Abstract
The threat posed by predation varies among predator species and with environmental context, and prey species often adjust their responses accordingly. We investigated such effects within an insect assemblage from a tropical Australian stream. These systems are frequently subjected to catastrophic floods, often suggested to reduce the importance of predation in streams, and invertebrate faunas are characterised by relatively broad environmental tolerances. Impacts of the hunting predator Australopelopia prionoptera (Diptera: Chironomidae) and an undescribed ambush predator from the Polycentropodidae (Trichoptera) on survival and development of two species of tubicolous Chironomidae, Echinocladius martini (Orthocladiinae) and Polypedilum australotropicus (Chironominae), were assessed in laboratory microcosms. A further experiment investigated how impacts of Australopelopia varied over a broad range of temperatures, exceeding that experienced annually by the studied populations. Neither predator impacted survivorship for E. martini, but the presence of the polycentropodid caused E. martini to spend longer as larvae and reduced adult longevity, and adult females were smaller-sized and had smaller oocytes. In contrast, both predators reduced survivorship of P. australotropicus, but only Australopelopia affected its development, causing reductions in pupal duration and oocyte size. The observed non-lethal impacts of predation reflect the threat each predator is known to pose to each prey species in situ. Impacts of predation varied little with temperature, reflecting the broad thermal tolerances of all study species. The predator-specific responses of the prey species imply that predation is a significant selective force in tropical Australian streams, although fluctuation in intensity of predation associated with flooding may limit its importance for community structure and prey diversity at larger scales. Our results indicate a more limited scope for environmental modification of predator–prey relationships in faunas characterised by broad physiological tolerances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic animals are able to detect predators via chemical, mechanical or visual means, and often respond by modifying their behaviour (e.g. reducing conspicuous feeding activity), morphology (investing in defensive structures) or life history (e.g. accelerating development to escape a risky environment) in order to minimise the risk of mortality (e.g. Hershey 1987; Laurila et al. 1998; McPeek and Peckarsky 1998). Such mechanisms typically have costs (e.g. reduced feeding limits resource acquisition), and it is becoming increasingly apparent that many prey species adjust the strength of their responses according to the threat posed (Brodin et al. 2006, and references therein). For example, both stonefly and trout predators cause reductions in resource acquisition for lotic Baetis mayflies, but nymphal development is extended in the presence of the stonefly only, suggesting that the advantages of a lengthened juvenile feeding stage outweigh the smaller mortality risk posed by the stonefly, but not the greater risk posed by trout (Peckarsky and McIntosh 1998). Because many individuals may respond to the presence of a single predator, implications of such predator-specific responses for prey assemblage structure and dynamics can be profound (Schmitz et al. 1997; Peacor and Werner 2001; Miner et al. 2005). However, the danger posed by predation depends not only on the predator species, but also varies with environmental context (e.g. Flecker and Allan 1984, Moore and Townsend 1998). An improved understanding of how such variation affects the responses of prey will yield insights into how predators impact prey population dynamics, and ultimately patterns of prey diversity and distribution (Juliano 1996, McPeek and Peckarsky 1998).
Environmental variation can alter the balance of predation by affecting the behaviour and physiology of either prey or predators (Flecker and Allan 1984; Juliano 1996; Weetman et al. 1999). For example, predatory activity of nymphal damselflies (Odonata: Zygoptera) appears retarded at higher temperatures due to an increased requirement to ventilate (Thompson 1978). When predators are so incapacitated, hardy prey can escape restrictions that would normally be imposed on their activity (Schmitz et al. 1997), with potential consequences for trophic structure (Kishi et al. 2005). At present, such effects have been documented for a small number of lentic (e.g. Moore and Townsend 1998), lotic (Kishi et al. 2005) and container-habitat (Juliano 1996) freshwater faunas from temperate and boreal regions of the world, and results may have limited applicability to faunas subjected to differing environmental regimes elsewhere. Predation has not previously been studied in the tropical rainforest streams of northern Australia, despite the notably high predator biomass that characterises these systems (Pearson et al. 1986; Pearson 1994), estimated to be 40% of total macroinvertebrate biomass in one food-web reconstruction (Cheshire et al. 2005). Australian tropical streams are subject to a regular wet–dry seasonality, with extreme summer flood events alternating with periods of stable low flow during the winter (Pearson 1994), and are inhabited by invertebrate faunas tolerant of broad variation in environmental parameters including temperature (McKie et al. 2004), dissolved oxygen (Connolly and Pearson 2004), and nutrient concentrations (Pearson and Connolly 2000). These characteristics have implications for the potential role of predation, since widespread density-independent mortality associated with extreme floods may dilute the importance of predation as a selective and structuring force (Hildrew and Giller 1994; Reice 1994), while the scope for environmental variation to modulate predator–prey relationships may be limited in a fauna characterised by broad environmental tolerances.
The three experiments presented here investigated lethal and non-lethal effects of predation on two species of chironomid (Diptera) larvae, Echinocladius martini Cranston (Orthocladiinae) and Polypedilum australotropicus Cranston (Chironominae), from Australian tropical rainforest streams, including consideration of temperature effects on predator–prey relationships. Experiments 1 and 2 investigated effects of predation by the actively hunting tanypod chironomid Australopelopia prionoptera Cranston (hereafter referred to as Australopelopia) on survival, development and fecundity of the prey species, with effects of temperature additionally investigated in experiment 1. Experiment 3 investigated prey responses to a retreat-building ambush predator from the Polycentropodidae (Trichoptera)—genus “I” species “Australian Voucher (AV)-7” (Cartwright 1998)—hereafter referred to as “the polycentropodid”. Both prey species inhabit tubes constructed using endogenously produced silk, affording some protection from predation (e.g. Dillon 1985; Hershey 1987) but otherwise contrast strongly in habitat use and behaviour. Polypedilum australotropicus inhabits accumulations of decaying leaves in pools, and builds robust tubes of particulate detritus which it ventilates using an undulatory body motion (McKie 2002), whereas E. martini does not ventilate its tube, which is constructed from silk only on cobbles and leaves in fast-flowing riffle/hygropetric habitats. Behaviour of the two predator species also contrasts. The free-roaming Australopelopia detects its prey mainly by movement (B. G. McKie, unpublished data), and can invade chironomid tubes. It feeds either by engulfing its prey whole or, if the prey is larger, by piercing the abdominal wall and sucking the body contents (personal observations). The polycentropodid is an ambush predator, and is too large to invade chironomid tubes. It builds a flattened messy-looking retreat using copious amounts of silk. When it detects prey in contact with its retreat, it darts out and seizes its victim with its mandibles (personal observations). Both predators co-occur with the prey species in situ, but are more common in the hygropetric habitats favoured by E. martini. In the stream inhabited by the studied population (see Materials and methods), mean density of the polycentropodid and Australopelopia is 0.87 and 0.17 individuals cm−3 of leaf material, respectively, in hygropetric leaf packs, compared with 0.15 and <0.01 individuals cm−3 in pools (B. G. McKie, unpublished data). However, densities of predacious Tanypodinae (comprising eight species including Australopelopia) overall are higher in pools (0.98 individuals cm−3 of leaf material) than in hygropetric packs (0.61 individuals cm−3, B. G. McKie, unpublished data). E. martini remains are found frequently in the guts of field-caught polycentropodids (found in 25% of dissected polycentropodids), but are rare in the guts of A. prionoptera and other tanypod chironomids (<0.1% occurrence, McKie 2002). In contrast, P. australotropicus remains are found more often in the gut contents of field-caught tanypods (10% occurrence) than polycentropodids (<0.1% occurrence, McKie 2002).
In all experiments, predators were caged so that induced non-lethal life-history effects were separable from direct mortality effects, and some experiments included a free (uncaged) predator treatment also, to quantify direct mortality effects. The experimental treatments were predicted to have the following effects on the prey species:
-
1.
Free predators should impose significant mortality, but may impact the prey species differentially according to their hunting behaviour.
-
2.
Prey reared in the presence of caged predators may suffer adult size and fecundity deficiencies, indicative of deficiencies in resource acquisition, and prey also may lengthen the larval stage, extending the feeding period to offset such deficiencies (e.g. Ball and Baker 1996); alternatively, prey may shorten the duration of the juvenile stages, to minimise mortality risk prior to reproductive maturity (e.g. Peckarsky et al 2001; McKie 2004).
-
3.
Prey responses may reflect habitat use and differential vulnerability to the predators in situ.
-
4.
Lethal and non-lethal impacts of Australopelopia on prey survivorship and development should vary with rearing temperature (e.g. Kishi et al. 2005), unless the broad tolerances of all species involved (McKie et al. 2004) militate against such effects.
Materials and methods
Animal collection and experimental set-up
Individuals of the prey and predator species were collected from leaf packs in Birthday and Camp creeks (18°59′S, 146°10′E, 800–850 m above sea level), which flow through tropical rainforest in the Paluma range, northern Queensland (further details in McKie et al. 2005). Collection protocol is detailed in McKie et al. (2004). The largest available predator individuals were collected, whereas third instar prey larvae were targeted (very small and more mature larvae were discarded). Animals were transported to James Cook University in Townsville for rearing in separate jars (diameter 5 cm) within a controlled-environment cabinet. Each jar contained 100 ml of water from Birthday Creek and five drops of a suspension of natural stream particulate detritus (preparation detailed in McKie 2004). This material was used by the prey species for food and in tube construction, and was supplemented as necessary during the experiments. For experiment 1, the cabinet was divided into two levels. On the top level were 12 polystyrene troughs (60×20×16 cm), within which the rearing jars were placed. Air-jets placed in the corners of each trough blew air over the jars, agitating the water surface. On the bottom level were three polystyrene water baths (walls 3.5 cm thick), each maintained at a different temperature (12, 18, or 26°C) using aquarium heaters. Each bath had four outlets, with water pumped to four of 12 troughs on the top level. Water returned by gravity from each trough to the original bath. Rearing jars were divided evenly among the 12 troughs, with the four troughs for each temperature spread evenly through the cabinet. Nocturnal temperatures were simulated by a 1.5°C reduction at night. Incandescent bulbs provided light from above, on a 12 h light–dark cycle. Minor gradients in temperature (<1°C variation) were overcome by rotating the jars daily at random through the replicate troughs for each temperature. Similar apparatus was used for experiments 2 and 3, except that all troughs were maintained at 18°C.
Experiments 1, 2 and 3 were conducted from September to December 1998, October to December 2000 and May to July 2000, respectively. In experiment 1, animals were exposed to one of three temperatures, 12, 18 and 26°C, representing a range slightly greater than that observed annually at Birthday Creek (15–22°C), but comparable to temperatures experienced by the prey species over their geographic range (McKie et al. 2004). Animals were also exposed to three levels of predation. One-third of jars contained no Australopelopia, a third contained one caged Australopelopia, and the remainder contained one free (uncaged) Australopelopia. In experiments 2 and 3, individuals of the study species were exposed to one of two treatments, one with a caged predator and the other without. Predators were always introduced on day 2, giving prey time to build tubes. Predator cages consisted of either inverted 5-ml (Australopelopia cages) or 25-ml (Polycentropodid cages) vials closed with 63-µm netting (the prey species were able to penetrate coarser mesh sizes). Caged predators were fed chopped Lumbriculus variegatus Müller (Oligochaeta: Lumbriculidae) worm fragments, which remain alive and active while regenerating into new individuals. All rearing jars contained a cage with worms, regardless of whether they also contained predators. Additional worms were added as needed to ensure ongoing presence in all jars.
Animal collection was insufficient to allow a free polycentropodid treatment as well-replicated as the other treatments in experiment 3. Instead, vulnerability of the prey species to the polycentropodid was assessed via a small trial, conducted simultaneously with experiment 3, in which E. martini and P. australotropicus were exposed to uncaged polycentropodid predators. Individuals were placed in separate jars containing particulate detrital material and given a day to construct tubes before exposure to the free predator. Jars were checked daily, with the trial maintained until all prey individuals had either been consumed or completed adult emergence (25 days).
Data collection
Jars were removed daily from the cabinet for inspection under a dissecting microscope, with each prey individualȁ9s status (larva, pupa and adult) recorded. More frequent observations were unnecessary because the prey species metamorphosed at night, and so days were the appropriate units for assessing life-stage durations. Emerging adults in experiment 1 were left alive for 1 day, to allow sclerotised parts to harden, and then killed and preserved in 70% ethanol. Reared individuals in experiments 2 and 3 were kept alive as adults and allowed to die naturally. The wings of emerged adults were mounted on slides, with wing length measured from the arculus to wing tip. Wing length of Chironomidae correlates with body mass, because of biophysical links between mass and the wing area required for flight (e.g. McLachlan 1986).
Fecundity characteristics were assessed in experiments 2 and 3 only. Ovary dissection protocol is detailed in McKie (2004). Egg production was assessed by counting the number of oocytes per female. Chironomid adult females are short-lived, essentially non-feeding and, with the exception of some pestiferous species, not known to produce multiple egg batches, and so oocyte number is a robust index of fecundity (Armitage et al. 1995). Oocyte maturity was classified according to a scheme modified from that commonly used by mosquito researchers (Table 1), and oocyte size was assessed by selecting randomly six oocytes per female and measuring their length and width, with oocyte cross-sectional area calculated using the formula for the area of an oval.
Analysis
Differences in the probability of individuals completing pupal development (i.e. emerging successfully) under the predation treatments were analysed using binary logistic regression. For experiment 1, the effect of temperature on hunting success (proportion of larvae killed) of Australopelopia in the free-predator treatment was assessed also using logistic regression, since a high proportion of low expected cell values in two-way contingency tables prevented robust log-linear analyses of interactions between temperature and predation. All individuals of both prey species that died in the free-predator treatment were clearly killed by the predator (see Results), obviating a need for comparison with control (no-predator) conditions.
Larval, pupal and adult duration data (recorded as days spent in each state), along with wing-length and oocyte number data, were investigated using ANOVA where possible. Tested factors included predator presence, sex, and temperature, where relevant, with log or square root transformation applied where necessary to satisfy parametric assumptions. In cases where transformation failed to normalise data, Mann–Whitney U-tests were used. Data from animals that died before pupation were not included in larval duration analyses, whilst pupal duration analyses included data both from adults that emerged successfully and pupae that died at the water surface while emerging (easily discernable as emerging pupae continue to float at the water surface even if they die). In experiment 1, few P. australotropicus exposed to free predators survived to emergence (see Results), and so this treatment was excluded from all P. australotropicus analyses excepting that for larval duration.
Oocyte cross-sectional area was analysed using mixed-model ANOVA, with predator presence treated as a fixed effect, and female individual (nested in predator presence) and the six oocytes measured per female (nested in individual) as random effects. Variation in oocyte maturity was assessed using cross tabulations with exact significance. Additionally, ANOVA and cross tabulation analyses tested whether pupal duration, a factor potentially influencing egg maturation, contributed significantly to variation in oocyte number and maturity. In experiments 2 and 3, most individuals (>95%) completed pupation in either 2 or 3 days. Accordingly, the “pupal period” factor comprised two levels, long (encompassing all individuals that took 3–4 days to complete pupation) and short (for individuals completing pupation in 2 days). All analyses were conducted using SPSS for Windows (version 10.0.5, 1989–1999; SPSS, Chicago, Ill.).
Results
Survivorship
In experiment 1, survivorship differed according to predation for P. australotropicus (logistic regression χ 22 =34.69, P<0.001), but not E. martini (χ 22 =2.67, P=0.26). Mortality of P. australotropicus was 82% in the presence of free Australopelopia, greater than levels observed in the caged- (28%) and no-predator (19%) treatments. In contrast, mortality of E. martini was 21% in the presence of free Australopelopia, only slightly higher than in the caged- (15%) and no-predator (12%) treatments. All individuals of both species that died under the free-predator treatment were clearly killed by the predator, recognisable by remains visible through the live predatorȁ9s abdomen wall, or by an obvious incision wound when the prey was attacked suctorially. All nine E. martini individuals that were consumed were taken as larvae, whereas four of 26 consumed P. australotropicus individuals were taken as pupae. The hunting success of Australopelopia on P. australotropicus in the free-predator treatment appeared greater at 18°C (100% prey individuals eaten) than at either 12 or 26°C (73% of individuals eaten), but this result was not significant at the 5% level (logistic regression χ 22 =5.10, P=0.078). Similar low numbers of E. martini were killed by Australopelopia at all temperatures (χ 22 =1.32, P=0.51). E. martini individuals taken by the predator survived for an average of 5.0±1.5 (mean±SE) days after the introduction of the predator, whereas consumed P. australotropicus survived for only 3.5±0.8 days , with 39% taken within 1 day. There was no significant temperature effect on prey survival time in the presence of free predators for P. australotropicus (ANOVA F 2,23=0.1, P=0.9). Insufficient E. martini individuals were taken to test for temperature effects on survival time.
In experiment 2, the presence of caged Australopelopia affected survivorship for P. australotropicus (logistic regression χ 22 =4.18, P=0.041) but not E. martini (χ 22 =0.27, P=0.602). Mortality of P. australotropicus was 18% in the caged-predator treatment, compared with 3% in the no-predator treatment. This was almost entirely attributable to elevated mortality during pupation, with 15% of larvae that survived until pupation in the caged-predator treatment failing to complete adult metamorphosis.
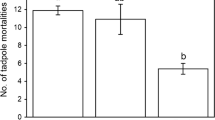
The presence of caged polycentropodids had no effect on survivorship for either species (logistic regression E. martini χ 22 =0.17, P=0.682; P. australotropicus χ 22 =0.517, P=0.475). However, survivorship differed between species in the polycentropodid free-predator trial (cross-tabulation χ 123 =7.08, P=0.008), as seven of ten P. australotropicus were taken by the predator, whereas only two of 13 E. martini were taken. Most P. australotropicus' deaths (five of seven) occurred within the first 5 days of exposure.
Larval, pupal and adult duration
Sex was never significant in any analyses of larval duration, either alone or in interaction (all F<2.6, all P>0.12), and is not fitted in larval duration analyses presented here, allowing inclusion of individuals of uncertain sex that died while undergoing pupal ecdysis. In experiment 1, temperature significantly affected larval duration for both species (ANOVA E. martini F 2,102=13.8, P<0.001; P. australotropicus F 2,70=5.73, P=0.005), with shortened durations at higher temperatures (mean±SE days E. martini at 12°C 14.9±1.5, at 18°C 9.1±0.9, at 26°C 5.8±0.6; P. australotropicus at 12°C 19.9±2.6, at 18°C 11.4±1.3, at 26°C 6.7±0.6). However, there was no effect of Australopelopia presence for either species (both F<1.5, P>0.24), nor any interaction between temperature and predation (both F<1.0, P>0.39). Similarly, the presence of caged Australopelopia did not affect larval duration for either species in experiment 2 (E. martini F 1,65=0.8, P=0.15, P. australotropicus F 1,62=0.1, P=0.71). However, in experiment 3, E. martini larval durations were longer for individuals reared in the presence of caged polycentropodids (8.6±0.6 days) than in the no-predator treatment (6.8±0.6 days, ANOVA F 1,57=4.2, P=0.045). No similar effects were apparent for P. australotropicus (F 1,66=0.2, P=0.667).
Pupal development proceeded faster at higher temperatures for both species in experiment 1 (Fig. 1; ANOVA E. martini F 2,87=278.6, P<0.001; P. australotropicus temperature F 2,51=229.2, P<0.001). Male E. martini spent longer in the pupal state than females (Fig. 1a; F 1,87=50.3, P<0.001). Although this difference appeared less apparent at 26°C (Fig. 1a), the temperature×sex interaction was not significant at the 5% level (F 2,87=2.8, P=0.068). Sex did not affect pupal duration for P. australotropicus (F 1,51=0.6, P=0.45). Pupal duration was unaffected by the presence of either caged or free Australopelopia for E. martini (F 2,87=0.4, P=0.68), but P. australotropicus reared with caged Australopelopia spent less time in the pupal state (Fig. 1b; F 1,51=8.2, P=0.006, insufficient larvae survived to test effects of free-predators on pupal duration). All other interactions between temperature, predation and sex were non-significant (ANOVA all F<1.7, P>0.20). Pupal duration was unaffected by the presence of caged predators for either prey species in either experiment 2 (Mann–Whitney U-test E. martini n=65, Z=−0.1, P=0.94; P. australotropicus n=58, Z=−0.8, P=0.60) or experiment 3 (E. martini, n=57, Z=−0.7, P=0.48; P. australotropicus n=61, Z=−1.2, P=0.25). In all experiments, male E. martini spent longer as pupae than females (data from experiment 1 in Fig. 1a; other experiments similar).
Adult duration did not vary with the presence of either free or caged Australopelopia for either species in experiment 2 (ANOVA E. martini F 1,51=1.7, P=0.20; P. australotropicus F 1,50=0.1, P=0.84). However, E. martini adult longevity was reduced in the presence of the polycentropodid (3.4±0.4 days) compared with controls (4.4±0.3 days) in experiment 3 (ANOVA F 1,39=4.6, P=0.039). No similar effect was apparent for P. australotropicus (F 1,58=0.1, P=0.763). In both experiments 2 and 3, P. australotropicus adult longevity was greater for females (e.g. experiment 2 mean±SE: 6.9±0.3 days) than males (4.1±0.2 days; ANOVA both F>6.3, P≤0.015). Sex was not significant for E. martini in either study (both F<0.6, P>0.45), and there were no interactions between sex and predator presence for either species (all F<2.7, P>0.11).
Adult size and fecundity characteristics
In experiment 1, wing lengths were shorter for animals reared at higher temperatures for both E. martini (F 2,76=44.9, P<0.001) and P. australotropicus (F 2,50=25.4, P<0.001, Fig. 2). Wing length also differed according to sex for both species (E. martini F 1,76=140.2, P<0.001; P. australotropicus F 1,50=5.6, P=0.022), but whereas male E. martini (Fig. 2b) had longer wings than females, the reverse was true for P. australotropicus (Fig. 2b). Australopelopia presence did not affect wing length for either species (ANOVA all F<2.3, P>0.14), and there were no significant interactions (all F<2.1, P>0.13). Similarly, wing length was unaffected by Australopelopia presence in experiment 2 (both species F<0.5, P>0.48), but did differ according to sex (E. martini F 1,58=102.3, P<0.001, P. australotropicus F 1,55=4.8, P=0.033), with differences identical to those at 18°C in Fig. 2 . There were no interactions between predator presence and sex (both F<0.5, P>0.48). In experiment 3, there was a significant interaction between predator presence and sex for E. martini (predator presence F 1,44=0.7, P=0.417, sex F 1,44=61.2, P<0.001; predator presence×sex F 1,44=4.1, P=0.048), with wing length of females, but not males, reduced in the presence of caged polycentropodids (Fig. 3). P. australotropicus wing length did not vary according to either polycentropodid presence or sex (all F<1.4, all P>0.25).
Predator presence affected neither oocyte number (ANOVA all F<0.3, all P>0.57) nor oocyte maturity (cross-tabulation all χ 22 <3.3, all P>0.42) for either prey species in experiments 2 and 3. Most E. martini oocytes were in maturity category IIIb, with 20% in stage IV, while 80% of P. australotropicus oocytes were in maturity categories IIb and IIIa. Australopelopia presence did not affect mean oocyte cross-sectional area for E. martini [nested ANOVA predator presence F 1,30=0.1, P=0.72; female(predator presence) F 30,160=57.6, P<0.001], but oocytes were smaller for P. australotropicus exposed to caged Australopelopia (18.3±0.7 µm) compared with controls [26.5±1.2 µm; predator presence F 1,33=9.14, P=0.005; female(predator presence) F 33,175=19.4, P<0.001]. In contrast, E. martini reared in the presence of caged polycentropodid predators in experiment 3 had smaller ovarioles (34.0±2.4 µm) than those reared under the no-predator treatment [53.9±3.2 µm; predator presence F 1,20=4.48, P=0.047; female(predator presence) F 20,108=23.93, P<0.001], whereas P. australotropicus ovariole size was unaffected [predator presence F 1,26=0.1, P=0.975; female(predator presence) F 26,137 = 10.9, P<0.001].
Oocyte number did not differ according to pupal period in experiment 2 (ANOVA both species F<1.8, P>0.19). However, E. martini oocyte maturity varied according to pupal period (cross-tabulation χ 22 =8.0, P=0.020), with seven of 14 E. martini that completed pupation in 3 days having oocytes in the most mature category IV, whereas no individuals completing pupation in 2 days had oocytes in this category. No similar effect was apparent for P. australotropicus (χ 22 =0.07, P=1.0). In experiment 3, pupal period had no effect on oocyte number (both species F<1.0, P>0.30) or maturity (χ 22 <2.4, P>0.39).
Discussion
Differential vulnerability and the predator-specific responses of the prey species
Sublethal effects of the predator species on the development and morphology of their potential prey were species specific. E. martini did not respond to the presence of Australopelopia, but its larval duration was lengthened and adult duration reduced in the presence of caged polycentropodids, with females emerging smaller-sized and with smaller oocytes. In contrast, P. australotropicus was unaffected by the presence of the polycentropodid, but suffered higher pupal mortality, spent less time in the pupal state, and emerged with smaller oocytes when reared in the presence of Australopelopia. The occurrence of predator-specific responses in other aquatic taxa varies among populations exposed to differing predation intensities (McIntosh and Peckarsky 1996; Rosenfeld 2000), and can have a genetic component (Juliano and Gravel 2002; Brodin and Johansson 2004). Maintenance of predator-specific responses by the prey species implies that the types of predator studied here exert significant selective pressure in Australian tropical streams, but that the pressure imposed by each predator impacts prey differentially in their favoured microhabitats.
Both prey species responded to the predator most likely to pose a significant threat in situ, regardless of their vulnerability in the laboratory. Unlike E. martini, P. australotropicus ventilates its tube in both larval and pupal stages (personal observations), rendering it vulnerable to tube-invading Tanypodinae, which are adept at discerning chironomid tubes via these ventilatory motions (B. G. McKie, unpublished data). The responses of P. australotropicus to Australopelopia reflect this vulnerability, apparent both in the laboratory and in situ (McKie 2002). In contrast, P. australotropicus is almost never found consumed by polycentropodids sampled from pool leaf packs in which both species occur (McKie 2002), despite its vulnerability in the laboratory. The polycentropodid does not invade chironomid tubes (B. G. McKie, unpublished data), and its threat may be further limited in the pool habitat favoured by P. australotropicus if the greater deposition of sediment and lack of current retards its predatory activity (Flecker and Allan 1984), obviating the need for P. australotropicus to routinely respond to its presence. However, E. martini is commonly found in the guts of field caught Polycentropodidae, including species AV−7 (McKie 2002, and see Introduction), contrasting with its lack of vulnerability in the laboratory. Densities of riffle-inhabiting chironomids and net-spinning predacious caddisflies are often correlated in situ, reflecting effects of caddis retreats on spatial complexity (Englund and Evander 1999 and references therein), and the responses of E. martini to the polycentropodid presumably reflect the close association between these species in hygropetric habitats in Australian tropical streams (B. G. McKie, unpublished data). These results demonstrate that species inhabiting running water environments, and from a single family appearing as morphologically uniform as the Chironomidae, possess antipredator responses matching the threat posed by different predator species in situ.
The observed effects of predator presence on adult size, longevity and fecundity characteristics for the prey species indicate inadequate resource (energy, nutrient) acquisition and/or storage. Reduced adult duration for E. martini reared with the polycentropodid suggests insufficient resource storage to support prolonged adult life. Similarly, the smaller wings and oocytes of E. martini females exposed to caged polycentropodids, and the smaller oocytes of P. australotropicus reared with Australopelopia, indicate stored resources were inadequate for maximal somatic and reproductive growth. Reduced oocyte size was not associated with changes to oocyte number or maturity, and so appears attributable to restrictions in nutrients available for allocation to each oocyte, though oocyte growth may have been additionally limited by reduced longevity for E. martini. Elevated pupal mortality for P. australotropicus reared with caged Australopelopia and lengthened larval development for E. martini reared with the polycentropodid also may reflect deficiencies in available resources. Other chironomids (Ball and Baker 1996) and aquatic insect taxa (McPeek and Peckarsky 1998) lengthen the larval stage when foraging is constrained by the presence of predators, to ensure sufficient resource acquisition for growth and reproduction. Similarly, compromised survivorship for chironomids (Ball and Baker 1995) and other invertebrates (Duvall and Williams 1995; Schmitz et al. 1997) reared in the non-lethal presence of predators has been attributed to changes in prey behaviour or development, with associated deficiencies in nutrient intake. Such effects also can reflect increased physiological stress induced by the presence of predators, if important physiological processes (e.g. digestion) are negatively impacted (Stoks et al. 2005). Whether results observed here are primarily attributable to reduced feeding in the presence of predators (as observed for other Chironomidae that limit foraging to minimise the risk from predation; Koperski 1998), increased resource expenditure on defensive structures (e.g. the extremely long setae grown by some chironomids in the presence of certain predators; Hershey and Dodson 1987), or some type of physiological stress remains unknown.
Effects of predator presence on pupal duration
Selection should favour individuals that spend the minimum period possible in vulnerable, non-feeding life stages (Wassersug and Sperry 1977), and both E. martini and P. australotropicus spent less time in the non-feeding pupal state when subjected to increased physical disturbance (McKie 2004). In this study, P. australotropicus reared with caged Australopelopia in experiment 1 spent less time in the pupal state, but E. martini pupal duration was not affected by the presence of either predator. The polycentropodid does not invade chironomid tubes (B. G. McKie, unpublished data), obviating a need for either species to respond to its presence during pupation, since pupae only leave their tubes on emergence. However, Australopelopia does invade chironomid tubes, and was observed to consume P. australotropicus pupae. Unlike the well-sclerotised, spinose and inactive pupae of E. martini, P. australotropicus pupae are soft-bodied and ventilate their tubes (personal observations). Reducing pupal duration limits the time available for Australopelopia to detect these movements and attack. However, P. australotropicus pupal duration was unaltered in experiment 2, implying that the threat posed by Australopelopia differed between the two studies. Australopelopia used in experiment 1 were large fourth instars (identifiable both by size and integument pigmentation), but in experiment 2, less than 50% were fourth instars, because fewer mature larvae were available in the source population at that time. Tanypods are largely detritivorous when younger (Baker and McLachlan 1979), and we observed that smaller caged Australopelopia ate less of the provided live worm food (personal observations). Aquatic prey often respond differently to predators fed different diets (Laurila et al. 1998; Turner 2000; Brodin et al. 2006), and differences in protein ingestion by large and small Australopelopia, presumably causing differences in composition of waste product kairomones, may explain the contrasting response of P. australotropicus between the two Australopelopia experiments.
More individuals, both in this study and that of McKie (2004), spent longer in the pupal state when the mortality threat was minimal. This implies that reducing pupal duration is costly in some way, unless pupal duration is not itself a plastic trait, but rather is dependent on variation in some other aspect of development or morphology, such as body size (Downie et al. 2004) or larval duration (McKie 2004), neither of which were affected for P. australotropicus in this study. McKie (2004) hypothesised that individuals leaving the pupal stage earlier may emerge with important adult structures, such as gametes, less developed. Since chironomid adults in situ are short-lived (Armitage et al. 1995), such delay in gamete maturation could have fitness consequences. In this study, E. martini that spent less time in the pupal state were more likely to have less mature oocytes (experiment 2). This result seems not confounded with any effect of predation, since the presence of Australopelopia did not affect any measured variable for E. martini. However, for this hypothesis to be properly assessed females should be killed immediately upon emergence, before ongoing oocyte maturation obscures potential deficiencies arising solely from differences in pupal development time.
The effect of temperature on predator–prey relationships
Temperature little impacted predator–prey relationships in experiment 1, reflecting the broad thermal tolerances of all species involved (McKie et al. 2004). The range of temperatures considered represents that over which the prey species can survive and complete development, and the observed eurythermic reaction norms (e.g. the asymptotic responses over a broad temperature range evident in Figs. 1 and 2) match those observed previously (McKie et al. 2004, McKie and Cranston 2005). Although there was weak evidence that hunting success of Austrolopelopia was reduced at 12 and 26°C, the time taken for Australopelopia to locate and attack its prey did not vary with temperature, and no interactions between temperature and predation were observed for any prey trait, indicating that anti-predator mechanisms were equally well implemented by the prey species at all temperatures. The broad tolerances of Australopelopia limit the potential for thermal variation to strongly impact its predatory behaviour, and only lethal temperatures may induce the prey species to alter behaviour in ways that increase their vulnerability (e.g. the increased rates of tube abandonment observed for E. martini at 32°C by McKie et al. 2004). There appear to be no genuinely stenothermic Australian Chironomidae, possibly a consequence of thermal unpredictability in streams throughout Australia (McKie et al. 2004, 2005). However, reaction norms are not identical across species (McKie et al. 2004; McKie and Cranston 2005), and thermal variation could have cumulative impacts on predators and prey over longer time scales than those considered here. Nevertheless, if tropical Australian macroinvertebrates are generally characterised by broader environmental tolerances (e.g. Pearson and Connolly 2000; Connolly and Pearson 2004), then the scope for environmental variation to modulate predator–prey relationships may be limited. These results indicate that the strong modifying effects of environmental variation on freshwater predator–prey interactions observed in temperate and boreal systems (e.g. Thompson 1978; Moore and Townsend 1998; Kishi et al. 2005) cannot necessarily be extended to systems with environmental characteristics that favour evolution of broader physiological tolerances.
Implications and conclusions
Extreme fluctuations in flow, such as those that characterise Australian tropical streams (Pearson 1994), are often suggested to undermine the importance of predation as a structuring force in some lotic communities through causation of widespread density-independent mortality (e.g. Hildrew and Giller 1994; Reice 1994). However, the sublethal effects of predation we observed have potential to influence individual fitness and habitat use, especially where both predator and prey are relatively sedentary. Because the polycentropodid builds fixed retreats, an E. martini larva living in a similarly fixed tube nearby should balance the risks of prolonged exposure to the predator, including associated sub-lethal costs, against benefits of inhabiting the more complex substrate engendered by polycentropodid retreats (potentially associated with enhanced entrapment of particulate detritus, Englund and Evander 1999) and the dangers of tube-abandonment and costs of tube reconstruction (McKie 2004). The sub-lethal consequences of exposure to retreat-building predators observed here might explain some of the remarkable within-population morphological variation documented for E. martini (McKie and Cranston 2005), if the extent of exposure varies widely among individuals. In contrast, threatened P. australotropicus may need to respond only briefly to the presence of the more mobile Australopelopia, unless population densities of Australopelopia and other similar tube-invading predators (Hershey 1987; Harkrider 2000) are high. In Australian tropical streams, such a situation may occur during the dry season, when populations grow without hindrance from flooding for months (Pearson 1994), a long period relative to the rapid life cycles of tropical invertebrates. Concurrently, low rainfall causes reductions in the wetted area of stream channels, concentrating organism densities (Douglas et al. 1995; Rosser and Pearson 1995), and potentially intensifying the impacts of predators on their prey (e.g. Dudgeon 1993).
Broader extension of these results requires consideration of factors that alter or obscure effects of small-scale processes at larger scales (Peckarsky et al. 1997). The lethal and non-lethal effects of predators on the life history and morphology of individuals can influence prey demographics, and hence patterns of species distribution (McPeek and Peckarsky 1998; Miner et al. 2005). However, to have a major influence, such effects need to be pervasive within one generation of a prey species, and persistent between generations. Australian tropical chironomids are multivoltine (McKie et al. 2004), and if predator pressure is intense only for part of each year (during the dry season), then its lethal and sub-lethal effects on prey life history may not accumulate sufficiently over multiple generations to threaten population persistence in isolation (Peckarsky et al. 1997). Nevertheless, the potential for predation to intensify in Australian tropical rainforest streams is indicated by the high biomass and diversity of predators in these systems (Pearson et al. 1986; Cheshire et al. 2005), and patchiness in the occurrence of important predator guilds, such as insectivorous fish (often absent from streams above waterfalls), is expected to have consequences for prey assemblage structure (Pearson 1994; Pusey and Kennard 1996). Further study of predation in Australian tropical streams should yield insights into the evolution of anti-predator mechanisms in prey populations, and into how environmental variation constrains predator regulation of community structure in regions subject to regular abiotic disturbances.
References
Armitage PD, Cranston PS, Pinder LCV (eds) (1995) The Chironomidae: biology and ecology of non-biting midges. Chapman and Hall, London
Baker AS, McLachlan AJ (1979) Food preferences of Tanypodinae larvae (Diptera: Chironomidae). Hydrobiologia 62:283–288
Ball SL, Baker RL (1995) The non-lethal effects of predators and the influence of food availability on life history of adult Chironomus tentans (Diptera: Chironomidae). Freshwater Biol 34:1–12
Ball SL, Baker RL (1996) Predator-induced life history changes: antipredator behaviour costs or facultative life history shifts? Ecology 77:1116–1124
Brodin T, Johansson F (2004) Conflicting selection pressures on the growth/predation-risk trade-off in a damselfly. Ecology 85:2927–2932
Brodin T, Mikolajewski DJ, Johansson F (2006) Behavioural and life history effects of predator diet cues during ontogeny in damselfly larvae. Oecologia 148(1):162–169
Cartwright D (1998) Preliminary guide to the identification of late instar larvae of Australian Polycentropodidae, Glossosomatidae, Dipseudopsidae and Psychomyiidae. Cooperative Research Centre for Freshwater Ecology identification guide no. 15, Albury, New South Wales
Cheshire K, Boyero L, Pearson RG (2005) Food webs in tropical Australian streams: shredders are not scarce. Freshwater Biol 50:748–769
Clements AN (1992) The biology of mosquitoes, vol 1. Development, nutrition and reproduction. Chapman & Hall, London
Connolly NM, Pearson RG (2004) Effect of low dissolved oxygen on survival, emergence, and drift of tropical stream macroinvertebrates. J N Am Benthol Soc 23:251–270
Dillon PM (1985) Chironomid larval size and case influences capture success achieved by dragonfly larvae. Freshwater Invert Biol 4:22–29
Douglas MM, Lake PS, McGuinness KA (1995) Fire management in tropical savanna: the effects on stream biota. In: Finlayson CM (ed) Wetland research in the wet-dry tropics of Australia. Office of the Supervising Scientist, Canberra, pp 69–73
Downie JR, Bryce R, Smith J (2004) Metamorphic duration: an understudied variable in frog life histories. Biol J Linn Soc 83:261–272
Dudgeon D (1993) The effects of spate-induced disturbance, predation and environmental complexity on macroinvertebrates in a tropical stream. Freshwater Biol 30:189–197
Duvall CJ, Williams DD (1995) Individuality in the growth of stonefly nymphs in response to stress from a predator. Arch Hydrobiol 133:273–286
Englund G, Evander D (1999) Interactions between sculpins, net-spinning caddis larvae and midge larvae. Oikos 85:117–126
Flecker AS, Allan JD (1984) The importance of predation, substrate and spatial refugia in determining lotic insect distributions. Oecologia 64:306–313
Harkrider JR (2000) Predation of Neoplasta Coquillett larvae (Diptera: Empididae) on larval midges in the genus Rheotanytarsus Bause (Diptera: Chironomidae). Pan-Pac Entomol 76:176–183
Hershey AE (1987) Tubes and the foraging behaviour in larval Chironomidae: implications for predator avoidance. Oecologia 73:236–241
Hershey AE, Dodson SI (1987) Predator avoidance by Cricotopus: cyclomorphosis and the importance of being big and hairy. Ecology 68:913–920
Hildrew AG, Giller PS (1994) Patchiness, species interactions and disturbance in the stream benthos. In: Giller PS, Hildrew AG, Rafaelli DG (eds) Aquatic ecology: scale, pattern and process. Blackwell, Oxford, pp 21–62
Juliano SA (1996) Geographic variation in Aedes triseriatus (Diptera: Culicidae): temperature dependent effects of a predator on survival of larvae. Environ Entomol 25:624–631
Juliano SA, Gravel ME (2002) Predation and evolution of prey behaviour: an experiment with tree hole mosquitoes. Behav Ecol 13:301–311
Kishi D, Murakami M, Nakano S, Maekawa K (2005) Water temperature determines strength of top-down control in a stream food web. Freshwater Biol 50:1315–1322
Koperski P (1998) Predator–prey interactions between larval damselflies and mining larvae of Glyptotendipes gripekoveni (Chironomidae): reduction in feeding activity as an induced defence. Freshwater Biol 39:317–324
Laurila A, Kujasalo J, Ranta E (1998) Predator-induced changes in life history in two anuran tadpoles: effects of predator diet. Oikos 83:307–317
McIntosh AR, Peckarsky BL (1996) Differential behavioural responses of mayflies from streams with and without fish to trout odour. Freshwater Biol 35:141–148
McKie BG (2002) Multiscale abiotic, biotic and biogeographic influences on the ecology and distribution of lotic Chironomidae (Diptera) in the Australian Wet Tropics. PhD thesis, School of Tropical Biology, James Cook University, Townsville
McKie BG (2004) Disturbance and investment: developmental responses of tropical lotic midges to repeated tube destruction in the juvenile stages. Ecol Entomol 29:457–466
McKie BG, Cranston PS (2005) Size matters: systematic and ecological responses of chironomid midge morphological ratios to experimental temperature manipulations. Can J Zool 83:553–568
McKie BG, Cranston PS, Pearson RG (2004) Gondwanan mesotherms and widespread eurytherms: effects of temperature on the development and survival of Australian Chironomidae (Diptera) from tropical and temperate populations. Mar Freshwater Res 55:759–768
McKie BG, Pearson RG, Cranston PS (2005) Does biogeographical history matter? Diversity and distribution of lotic midges (Diptera: Chironomidae) in the Australian Wet Tropics. Aust Ecol 30:1–13
McLachlan AJ (1986) Sexual dimorphism in midges: strategies for flight in the rainpool dwellerChironomus imicola (Diptera: Chironomidae). J Anim Ecol 55:261–267
McPeek MA, Peckarsky BL (1998) Life histories and the strengths of species interactions: combining mortality, growth and fecunditiy effects. Ecology 79:867–879
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trees 20:685–692
Moore MK, Townsend VRJ (1998) The interaction of temperature, dissolved oxygen and predation pressure in an aquatic predator–prey system. Oikos 81:329–336
Peacor SD, Werner EE (2001) The contribution of trait-mediated indirect effects to the net effects of a predator. Proc Natl Acad Sci USA 98:3904–3908
Pearson RG (1994) Limnology in the northeastern tropics of Australia, the wettest part of the driest continent. Mitt Int Verein Limnol 24:155–163
Pearson RG, Connolly NM (2000) Nutrient enhancement, food quality and community dynamics in a tropical rainforest stream. Freshwater Biol 43:31–42
Pearson RG, Benson LG, Smith REW (1986) Diversity and abundance of the fauna in Yuccabine Creek, a tropical rainforest stream. In: De Decker P, Williams WD (eds) Limnology in Australia. CSIRO, Melbourne, pp 329–342
Peckarsky BL, McIntosh AR (1998) Fitness and community consequences of avoiding multiple predators. Oecologia 13:565–576
Peckarsky BL, Cooper SD, McIntosh AR (1997) Extrapolating from individual behaviour to populations and communities in streams. J N Am Benthol Soc 16:375–390
Peckarsky BL, Taylor BW, McIntosh AR, McPeek MA, Lytle DA (2001) Variation in mayfly size at metamorphosis as a developmental response to risk of predation. Ecology 82:740–757
Pusey BJ, Kennard MJ (1996) Species richness and geographical variation in assemblage structure of the freshwater fish fauna of the wet tropics region of northern Queensland. Mar Freshwater Res 47:563–573
Reice SR (1994) Nonequilibrium determinants of biological community structure. Am Sci 82:424–435
Rosenfeld JS (2000) Contrasting effects of fish predation in a fishless and fish-bearing stream. Arch Hydrobiol 147:129–142
Rosser ZC, Pearson RG (1995) Responses of rock fauna to physical disturbance in two Australian tropical rainforest streams. J N Am Benthol Soc14:183–196
Schmitz OJ, Beckerman AP, Oȁ9Brien KM (1997) Behaviorally meditated trophic cascades: effects of predation risk on food web interactions. Ecology 78:1388–1399
Stoks R, De Block M, Van De Meutter F, Johansson F (2005) Predation cost of rapid growth: behavioural coupling and physiological decoupling. J Anim Ecol 74:708–715
Thompson DJ (1978) Toward a realistic predator-prey model: the effect of temperature on the functional response and life history of larvae of the damselfly, Ischnura elegans. J Anim Ecol 47:757–767
Turner AM (2000) Chemical cues modify species interactions: the ecological consequences of predator avoidance by freshwater snails. Oikos 88:148–158
Wassersug RJ, Sperry DG (1977) The relationship of locomotion to differential predation on Psuedacris triseriata (Anura: Hylidae). Ecology 58:830–839
Weetman D, Atkinson D, Chubb JC (1999) Water temperature influences the shoaling decisions of guppies, Poecilia reticulata, under predation threat. Anim Behav 58:735–741
Acknowledgements
We are grateful to Joe Holtum and Jack Christopher for freely given assistance with the controlled environment chambers, and to Niall Connolly and Mayuri Ando for help in the field and laboratory. Thanks also to Frank Johansson and three anonymous referees, who commented insightfully on this manuscript, and Leon Barmuta, Andrew Boulton and Athol McLachlan, whose detailed assessment of the doctoral thesis from which this manuscript arose greatly improved its quality. Finally, we are indebted to Peter Cranston, who as supervisor was involved at every stage of this work. The senior author was supported by an Australian Postgraduate Award, with research funding from the Rainforest Cooperative Research Centre. All experiments complied with relevant Australian regulations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Roland Brandl
Rights and permissions
About this article
Cite this article
McKie, B.G., Pearson, R.G. Environmental variation and the predator-specific responses of tropical stream insects: effects of temperature and predation on survival and development of Australian Chironomidae (Diptera). Oecologia 149, 328–339 (2006). https://doi.org/10.1007/s00442-006-0454-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0454-8