Abstract
In common with many habitat elements of riverine landscapes, exposed riverine sediments (ERS) are highly disturbed, naturally patchy and regularly distributed, whose specialists are strongly adapted to flood disturbance and loss of habitat due to succession. Investigations of dispersal in ERS habitats therefore provide an important contrast to the unnaturally fragmented, stable systems usually studied. The present investigation analysed the three interdependent stages of dispersal: (1) emigration, (2) inter-patch movement and (3) immigration of a common ERS specialised beetle, Bembidion atrocaeruleum (Stephens 1828) (Coleoptera, Carabidae), in a relatively unmodified section of river, using mark–resight methods. Dispersal was correlated with estimates of local population size and density, water level and patch quality in order to test for condition-dependent dispersal cues. Flood inundation of habitat was found to increase strongly the overall rate of dispersal, and the rate of emigration was significantly higher from patches that were heavily trampled by cattle. Strongly declining numbers of dispersers with distance suggested low dispersal rates during periods of low water level. Dispersal in response to habitat degradation by cattle trampling would likely lead to a higher overall population fitness than a random dispersal strategy. Dispersal distances were probably adapted to the underlying habitat landscape distribution, high-flow dispersal cues and ready means of long-distance dispersal through hydrochory. Species whose dispersal is adapted to the natural habitat distribution of riverine landscapes are likely to be strongly negatively affected by reduced flood frequency and intensity and habitat fragmentation through flow regulation or channelisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal between patches of habitat can have profound consequences for the population and distribution dynamics of a species (Harrison 1991; Hanski and Gilpin 1997). It is a key mechanism underlying theories of risk spreading (den Boer 1968), metapopulation dynamics (Levins 1969) and island biogeography (MacArthur and Wilson 1967) and is of considerable importance for conservation management, especially the assessment of population viability (Shaffer 1981) and fragmentation sensitivity (Henle et al. 2004). As a consequence, there are a large number of studies investigating the dispersal characteristics of rare and threatened species (e.g. Nève et al. 1996; Purse et al. 2003). In comparison, there are relatively few investigations of the dispersal of more common species (Sutcliffe et al. 1997), despite the importance of such information as a baseline against which the factors driving relative rarity can be assessed (e.g. Thomas et al. 1999). Conservation objectives, and the highly managed nature of most developed landscapes, have meant that most dispersal studies concentrate on species in unnaturally fragmented habitats (Dennis et al. 2003). Research on the dispersal characteristics of species adapted to naturally patchy habitats should highlight characteristics that enhance survival in fragmented habitats.
Globally, riverine landscapes cover in excess of 2 million km2 and, in their natural condition, are some of the most biologically productive and diverse ecosystems on earth (Tockner and Stanford 2002). Natural riverine landscapes consist of mosaics of habitats that are patchy, typically linear and, often, regularly distributed (e.g. riffles, pools, meanders and their associated point bars and eroding banks) due to the influence of underlying hydraulic processes (Richards 1982; Ward et al. 2002). Dispersal within linear habitats should be more limited than that expected in habitat networks (Roland et al. 2000), but, in riverine landscapes, flood and flow pulses not only cause disturbances and the modification of habitat mosaics (Junk et al. 1989; Tockner et al. 2000) but can also potentially provide downstream dispersal through hydrochory (e.g. Lude et al. 1999; Goodson et al. 2003). These factors mean that the dispersal processes of riverine habitat specialists are likely to differ from those in other habitat networks.
Exposed riverine sediments (ERS) are frequently inundated areas of relatively un-vegetated, fluvially deposited sand and gravel, which are distributed in distinct patches that are easily delineated from neighbouring lotic and heavily vegetated riparian habitats. In contrast with most riparian habitats (e.g. wet woodland, ox-bow lakes) in heavily modified landscapes, ERS are often relatively unmodified because of their highly disturbed, within-channel nature (but cf. Plachter 1986; Kondolf 1997) and often maintain the patchy but regular distribution typical of natural riverine landscapes (Naiman et al. 1993; Ward et al. 2002). Research into the dispersal and spatial population dynamics of specialised invertebrates of ERS can, therefore, potentially provide highly valuable information on the dispersal adaptations that drive population and distribution dynamics in natural riverine landscapes, in addition to highlighting the likely response of ERS specialists to the degradation of their own habitat.
The availability and distribution of ERS habitat is in a constant state of adjustment in response to flood disturbances, which rejuvenate and redistribute patches, and vegetation succession, which stabilise and therefore remove patches (Plachter and Reich 1998; Brewer et al. 2000). The associated habitat turnover and spatio-temporal variation in patch quality, combined with the patchy distribution of ERS, should select for high rates of dispersal in ERS specialists (cf. Southwood 1962; Henle et al. 2004; Bowler and Benton 2005). Importantly, the high level of variation and relatively low level of isolation in these regularly distributed habitats might favour condition-dependent dispersal in response to environmental quality, as the selective advantage gained from dispersal from low-quality patches is likely to outweigh the costs of movement between patches. Such condition-dependent dispersal is expected to be selected for over a wide range of circumstances and has been observed in a number of empirical studies (Travis and French 2000; Bowler and Benton 2005).
This investigation used mark–resight techniques to study the dispersal dynamics of adults of the specialised, but widespread, carabid beetle Bembidion atrocaeruleum (Stephens 1828) (Coleoptera, Carabidae) in a relatively natural ERS system. The three interdependent stages of dispersal: (1) emigration, (2) inter-patch movement and (3) immigration (Bowler and Benton 2005) were studied separately and correlated with estimates of local population size and density, water level and patch quality in order to test for condition-dependent dispersal cues. The study aimed to understand the factors driving the dispersal dynamics of B. atrocaeruleum and to describe their likely effects on the spatial population structure, and sensitivity to anthropogenic threats.
Materials and methods
Study species
Bembidion atrocaeruleum is almost exclusively associated with ERS habitat, has a widespread distribution and is numerically dominant on ERS in the study area. In the UK its lifecycle is generally thought to be annual, with breeding in the early spring and larvae and teneral adults present during the summer (June–August inclusive). It over-winters as an adult, most probably in less frequently inundated habitats away from the river channel (cf. Andersen 1968). It is a constantly macropterous species, possessing functional flight muscles, and is always capable of flight (Desender 1989). The species ranges over most micro-habitats within patches of ERS (Bates and Sadler 2005) and is known to have an inter-patch dispersal rate indicating that each patch supports a local population (Bates et al. 2005; cf. Harrison 1991).
Design considerations
The above findings of Bates et al. (2005) and Bates and Sadler (2005) showed that using patches of ERS as the main sampling and descriptive unit provides an appropriate study resolution. However, other habitats (e.g. adjoining pasture and woodland) are most probably used as flood refugia and over-wintering sites, so the distribution of ERS patches do not completely correlate with the distribution of the species’ habitat per se (cf. Dennis et al. 2003). Nonetheless, B. atrocaeruleum is only found in riverine landscapes with ERS, and the species spends the vast majority of its active phase living on ERS patches, so the focus on these patches was reasonable.
The extent of the study area strongly influences the characteristics of the measured dispersal parameters (Schneider 2003). Dispersal success is dependent on both the dispersal capabilities of the study species and the landscape distribution of its habitat patches (Addicott et al. 1987). Accordingly, the distribution of patches in the landscape should, in part, determine the scale of study. The relatively regular and tight distribution of patches within the study system enabled a relatively small (∼300 m of river) spatial extent of sampling to be used. This facilitated a high sampling intensity but reduced the range over which distance effects on inter-patch movements could be characterised.
Site description and environmental variation
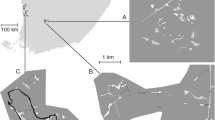
Six patches of ERS in a stretch of the River Severn in the UK (3°25′ E, 52°30′ N) were originally chosen for study, but sampling on one patch (Bar US) was discontinued midway through the investigation (Fig. 1). Despite upstream flow regulation of the Clywedog tributary and historical river engineering works, the stretch has retained its semi-natural, wandering gravel bed (sensu Church 1983) configuration, which is characterised by a range of bar forms.
Eight environmental variables were measured for each bar (Table 1): bar area, bar edge, edge-to-area ratio, distance from study centre, typical sediment size, vegetation cover, elevation range and the presence of heavy cattle trampling. Sediment size was estimated from several photographs of surface sediments using a photo-sieving method (see Petts et al. 2000). Bar area, bar edge, edge-to-area ratio, elevation range (height above base flow), distance from study centre (from the centre of bars to the centre of the study area) and distance between patches (edge to edge) were estimated from a digital elevation model (DEM) of the study reach, created with a Leica differential global positioning system. Patches fell within two categories related to trampling intensity: (1) those where there was no trampling, or trampling of minimal spatial extent (<5% by area), and (2) those that were heavily trampled so that the natural sediment structure was destroyed over most (>50%) of the patch.
Water levels were measured at the Environment Agency stage measurement facility at Caersws (∼250 m downstream of the study site). Daily total rainfall and daily maximum and minimum temperature data were taken from the Meteorological Office sampling stations at Llandinam (3°27′ E, 52°29′ N, 131 m.a.s.l., ∼2.5 km from the study site) and Llanfair Caereinion (3°16′ E, 52°38′ N, 243 m.a.s.l., ∼28 km from the study site), respectively. Air temperature and rainfall varied markedly over the study period, and water levels responded both to reservoir releases (9–17 July 2003) and to rainfall events (Figure in supplementary material). Two events were likely to be of particular significance for the dispersal of B. atrocaeruleum: (1) the complete, extended inundation of all patches following prolonged rainfall in mid-May and (2) the heavy rainfall and associated near inundation of bars 1 and 5 on 24 July 2003 (Figure in supplementary material, Table 1).
Sampling
An earlier investigation (Bates et al. 2005) indicated that a mark–resight approach (e.g. Dreitz et al. 2002) was the most appropriate mark–recapture method, so sampling was separated into marking and recapturing periods. Three mark−recapture periods were conducted over May, June and July 2003 (Table 2), as mark losses after periods of >30 days (Bates et al. 2005) would have biased population estimates. During recapture periods, all traps were open for ∼24 h except for the first recapture period in July, when a high rate of capture meant that some traps could not be processed for ≤48 h. The May marking period was not followed by any recapture periods because the study reach was completely inundated shortly afterwards.
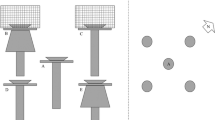
Beetles were captured in 381 modified dry pitfall traps (Bates et al. 2005), which were arranged in grids across the bars at a density of ∼0.06 m−2 (Table 1). The traps were designed to minimise mortality and stress in captured beetles by protecting them from aerial predation, within-trap predation by large arthropods, desiccation, flooding and to prevent beetles escaping. Six dots of non-toxic (Bates and Sadler 2004) paint (extracted from Mark-Tex Tech-Pen ITW Dymon, Kan., USA) applied to the beetles elytra were used to individually mark beetles.
Tipping beetles into water allowed them to be easily picked up and held for marking, after which they were transferred into containers with damp sediment and food (Bates et al. 2005). In marking periods beetles were released in the centre of the bar upon which they had been captured. In recapture periods, beetles were released <0.5 m away from the point of capture. Stress and consequent unusual movement behaviour (Singer and Wedlake 1981) would be more likely after marking, and more time was needed for beetles to re-establish their natural distribution following release in the centre of bars, so longer intervals (at least 5 days) were left between release and recapture periods than between recapture periods (at least 2 days).
Analytical techniques
Bembidion atrocaeruleum disperses between patches (Bates et al. 2005), so the open population programme JOLLY (Pollock et al. 1990a, b), which uses variants of the Cormack–Jolly–Seber model (Cormack 1964; Jolly 1965; Seber 1965), was used to analyse the mark–resight data. The programme tests the fit of a variety of models and highlights when data violate model assumptions. When more than one model was found to fit the data adequately, the most parsimonious one was used. Two models were selected: (1) ‘Jolly A’, which is the standard Cormack–Jolly–Seber model, and (2) ‘Jolly B’, which is the Cormack–Jolly–Seber model with survival rate assumed to be constant per unit time and time-specific capture probability (Table 3). Population densities were calculated by dividing estimated local population sizes by patch area.
Emigration was measured as the percentage of all recaptured individuals that were recaptured on a different patch. Scatter plots of the percentage of dispersing individuals against the distance between patches and comparisons of the number of upstream and downstream dispersers were used to investigate inter-patch movement patterns. Immigration was measured as a percentage of emigrating individuals that moved from one patch to another, and total immigration was measured as a percentage of all individuals that dispersed to a patch. The proximate causes of dispersal in June and July were investigated for the emigration and total immigration variables using Spearman’s rank correlations with local population size, density, bar area, bar edge, edge-to-area ratio, distance from study centre, sediment size, vegetation cover and trampling.
Results
Rate of recapture and dispersal dynamics
The overall rate of recapture was lowest for the May release (11.7%), compared with June (29.6%) and July (25.6%), despite the longer time period for recaptures. Both the overall and daily adjusted emigration rates were highest for the May release (39.5%, 0.5% per day) compared with those for June (6.43%, 0.1% per day) and July (7.84%, 0.3% per day). Total immigration was mainly into bar 2 in May and, to a lesser extent in June, and into bar 2 and 3 in July (Fig. 1, Table 4).
Figure 2 compares the relationships between dispersal distance and the percentage of dispersers in May, June, July, and June and July (overall relationship at low water) combined. Significant negative exponential relationships were found for July (F=17.9, significant at 1% level; R 2=0.69) and June and July (F=8.4, significant at 5% level; R 2=0.51), and both curves suggested a fairly rapid reduction in the percentage of dispersers with distance. For May this reduction in the percentage of dispersers with distance was barely apparent.
Exponential fits for the relationship between dispersal distance and the percentage of dispersing B. atrocaeruleum for (a) May [F=0.4, not significant (NS); R 2=0.05] and June (F=1.7, NS; R 2=0.18) and (b) July (F=17.9, significant at 1% level; R 2=0.69) and June and July (F=8.4, significant at 5% level; R 2=0.51)
Of the 60 emigrating individuals marked in May, 41 (68.3%) dispersed upstream and 19 (31.7%) dispersed downstream within the study area, although most of these upstream movements were to bar 2 (35, 85.4%). The percentage of emigrating individuals that dispersed upstream and downstream in the June (upstream 13, 44.8%; downstream 16, 55.2%) and July (upstream 20, 50%; downstream 20, 50%) releases were much more balanced.
Dispersal correlates for the June and July releases
The mean estimated population size and density on each bar for the June and July mark–recapture periods are shown in Fig. 3. Emigration was: (1) significantly positively correlated with heavy trampling, which negatively co-varied with local population size, local population density and vegetation cover, and (2) significantly negatively correlated with local population size, which negatively co-varied with sediment size and heavy trampling and positively co-varied with vegetation cover and local population density. Immigration was significantly negatively correlated with the area-to-edge ratio, which positively co-varied with heavy trampling and negatively co-varied with local population size, vegetation cover, edge and area (Table 5).
Discussion
Inundation disturbance response
Spring and summer bankfull flows in temperate rain-fed systems are rare when the lifespan of ERS beetles are considered and are also highly unpredictable. However, such events are a fundamental characteristic of these systems and are common in longer hydrological time series. The May inundation provided a unique opportunity to investigate the dispersal responses of individual B. atrocaeruleum to this short-term unavailability of their main habitat. There were three inter-related lines of evidence that suggested that the dispersal of beetles released in May was markedly different from that observed in June and July. Firstly, there was a far higher overall total rate of emigration in the May release (39.5%) than in June (6.4%) and July (7.8%). This was also far higher than the rates observed by Bates et al. (2005) in June (5.7%) and August (3.0%) 2002 in the same stretch of river when water levels were low. Secondly, there were markedly different inter-patch movement patterns between the May and June/July releases. A larger percentage of beetles dispersed upstream from the May release, and the relationship between percentage of dispersers and dispersal distance was also weak for the May release.
The final line of evidence was the low rate of recapture from the May release (11.69%, compared with 29.58% in June and 25.59% in July). This could have been due to a variety of factors, acting individually or in association: (1) enhanced mark loss over the longer time period over which recaptures could be made; (2) a longer time period over which the background rate of mortality could act; (3) higher migration of individuals from the study area during the inundation event and (4) higher mortality during the inundation event. Large floods can markedly reduce the abundance of ERS specialised carabids (Hering et al. 2004), most probably through direct mortality. However, it is unlikely that smaller inundation events cause substantial mortality in adult ERS specialised carabids because they can swim and survive inundation (Andersen 1968; Hammond 1998). However, if individuals did enter the river as the water rose, many could have been carried out of the study area before they could get to shore. Given the higher rate of emigration in the May event, it seems probable that at least some of the lower rate of recapture can be explained by migration out of the study area.
It is possible that earlier in the season B. atrocaeruleum have a dispersal phase associated with reproduction, or emergence from over-wintering diapause, or that flight conditions were favourable due to higher temperatures and low rainfall (Southwood 1962), and that the enhanced migration rate following habitat inundation was coincidental. However, given the known higher rate of flight activity by riparian carabids during and after floods (Bonn 2000) and the low temperatures and high levels of rainfall during the early summer (Figure in supplementary material), this seems unlikely.
In contrast to the bankfull event in May, the incomplete inundation that occurred on 24 July 2003 did not clearly increase the rate of emigration. The overall percentage movement rate was higher in July (0.27% per day) than in June (0.11%), but mark loss after more than 1 month would have been the cause of some of this difference. Therefore, the partial inundation, or heavy rainfall (Figure in supplementary material, cf. Lytle 1999), did not increase the rate of emigration, but total inundation acted as the condition-dependent dispersal cue. Dispersal has costs, such as energy loss and dispersal mortality (e.g. Waser et al. 1994), so individuals are unlikely to disperse unless the increase in fitness gained through the dispersal outweighs the costs of that dispersal (Bowler and Benton 2005). In this case, dispersal seems to have been the result of movements to and from flood refugia, where the costs associated with entering the river at high flow are potentially high. Andersen (1968) observed ERS specialised Bembidion species allowing themselves to be taken by rising flow levels when there was no more chance of escape by running. This could have been because of low air temperature at the time, which can prevent insects from taking flight (Southwood 1962), suggesting that the condition-dependent inundation response might vary with changes in air temperature.
Trampling disturbance emigration response
Over the June and July releases, the emigration rate was negatively correlated with local population size and positively correlated with trampling. However, these two variables significantly co-varied with each other, so potentially either or both variable(s) could have influenced the emigration rate. It is unlikely that an individual would be able to perceive the total size of its local population, so this variable in itself was unlikely to have influenced the emigration rate. However, the strong positive correlation between local population size and density suggests that density might have influenced the emigration rate. Negative density-dependent relationships, where emigration rate increases at lower densities, have been observed (e.g. Kuussaari et al. 1996; Bonte et al. 2004) but are much less common than positive density-dependent emigration (Bowler and Benton 2005). Negative density-dependent emigration can be selected for if the advantages of group living outweigh the costs of competition (Bowler and Benton 2005) or can result from using the presence of conspecifics as a determinant of habitat quality (Reed and Dobson 1993; Kuussaari et al. 1996). However, it will weaken self-limitation, lead to some suitable patches becoming unoccupied, and will reduce the ability of a species to increase when rare (Sæther et al. 1999; Amarasekare 2004). Therefore, such negative density-dependent migration is unlikely for the common B. atrocaeruleum and is unlikely to be selected for in the highly unstable ERS environment. It is most likely, therefore, that the significant negative relationship between local population size and emigration rate was an artefact of the negative co-variation between population size and trampling.
Livestock grazing and trampling are known to negatively affect lotic and riparian biotic communities (e.g. Jansen and Robertson 2001). Livestock trampling can negatively influence the conservation value of ERS beetle communities (Bates et al. 2006), probably through sediment compaction or defecation, which can damage ERS habitats by destroying the interstitial cavities that many ERS specialised beetles use for shelter (Sadler et al. 2004). Some negative effect of trampling was therefore expected for B. atrocaeruleum, but the observed enhanced rate of dispersal from trampled patches is particularly interesting as it can influence the spatial distribution of the species over an area that is more extensive than the area directly impacted upon by the trampling. Similar to the inundation dispersal response, there was a clear cost associated with staying in the degraded habitat patch, and the cost of dispersal to another patch might well have been outweighed by the increased population fitness gained.
Effect of the edge-to-area ratio on the rate of immigration
A large edge-to-area ratio can increase dispersal when the edge encounter rate influences the decision to emigrate from a patch, and a large edge can increase the immigration of dispersing individuals into a patch by increasing the likelihood of encountering a patch. However, immigration was found to be negatively associated with edge-to-area ratio in this investigation. This was unlikely to be a valid effect and might rather have been the result of one of the co-variations (Table 5) such as heavy trampling, or the distance effect on inter-patch movements (Fig. 2).
Spatial dynamics and its implications
Populations can be classified as patchy (Harrison 1991) when: (1) movement between patches is not so frequent that individuals occupy many patches in their lifetime, (2) all suitable patches in a system are occupied because local extinctions do not occur or are not important due to high rates of dispersal and (3) on average, an individual inhabits more than one patch in its lifetime. B. atrocaeruleum was present on every ERS patch in the study area and in the ∼6 km stretch of river upstream of the site (J. P. Sadler et al., unpublished data). Furthermore, the rates of migration observed in this and the previous study (Bates et al. 2005) suggest that the average individual is likely to inhabit more than one patch in its lifetime, especially when inundation responses are taken into account. Consequentially, B. atrocaeruleum can be classified as having one patchy population connected by migration.
The rate of dispersal and the dynamic spatial population structure are both dependent on the dispersal capabilities of the species and the underlying distribution of the habitat (Addicott et al. 1987). Although B. atrocaeruleum had a patchy population structure, characterised by a high rate of dispersal, the actual number of dispersing individuals under stable water levels declined rapidly with distance and was likely to be zero at a separation of ∼210 m (July) to ∼280 m (June and July). This dispersal distance is relatively low (e.g. Matter 1996; Schneider 2003), given the naturally patchy and disturbed nature of the environment (Southwood 1962; Henle et al. 2004; Bowler and Benton 2005). There are two explanations for this relatively low dispersal distance.
Firstly, habitat inundations raise the rate and most probably the dispersal distance of B. atrocaeruleum both through active flight and hydrochory of some individuals. The ability of many ERS specialised beetles to swim and survive underwater for long periods (Andersen 1968) suggests that long distance dispersal downstream by hydrochory is likely. Indeed, many ERS specialised beetles can be found in stranded flood debris following high water levels (Hammond 1998). Secondly, the dispersal capabilities of a species are likely to be adapted to the natural distribution of their habitat. In natural systems, patches of ERS are typically regularly and closely distributed like beads along a necklace; therefore, there might be little evolutionary pressure for the selection of large dispersal distances.
Any reduction in the frequency and magnitude of high flow events could reduce the level of dispersal both through flight and hydrochory, and any fragmentation of the naturally regular and closely distributed arrangement of habitat patches could reduce the rate of dispersal by flight. This will be particularly detrimental for upstream populations where immigration by hydrochory is not possible. The direct costs of dispersal will also increase with increasing fragmentation and might reach the point where the costs of dispersal outweigh the enhanced fitness gained by that dispersal (Bowler and Benton 2005). Another implication is that reductions in the number of flow pulses will reduce the selective advantage accrued by flight ability (Denno et al. 1989). Both these increases in costs will favour more generalised riparian species over those, such as B. atrocaeruleum, that are full-winged and constantly associated with ERS. In addition, if the level of dispersal is reduced to a level that isolates populations then local extinctions through demographic stochasticity are also likely to occur.
The implications for less common specialists, particularly those with lower dispersal capability, are likely to be more extreme (cf. Stelter et al. 1997). Not surprisingly, therefore, the widespread modification of natural hydrological regimes through river regulation and channelisation has reduced the extent of connectivity of ERS habitat and is regarded as being the main contributor towards the considerable rarity of many ERS specialised invertebrates (Plachter 1986; Stelter et al. 1997; Sadler et al. 2004; Bates et al. 2005).
References
Addicott JF, Aho JM, Antolin MF, Padilla DK, Richardson JS, Soluk DA (1987) Ecological neighbourhoods: scaling environmental patterns. Oikos 49:340–346
Amarasekare P (2004) The role of density-dependent dispersal in source—sink dynamics. J Theor Biol 226:159–168
Andersen J (1968) The effect of inundation and choice of hibernation sites of Coleoptera living on river banks. Norsk Tidsskr 15:115–133
Bates AJ, Sadler JP (2004) A long-lasting method for marking beetles (Coleoptera), which does not enhance mortality. Entomol News 115:49–51
Bates AJ, Sadler JP (2005) The ecology and conservation of beetles associated with exposed riverine sediments. CCW Contract Science Report no. 688
Bates AJ, Sadler JP, Fowles AP, Butcher CR (2005) Spatial dynamics of beetles living on exposed riverine sediments in the Upper River Severn: method development and preliminary results. Aquat Conserv 15:159–174
Bates AJ, Sadler JP, Fowles AP (2006) Livestock trampling reduces the conservation value of beetle communities on high quality exposed riverine sediments. Biodivers Conserv (in press). DOI 10.1007/s10531-006-9028-7
den Boer PJ (1968) Spreading of risk and stabilisation of animal numbers. Acta Biotheor 18:165–194
Bonn A (2000) Flight activity of carabid beetles on a river margin in relation to fluctuating water levels. In: Brandmayr P, Lövei G, Brandmayr TZ, Casale A, Vigna Taglianti A (eds) Natural history and applied ecology of Carabid beetles. Pensoft, Sofia, Moscow, pp 147–160
Bonte D, Lens L, Maelfait J-P (2004) Lack of homeward orientation and increased mobility result in high emigration rates from low-quality fragments in a dune wolf spider. J Anim Ecol 73:643–650
Bowler DE, Benton TG (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225
Brewer PA, Maas GS, Macklin MG (2000) A fifty-year history of exposed riverine sediment dynamics on Welsh rivers. BHS Occas Pap 11:245–252
Church M (1983) Pattern of instability in a wandering gravel bed channel. Int Assoc Sedimentol Spec Publ 6:169–180
Cormack RM (1964) Loglinear models for capture–recapture. Biometrics 45:395–413
Dennis RLH, Shreeve TG, van Dyck H (2003) Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos 102:417–426
Denno RF, Olmstead KL, McCloud ES (1989) Reproductive cost of flight capability: a comparison of life history traits in wing dimorphic planthoppers. Ecol Entomol 14:31–44
Desender K (1989) Ecomorphological adaptations of riparian carabid beetles. In: Wouters K, Baert L (eds) Comptes Rendus du Symposium ‘Invertèbres de Belgique’. Royal Institute of Natural Sciences, Brussels, pp 309–314
Dreitz VJ, Nichols JD, Hines JE, Bennetts RE, Kitchens WM, Deangelis DL (2002) The use of resighting data to estimate the rate of population growth of the snail kite in Florida. J Appl Stat 29:609–623
Goodson JM, Gurnell AM, Angold PG, Morrissey IP (2003) Evidence for hydrochory and the deposition of viable seeds within winter flow-deposited sediments: the River Dove, Derbyshire, UK. River Res Appl 19:317–334
Hammond PM (1998) Riparian and floodplain arthropod assemblages: their characteristics and rapid assessment. In: Bailey RG, José PV, Sherwood BR (eds) United Kingdom floodplains. Westbury Press, Otley, pp 237–282
Hanski I, Gilpin ME (1997) Metapopulation biology: ecology, genetics and evolution. Academic, San Diego
Harrison S (1991) Local extinction in a metapopulation context: an empirical evaluation. Biol J Linn Soc 42:73–88
Henle K, Davies KF, Kleyer M, Margules C, Settele J (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251
Hering D, Gerhard M, Manderbach R, Reich M (2004) Impact of a 100-year flood on vegetation, benthic invertebrates, riparian fauna and large woody debris standing stock in an Alpine floodplain. River Res Appl 20:445–457
Jansen A, Robertson AI (2001) Relationship between livestock management and the ecological condition of riparian habitats along an Australian floodplain river. J Appl Ecol 38:63–75
Jolly GM (1965) Explicit estimates from capture–recapture data with both death and immigration-stochastic model. Biometrika 52:225–247
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. Can J Fish Aquat Sci 106:110–127
Kondolf GM (1997) Hungry water: effects of dams and gravel mining on river channels. Environ Manag 21:533–551
Kuussaari M, Nieminen M, Hanski I (1996) An experimental study of migration in the Glanville fritillary butterfly Melitaea cinxia. J Anim Ecol 65:791–801
Levins RA (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am 15:237–240
Lude A, Reich M, Plachter H (1999) Life strategies of ants in unpredictable floodplain habitats of Alpine rivers (Hymenoptera: Formicidae). Entomol Gen 24:75–91
Lytle DA (1999) Use of rainfall cues by Abedus herberti (Hemiptera: Belostomatidae): a mechanism for avoiding flash floods. J Insect Behav 12:1–12
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Matter SF (1996) Interpatch movement of the red milkweed beetle, Tetraopes tetraophthalmus: individual responses to patch size and isolation. Oecologia 105:447–453
Naiman RJ, Decamps H, Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecol Appl 3:209–212
Nève G, Barascud B, Hughes R, Aubert J, Descimon H, Lebrun P, Baguette M (1996) Dispersal, colonisation power and metapopulation structure in the vulnerable butterfly Proclossiana eunomia (Lepidoptera, Nymphalidae). J Appl Ecol 33:14–22
Petts GE, Gurnell AM, Gerrard AJ, Hannah DM, Hansford B, Morrissey I, Edwards PJ, Kollmann J, Ward JV, Tockner K, Smith BPG (2000) Longitudinal variations in exposed riverine sediments: a context for the ecology of the Fiume Tagliamento, Italy. Aquat Conserv 10:249–266
Plachter H (1986) Composition of the carabid beetle fauna of natural riverbanks and man-made secondary habitats. In: den Boer PJ, Luff ML, Mossakowski D, Weber F (eds) Carabid beetles: their adaptations and dynamics. Fischer, Stuttgart, pp 509–538
Plachter H, Reich M (1998) The significance of disturbance for populations and ecosystems in natural floodplains. In: Proceedings of the international symposium on river restoration, 26–27 May, Tokyo
Pollock KH, Nichols JD, Brownie C, Hines JE (1990a) JOLLY—computes estimates of survival and capture probability for 1-age class open population capture–recapture models. US Geological Survey, Patuxent Wildlife Research Centre. http://www.mbr-pwrc.usgs.gov/software.html#jolly
Pollock KH, Nichols JD, Brownie C, Hines JE (1990b) Statistical inference for capture–recapture experiments. Wildlife Monogr 107:1–97
Purse BV, Hopkins GW, Day KJ, Thompson DJ (2003) Dispersal characteristics and management of a rare damselfly. J Appl Ecol 40:716–728
Reed JM, Dobson AP (1993) Behavioural constraints and conservation biology: conspecific attraction and recruitment. Trends Ecol Evol 8:253–256
Richards K (1982) Rivers: form and process in alluvial channels. Methuen, London New York
Roland J, Keyghobadi N, Fownes S (2000) Alpine Parnassius butterfly dispersal: effects of landscape and population size. Ecology 81:1642–1653
Sadler JP, Bell D, Fowles AP (2004) The hydroecological controls and conservation value of beetles on exposed riverine sediments in England and Wales. Biol Conserv 118:41–56
Sæther B-E, Engen S, Lande R (1999) Finite metapopulation models with density-dependent migration and stochastic local dynamics. Proc R Soc B Biol Sci 266:113–118
Schneider C (2003) The influence of spatial scale on quantifying insect dispersal: an analysis of butterfly data. Ecol Entomol 28:252–256
Seber GAF (1965) A note on the multiple-recapture census. Biometrika 52:249–259
Shaffer ML (1981) Minimum viable population sizes for species conservation. Bioscience 31:131–134
Singer MC, Wedlake P (1981) Capture does affect probability of recapture in a butterfly species. Ecol Entomol 6:215–216
Southwood TRE (1962) Migration of terrestrial arthropods in relation to habitat. Biol Rev 37:171–214
Stelter C, Reich M, Grimm V, Wissel C (1997) Modelling persistence in dynamic landscapes: lessons from a metapopulation of the grasshopper Bryodema tuberculata. J Anim Ecol 66:508–518
Sutcliffe OL, Thomas CD, Peggie D (1997) Area-dependent migration by ringlet butterflies generates a mixture of patchy population and metapopulation attributes. Oecologia 109:229–234
Thomas JA, Rose RJ, Clarke RT, Thomas CD, Webb NR (1999) Intraspecific variation in habitat availability among ectothermic animals near their climatic limits and their centres of range. Funct Ecol 13 [Suppl 1]:55–64
Tockner K, Stanford JA (2002) Riverine flood plains: present state and future trends. Environ Conserv 29:308–330
Tockner K, Malard F, Ward JV (2000) An extension of the flood pulse concept. Hydrol Process 14:2861–2883
Travis JMJ, French DR (2000) Dispersal functions and spatial models: expanding our dispersal toolbox. Ecol Lett 3:163–165
Ward JV, Tockner K, Arscott B, Claret C (2002) Riverine landscape diversity. Freshw Biol 47:517–539
Waser PM, Creel SR, Lucas JR (1994) Death and disappearance—estimating mortality risks associated with philopatry and dispersal. Behav Ecol 5:135–141
Acknowledgements
We thank the School of Geography, Earth and Environmental Sciences at the University of Birmingham and the Countryside Council for Wales for funding the study; Alan Jones (Environment Agency) for obtaining landowner contact details; Tom Kinsey, Gordon Trow and David Davies for allowing site access; Cat Butcher and Richard Johnson for help with fieldwork and equipment preparation, and Kevin Burkhill and Anne Ankorn for help with Fig. 1. We are grateful to the British Atmospheric Data Centre for access to the Meteorological Office Land Surface Observation Stations Data, to the Patuxent Wildlife Centre for provision of the JOLLY model, and to Klement Tockner and Gavin Stewart for useful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Matthias Schaefer
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Bates, A.J., Sadler, J.P. & Fowles, A.P. Condition-dependent dispersal of a patchily distributed riparian ground beetle in response to disturbance. Oecologia 150, 50–60 (2006). https://doi.org/10.1007/s00442-006-0508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0508-y