). Note that positions located on the African continent are inaccuracies of the geolocation method
Nutritional condition, muscular damage and trophic ecology of adults
None of the mean increment values in blood biochemistry, haematological, body mass and muscular damage variables differed significantly between control and handicapped adults throughout the experiment except for the cholesterol increment during the chick-rearing period, which was significantly greater for handicapped than for control birds (Table 3). Isotopic signatures of N and C did not differ between both groups (Table 3).
Between sexes, change in haematocrit, haemoglobin and body mass during incubation were significantly greater for females than for males (haematocrit: males=−2.67±5.21%, females=6.36±10.33%, F 1,46=15.65, P<0.001; haemoglobin: males=−9.03±9.01%, females=8.48±13.01%, F 1,46=30.35, P<0.001, body mass: males=−2.71±5.182%, females=8.54±8.02%, F 1,46=15.77, P<0.001). Creatinine increment during the incubation period was significantly greater for males than females (males=63.04±41.10%, females=41.22±27.08%, F 1,45=4.48, P=0.04).
Effect on the chicks
Body size and mass were significantly greater in chicks reared by control than those by treated birds (Table 4) and for male than for female chicks (body size: males=0.63±0.79, females=−0.54±0.83, F 1,24=6.41, P=0.02; body mass: males=929.60±137.07 g, females=761.00±78.81 g, F 1,24=11.40, P=0.002). Cell mediated immune response, measured by the PHA assay, was significantly greater in chicks reared by control than by treated pairs (Table 4). Blood biochemistry, haematology, muscular damage and stable isotope signatures did not differ between chicks (Table 4).
Effects in the subsequent breeding season
The frequency of pairs that returned and bred in the subsequent season after the experiment and their laying dates did not differ between control and treated pairs (return rate: control pairs=75%, treated pairs=71%, n=27, χ 2=2.20, df=1, P=0.12; increment in laying dates between the two consecutive years: control pairs=−0.65±2.05%, treated pairs=−1.65±2.7%, F 1,27=1.81, P=0.21).
Discussion
The results of this study clearly support the ‘fixed investment hypothesis’ as the main strategy in Cory’s shearwaters to balance current and future reproduction, that is, parents invest a fixed amount of effort to a certain reproduction regardless of the breeding requirements of this event. Handicapped shearwaters fulfilled all predictions of this hypothesis: in response to the experimentally increased costs, handicapped birds adjusted their foraging strategies to maintain their body condition, physiological state and survival prospects, and extra costs were partly shared with the partner and mainly transferred to the chick (see Table 2). Changes in foraging strategies of handicapped birds included an increase in foraging trip duration periods and a decrease in incubating time in comparison to the control birds. Moreover, because males and females share the incubation duties, the experimental increase in flight costs also resulted in a compensation of the shorter incubation bouts by the partner, who had to wait for the handicapped bird before engaging in a new foraging trip. In addition, resource predictability (Fig. 2), as indicated by the strength in the relationship between the mass gain and the number of days foraging, was lower in handicapped than in control birds, suggesting a difference in the type or catchability of the resources used. Similar adjustments in foraging strategies were also detected in other experimental studies in seabirds (see Table 1). In addition, we also detected changes in the foraging trips tracked by light level geolocation (GLS). Handicapped birds increased foraging effort: they travelled more days, longer distances and covered greater foraging areas compared to control birds. This increase is presumably related to the need to gather more food for covering the greater energy demands imposed by a greater wing loading (Freed 1981). However, despite the increase in foraging effort and the lower resource predictability of handicapped birds, GLS tracking and stable isotope analyses most likely indicate that there was no shift in their diet or in the location of their foraging areas. All instrumented shearwaters tracked by GLS, control and handicapped, foraged on similar feeding grounds, essentially located on the Western Sahara coast (Fig. 3). This area is one of the main upwelling zones of the Atlantic Ocean, with a high biological productivity and high food availability for seabirds (Barton et al. 1998; Davenport et al. 2002). This result was further corroborated by the absence of differences in δ13C signatures, which are sensitive to latitudinal changes in feeding areas or to changes in offshore-inshore feeding grounds (Forero and Hobson 2003). Moreover, handicapped and control birds did not differ in the δ15N signatures, which are sensitive to changes in diet composition, suggesting that both groups fed on similar prey types (Forero and Hobson 2003). Thus, although handicapped shearwaters increased their foraging effort, all birds foraged on similar areas and obtained similar prey. Probably, the high food availability of the Western Sahara coast helped handicapped birds to maintain body condition to the same extent as control birds.
The maintenance of body condition is a common feature of most experimental studies that manipulated reproductive costs in long-lived bird species (see Table 1). Similar to the results found in the present study, in most cases handicapped birds maintained their body mass during the incubation and during the chick-rearing period (Mauck and Grubb 1995; Tveraa et al. 1997; Weimerskirch et al. 1999; Duriez et al. 2000; Nisbet et al. 2004; Ewing et al. 2005), and apparently transferred the extra breeding cost to the partner or to the chicks. Unlike previous studies, we assessed the nutritional state of the birds through the analysis of blood biochemical parameters related to the dynamics of protein (total protein, uric acid and urea) and fat stores (triglycerides) (Jenni-Eiermann and Jenni 1998; Totzke et al. 1999; Alonso-Álvarez et al. 2002). Apart from cholesterol, none of these differed between control and handicapped birds, confirming that handicapped birds were able to maintain their health state despite of the imposed increase in flying costs.
Cholesterol is a fat store and its increment during chick-rearing phase was greater in handicapped than in control adult birds (Table 3). Variation in this metabolite is generally related to a diet change, body mass change or to endocrino or renal damage (Duncan et al. 1994; Alonso-Álvarez et al. 2002). Causes of the difference in our study are difficult to envisage, since none of the potential explanations agree with other aspects examined in the present study. Body mass did not differ between control and handicapped birds along the breeding season. Diet was also similar between groups according to the analyses of stable isotopes. Finally, possible endocrine or renal damage would be expressed in parallel by an elevation of other metabolites such as alanin aminotransferase or creatinine (Fudge 2000), but these enzymes did not differ between control and handicapped birds. Perhaps cholesterol is more sensitive to subtle changes in diet than stable isotopes, but further experimental research is required to confirm this suggestion.
The increase in foraging effort of handicapped birds may affect oxygen demands and muscular condition. Extra efforts could lead to a decrease in the haematocrit and haemoglobin values as a consequence of an increase in blood oxygen demand (Davey et al. 2000). Besides, muscular damage could be reflected by an increment in muscular enzymes (Rosskopt et al. 1982; Guglielmo et al. 2001). However, none of the haematological parameters and muscular enzymes differed between control and handicapped animals, although handicapped birds tended to increase hamatocrit, which is probably related to the increase in foraging effort caused by the manipulation of flight costs.
Besides the cost transferred to the incubating partner, increased flying costs in handicapped birds can also be reflected in the condition of their chicks. Chicks reared by treated pairs showed lower body size and mass to chicks reared by control pairs. This is probably a consequence of less food being contributed by the handicapped bird, which was not fully compensated by the partner. This result agrees with previous experimental studies on other Procellariiform species where flight costs were experimentally increased. Handicapped birds reduced chick feeding frequency and the chicks of treatment pairs grew less and were lighter than in control pairs (Mauck and Grubb 1995; Weimerskirch et al. 1995). In addition, we found that immune response of chicks reared by treated pairs was lower than those reared by control pairs, suggesting that the development of the immune system of the chicks was adversely affected by the lower food intake (Alonso-Álvarez and Tella 2001). These results show that handicapped birds did not increase the effort to maintain the average level of reproductive investment, but to maintain their body condition at the expense of chick condition.
In conclusion, although handicapped birds had to increase their foraging effort, they maintained body condition by reducing parental investment and transferring the experimentally increased costs to their partners and the chick. Thus, the present study supports the fixed investment hypothesis for shearwaters and is consistent with life history theory predictions.
References
Afanasyev V (2004) A miniature daylight level and activity data recorder for tracking animals over long periods. Memoir Natl Inst Polar Res C Earth Sci 58:227–233
Alonso-Álvarez C, Tella JL (2001) Effects of experimental food restriction and body-mass changes on avian T-cell mediated immune response. Can J Zool 79:101–105
Alonso-Álvarez C, Ferrer M, Velando A (2002) The plasmatic index of body condition in Yellow-legged Gulls (Larus cachinnans): a food-controlled experiment. Ibis 144:147–149
Barton ED, Arístegui J, Tett P, Cantón M, García-Braun J, Hernández-León S, Nykjaer L, Almeida C, Almunia J, Ballesteros S, Basterretxea G, Escánez J, García-Weill L, Hernández-Guerra A, López-Laatzen F, Molina R, Montero MF, Navarro-Pérez E, Rodríguez JM, van Lenning K, Vélez H, Wild K (1998) The transition zone of the Canary Current upwelling region. Prog Oceanogr 41:455–504
BirdLife International (2004) Tracking ocean wanderers: the global distribution of albatrosses and petrels. Results from the Global Procellariiform Tracking Workshop, 1–5 September, 2003, Gordon’s Bay, South Africa. BirdLife International, Cambridge
Bustamante J, Travaini A (1994) Effect of keeping plasma at −20°C on the concentration of blood metabolites. Comp Biochem Physiol 107:661–664
Chaurand T, Weimerskirch H (1994) Incubation routine, body mass regulation and egg neglect in the blue petrel (Halobaena caerulea). Ibis 136:285–290
Croxall JP (1982) Energy costs of incubation and moult in petrels and penguins. J Anim Ecol 63:275–282
Davenport R, Neuer S, Helmke P, Perez-Marrero J, Llinas O (2002) Primary productivity in the northern Canary Islands region as inferred from SeaWiFS imagery. Deep-Sea Res 49:3481–3496
Davey C, Lill A, Baldwin J (2000) Variation during breeding in parameters that influence blood oxygen carrying capacity in shearwaters. Aust J Zool 48:347–356
Drabkin DL, Austin JH (1936) Spectrophotometric studies. V.A. technique for the analysis of undiluted blood and concentrated hemoglobin solution. J Biol Chem 112:105–115
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 80:225–252
Duncan RJ, Prasse KW, Mahaffey FA (1994) Veterinary laboratory medicine clinical pathology, 2nd edn. Iowa State Press, Ames
Duriez O, Weimerskirch H, Fritz H (2000) Regulation of chick provisioning in the thin-billed prion: an interannual comparison and manipulation of parents. Can J Zool 78:1275–1283
Ellegren H (1996) First gene on avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proc R Soc Lond B 263:1635–1641
Ewing AD, Norman FI, Ward SJ, Bunce A (2005) Preliminary investigation of the costs of incubation in the Australasian gannet (Morus serrator) breeding in Port Phillip Bay, Victoria. Emu 105:137–144
Forero MG, Hobson KA (2003) Using stable isotopes of Nitrogen and Carbon to study seabird ecology: applications in the Mediterranean seabird community. Sci Mar 67:23–32
Forero MG, Hobson KA, Bortolotti GR, Donázar JA, Bertellotti M, Blanco G (2002) Food resource utilisation by the Magellanic penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Mar Ecol Prog Ser 234:289–299
Freed LA (1981) Loss of mass in breeding wrens: stress or adaptation? Ecology 62:1179–1186
Fudge AM (2000) Laboratory medicine: avian and exotic pets. Saunders, Philadelphia
González-Solís J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels (Macronectes halli) during the incubation period. Oikos 90:390–398
Granadeiro JP, Nunes M, Silva MP, Furness RW (1998) Flexible foraging strategy of Cory’s Shearwater (Calonectris diomedea) during the chick-rearing period. Anim Behav 56:1169–1176
Guglielmo CG, Piersma T, Williams TD (2001) A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. J Exp Biol 204:2683–2690
Hobson KA (1993) Trophic relationships among high Arctic seabirds: insights from tissue-dependent stable-isotope models. Mar Ecol Prog Ser 95:1–78
Hooge PN, Eichenlaub B (1997) Animal movement extension to Arcview, version 1.1. US Geological Survey, Anchorage
Jenni-Eiermann S, Jenni L (1998) What can plasma metabolites tell us about the metabolism, physiological state and condition of condition of individual birds? An overview. Biol Conserv Fauna 102:312–319
Johnsen I, Erikstad KE, Sæther BE (1994) An experimental study of the costs of reproduction in the kittiwake (Rissa tridactyla). Ecology 76:1636–1642
Linden M, Møller AP (1989) Cost of reproduction and covariation of life history traits in birds. Trends Ecol Evol 4:367–371
Mauck RA, Grubb TC (1995) Petrel parents shunt all experimentally increased reproductive costs to their offspring. Anim Behav 49:999–1008
McConnell BJ, Chambers C, Fedak MA (1992) Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarctic Sci 4:393–398
Mínguez E (1998) The costs of incubation in the British Storm-petrel: an experimental study in a single-egg layer. J Avian Biol 29:183–189
Nisbet ICT, Arnold JM, Galbraith H, Hatch JJ (2004) Responses of known-aged common terns to experimental shortering of the wings. Waterbirds 27:13–20
Partridge PA, Harvey P (1988) The ecological context of life history evolution. Science 241:1449–1455
Pennycuick CJ (1989) Variables needed for flight calculations. In: Pennycuick CJ (ed) Bird flight performance. Oxford University Press, New York, pp 7–17
Phillips RA, Silk JRD, Croxal JP, Afanasyev V, Briggs DR (2004) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:262–272
Reid WV (1987) The cost of reproduction in the glaucous-winged gull. Oecologia 74:458–467
Ricklefs RE (1987) Response of adult Leach’s storm-petrels to increased food demand at the nest. Auk 104:750–756
Rosskopt WJ, Woerpel RW, Rosskopf GA (1982) Haematological and blood chemistry values for commonly kept cockatoos. Calif Vet 36:11–13
Silva MP, Favero M, Copello S, Bastida R (2001) Does access top high-quality pelagic prey increase the breeding success of Kelp Gulls (Larus dominicanus) in the Antarctic Peninsula? Mar Ornithol 29:85–88
Smits JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
SPSS for Windows (1999) SPSS, Chicago
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Thibault JC, Bretagnolle V, Rabouam C (1997) Cory’s shearwater. BWP Update. Oxford University Press, Oxford
Totzke U, Fenske M, Hüppop O, Raabe H, Schach N (1999) The influence of fasting on blood and plasma composition of Herring Gulls (Larus argentatus). Physiol Biochem Zool 72:426–437
Tveraa T, Lorentsen S, Saether B (1997) Regulation of foraging trips and costs of incubation shifts in the Antarctic petrel (Thalassoica antarctica). Behav Ecol 8:465–469
Velando A (2002) Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behav Ecol 13:443–449
Velando A, Alonso-Álvarez C (2003) Differential body condition regulation by males and females in response to experimental manipulations of brood size and parental effort in the blue-footed booby. J Anim Ecol 72:846–856
Warham J (1990) The Petrels: their ecology and breeding systems. Academic, London
Weimerskirch H, Chastel O, Ackermann L (1995) Adjustment of parental effort to manipulate foraging ability in pelagic seabird, the thin-billed prion (Pachyptila belcheri). Behav Ecol Sociobiol 36:11–16
Weimerskirch H, Mougey T, Hindermeyer X (1997) Foraging and provisioning strategies of black-browed albatrosses in relation to the requirements of the chick: natural variation and experimental study. Behav Ecol 8:635–643
Weimerskirch H, Fradet G, Cherel Y (1999) Natural and experimental changes in chick provisioning in a long-lived seabird, the Antarctic Prion. J Avian Biol 30:165–174
Weimerskirch H, Prince PA, Zimmermann L (2000) Chick provisioning by the Yellow- nosed albatross (Diomedea chlororynchos): response of foraging effort to experimentally increased costs and demands. Ibis 142:103–110
Williams GC (1966) Natural selection, the costs of reproduction and refinement of Lack’s principle. Am Nat 100:687–690
Zar JH (1984) Biostatistical analysis. Prentice-Hall, London
Acknowledgments
We thank Suni García, Alejo Pastor and Raül Ramos their field support; Yaiza Amescoa, Pascual Calabuig and Cabildo de Gran Canaria for their logistical support; Gines Viscor for supervising biochemical analyses; Elena Gómez for sexing the chicks; Lluis Jover, Marc Franch, Maria del Mar, and the Asociación Amigos de las Pardelas for their help on different aspects; John Croxall, Vsevolod Afanasyev, Dirk Briggs and Xavier Ruiz for making available the geolocators. Joan Navarro was supported by a postgraduate grant of the Ministerio de Educación y Ciencia (MEyC) of Spain and Jacob González-Solís was supported by a contract of the Program Ramon y Cajal of MEyC and Fondos FEDER. Financial support was provided by the projects REN2002-01164 and BOS2000-0569-CO2-01 from MEyC and by 2001SGR-00091 from the Generalitat de Catalunya. All methods used in this study comply with the current laws of Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl.
Rights and permissions
About this article
Cite this article
Navarro, J., González-Solís, J. Experimental increase of flying costs in a pelagic seabird: effects on foraging strategies, nutritional state and chick condition. Oecologia 151, 150–160 (2007). https://doi.org/10.1007/s00442-006-0559-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0559-0