Abstract
The presence of annual and biennial individuals within the same population has been recently demonstrated in the myrmecophilous butterflies Maculinea rebeli and Maculinea alcon, which present a cuckoo strategy inside Myrmica nests, and Maculinea arion which is a predatory species. Here, we present field and laboratory data on polymorphic larval growth in two other predatory species of Maculinea: M. teleius and M. nausithous. Body mass distributions of pre-pupation larvae were bimodal in both species. These results point to the existence of larvae that develop in 1 or 2 years. We also showed that the probability of pupation depended on larval body mass. In the case of M. teleius, the critical body mass at which larvae have a 50% probability of pupation is about 80 mg. We suggest that polymorphism in Maculinea may have evolved as an adaptation to life in ant nests, a habitat which protects them from predators and provides food. However, the quality of this resource is highly variable and unpredictable. According to the bet-hedging hypothesis, if the habitat is unpredictable, females should have an advantage by producing more variable offspring. In the case of Maculinea butterflies, this may involve maintaining larvae that develop in 1 or 2 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of annual and biennial individuals within the same population is very rare in the animal kingdom and it has been found only in salmonid fish (Gross 1985), and Maculinea butterflies (Thomas et al. 1998a; Schönrogge et al. 2000). Schönrogge et al. (2000) found biennial larvae in the syrphid fly Microdon mutabilis, although larvae which develop within 1 year have not been confirmed for this species yet. The existence of biennialism in the Maculinea genus is surprising because these species do not possess traits associated with prolonged growth in insects, such as a short seasonal growth period, and a large body size, or feed on food which is not nutritious or inhabit a stable but inhospitable environment (Stearns 1992).
Maculinea butterflies possess highly specialised life cycles (Thomas et al. 1998b). Young caterpillars first feed on specific food plants, then drop to the ground and are carried by Myrmica ant workers into their nests. While living in Myrmica nests they feed in two different ways. Caterpillars of M. rebeli and M. alcon mimic the behaviour of ant grubs and are fed directly by nurse ants (cuckoo species), whereas caterpillars of M. teleius and M. arion prey on Myrmica brood (predatory species) (Elmes et al. 1991; Thomas and Wardlaw 1992; Thomas and Elmes 1998). M. nausithous is generally thought to feed as a predator inside Myrmica nests, but it has also certain characteristics typical of a cuckoo species (Fiedler 1990; Thomas and Elmes 1998). Predation is a less efficient way of feeding compared to the cuckoo strategy and results in high intra-specific competition and high mortality of predacious caterpillars in the host ant nests (Thomas and Wardlaw 1992; Thomas and Elmes 1998). The caterpillars of cuckoo species are also integrated to a greater extent in their host ant colonies and thus receive better protection than the predatory species (Thomas et al. 1998a). These differences make biennialism much more likely in cuckoo species than in predatory ones (Thomas et al. 1998a).
The existence of this phenomenon in Maculinea butterflies was initially confirmed for M. rebeli (Thomas et al. 1998a) and later for M. alcon (Schönrogge et al. 2000). However, Schönrogge et al. (2000) also provided evidence, though it was less strong than in the case of the two cuckoo species, for biennialism in the predatory species M. arion, and in the myrmecophilous syrphid fly, M. mutabilis L., which spends its larval life inside an ant nest and preys upon ant larvae (Elmes et al. 1999). Thus, Schönrogge et al. (2000) hypothesised that polymorphic growth rates may be a more general phenomenon in myrmecophilous social parasites.
Proving that larval polymorphism exists in other predatory species of Maculinea such as M. teleius and M. nausithous appears crucial for validation of this hypothesis. It may also shed light on the evolution of this phenomenon in this genus. In this paper, we present field and laboratory evidence for polymorphic growth in larvae of M. teleius. We compare body mass of larvae and pupae of M. teleius and assess the larval body mass when it pupates. We also present field data suggesting that larval polymorphism exists in M. nausithous larvae as well.
Materials and methods
The study was conducted on a wet meadow complex located in southern Poland (50°01′N, 19°54′E), 4 km southwest of Kraków city centre (Figurny and Woyciechowski 1998). The meadows comprise over 200 ha covered with Sanguisorba officinalis, the food plant of M. teleius and M. nausithous. Myrmica ant nests were searched for in 2003 and 2004 from June to the beginning of July, shortly before the butterflies started to eclose. Altogether 610 nests were found in 2003 and 1617 in 2004. All were opened to check for the presence of M. teleius and M. nausithous caterpillars or pupae; species identification was done using the key by Sliwinska et al. (2006). The larvae and pupae were taken to the laboratory where they were immediately weighed with 0.1-mg precision using a Radwag analytical balance. Subsequently, they were placed in small plastic boxes with a moistened sponge and kept at 20°C until butterfly eclosion. Larvae with body mass <60 mg were initially stimulated to reach the pupal stage by placing them together with ant workers and ant brood. However, none of them pupated, so later we gave up such attempts and used the small larvae for a genetic study (the results of which will be published elsewhere). The same happened to larvae which visibly suffered from fungal infection or physical damage.
In addition, we analysed the body mass of M. teleius larvae found in 94 Myrmica nests that were excavated in mid August 2004 for an adoption experiment (the results of which will be published elsewhere) as well as body mass of M. teleius larvae used in this experiment that were obtained from S. officinalis flower heads. During the experiment, the larvae were kept in standard laboratory ant nests under natural daily and seasonal cycles of light and temperature (Wardlaw 1991; Wardlaw et al. 1998). For comparison, we used the body mass of larvae recorded 5 weeks after adoption which corresponded to the maximum possible age of the larvae obtained from excavated nests in August provided that they entered ant nests that summer. The maximum age of caterpillars from excavated nests was estimated on the basis of the known date of the start of the imago flight period including 3 weeks of larval development in S. officinalis flower heads.
All standard statistical tests were conducted with Statistica 6.0 software (StatSoft 2003). Macdonald’s (1980) optimisation routine was used for the analysis of body mass distributions.
Results
The total number of larvae found was 200 for M. teleius and 20 for M. nausithous. For pupae, the respective figures were 105 and 11. The Kolmogorov–Smirnov test revealed no significant differences in body mass distribution of larvae of M. teleius obtained from the nests of different host ant species (D=0.28, P>0.05, in 2003; D=0.17, P>0.05, in 2004) thus the data were pooled together in all subsequent analyses. The body mass distributions of M. teleius larvae taken from wild host nests at pre-pupation time clearly showed a bimodal pattern (Fig. 1). According to Macdonald’s optimisation routine, these distributions were unlikely to originate from a single normal distribution (χ 2=50.64, P<0.001 in 2003; and χ 2=25.3, P=0.003 in 2004), but instead had a high probability of originating from two normal distributions (χ 2=16.86, P=0.39 in 2003; and χ 2=8.97, P=0.17 in 2004). The best-fit parameters (means±SE) of these distributions were 27.9±16.15 mg for smaller larvae, 103.5±6.32 mg for bigger larvae in 2003; and, respectively 55.9±35.7, 98.5±5.88 mg in 2004. Because the caterpillars were collected in the pre-pupation period, we conclude that the smaller larvae were 2-year developers that had entered Myrmica nests in the previous season. Despite the small sample size, a similar pattern could be traced for M. nausithous (Fig. 2) with Macdonald’s optimisation routine showing that data came rather from two normal distributions (χ 2=9.68, P=0.14) than a single one (χ 2=18.31, P=0.03). Mean body masses (±SE) were 41.1±52.74 mg for smaller larvae, and 74.0±6.34 mg for bigger larvae.
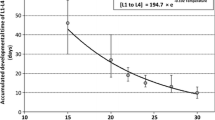
In the laboratory experiment, none of the M. teleius larvae reared in artificial Myrmica nests reached more than 18 mg in their fifth week (Fig. 3). Meanwhile, seven out of 23 larvae of this species found in the field in August 2004 were heavier than 20 mg, and two of them exceeded 30 mg, which may imply that they had been developing in ant nests since the previous summer. In contrast, it may seem striking that none of these larvae reached the mean body mass estimated for smaller larvae in late spring of the same year (see above); however, we believe that this is an artefact originating from very limited precision of the estimate as well as a rather small sample of August larvae.
Six larvae of M. teleius reared in the laboratory survived till spring 2005; they all weighed between 10 and 15 mg in April that year. One month later body mass of three of them remained almost unchanged at 13–20 mg, while the other three had grown rapidly reaching 45–66 mg, which once again suggests the existence of slow- and fast-developers among M. teleius larvae.
The comparison of M. teleius larvae that pupated in the laboratory with those that failed to do so, showed that the probability of pupation depended on larval body mass (logistic regression, χ 2=34.18, df=1, P<0.001 in 2003; χ 2=6.46, df=1, P<0.01 in 2004). Hypothetical body mass corresponding to the 50% probability of pupation was estimated at 107 and 88 mg during 2 consecutive years. The smallest larvae that pupated weighed 84.5 and 76.6 mg, respectively. They were considerably heavier than the smallest pupae found in ant nests (46 and 47.5 mg, respectively). However, it must be noted that Maculinea larvae obviously lose body mass during pupation (Thomas and Wardlaw 1992). For laboratory-reared M. teleius larvae, we recorded a significant body mass loss of up to 60.5 mg between the larval and pupal stage (paired-sample t-test, t 39=4.64, P<0.001 in 2003; t 40=4.72, P<0.001 in 2004.
Discussion
The previous studies of larval polymorphism in Maculinea butterflies proved that annual and biennial larvae within the same population exist in two cuckoo-feeding species of M. rebeli and M. alcon (Thomas et al. 1998a; Schönrogge et al. 2000). Schönrogge et al. (2000) also presented data for the presence of polymorphism in M. arion larvae which behave as predators inside Myrmica nests. Several of our results clearly show that annual and biennial larvae exist within the same populations of M. teleius, another predatory species, and M. nausithous, which shares characteristics of both predatory and cuckoo species. An additional indication of the existence of two larval cohorts in these species is their bimodal patters of adult eclosion within a season (Nowicki et al. 2005). All this implies that larval growth polymorphism is present in all five European species of Maculinea representing three types of Maculinea–Myrmica interactions. Consequently, we suggest similarly to Schönrogge et al. (2000), that it is an evolutionary trait common for the entire Maculinea genus and reflects ancestral life history. Recent phylogenies of Maculinea (Fiedler 1991; Als et al. 2004) indicate that their ancestors had a predatory strategy inside ant nests, just like the contemporary M. arion and M. teleius.
Thomas et al. (1998a) have presented two possible explanations for the evolution of larval polymorphism in M. rebeli, which is a cuckoo species. The first relates to ergonomic adaptation to its population structure and style of feeding. According to current knowledge, we conclude that the ergonomic hypothesis is not feasible, because annual and biennial larvae exist in predatory species as well. Another explanation given for polymorphic growth in Maculinea is associated with bet-hedging benefits. Thomas et al. (1998a) pointed out that individuals producing a mixture of 1- and 2-year developing larvae could benefit if occasional catastrophes in their habitat occur or if there is strong pressure from parasitoids. Recently, Hovestadt et al. (2005) developed an ESS model for evolution of growth polymorphism in M. rebeli and underlined that kin competition could be an important driving factor in this respect. Such an explanation may indeed be true for this particular species as well as for M. alcon, which both oviposit their eggs on gentian food plants that are often scarce and grow in clumps, and consequently several sibling larvae are likely to end up in the same ant nest. However, with their S. officinalis food plant typically being relatively abundant, females of M. teleius and M. nausithous rarely lay more than one egg per single plant (Figurny and Woyciechowski 1998). Hence the probability of kin competition appears to be extremely low in these species.
We suggest that the quality of the ant nest, a fundamental source of food and protection for larvae, should be more important. The quality of Myrmica nests can be highly variable, thus nests may be treated as quite unpredictable habitats for Maculinea larvae. Most Myrmica nests are small colonies consisting of 200–500 workers (Elmes et al. 1998), but in many species large colonies reaching >6,000 individuals may also be found occasionally (Wardlaw and Elmes 1996). It seems that variation in the size of nests is extremely high even within a single Myrmica species (Skórka et al 2006). The number of workers is correlated with the number of brood ants (Elmes and Wardlaw 1981) and both these traits have an effect on the survival of Maculinea larvae. Caterpillars may be adopted by a large colony with enough food for fast growth but may also end up in a small colony where resources are limited. In the latter case, there may be not enough food for fast development and consequently 1 year is insufficient for pupation. Our results indicate that the critical body mass at which M. teleius larvae have a 50% probability of pupation exceeds 80 mg (although this estimate should be treated with caution, because it was obtained under laboratory conditions that may different from the ones experienced by larvae in the field). It is also known that Myrmica colonies switch nest sites regularly (Elmes et al. 1998) and very often disperse after their brood is destroyed, e.g. by Maculinea parasitism (Thomas and Wardlaw 1992). Under these circumstances, the larvae are left behind in the empty nests and have to wait until another Myrmica colony arrives (Schönrogge et al. 2000) often enduring a long period of starvation.
Taking all these facts into consideration it may be beneficial for a single female to produce both 1- and 2-year developing larvae. Because most Myrmica nests are small, the majority of Maculinea larvae should be slow developers. This prediction is confirmed by the findings of Thomas et al. (1998a) according to which 75% of the adult population of M. rebeli consists of individuals that take 2 years to develop, and the remaining 25% take 1 year.
Finally, it has to be underlined that basically all the arguments explaining evolutionary benefits of larval polymorphism in Maculinea butterflies are also true for any species of social parasites living in ant nests. As the number of such insect species is close to 100,000 (Schönrogge et al. 2000) larval polymorphism is likely to be much more widespread than initially expected.
References
Als TD, Vila R, Kandul NP, Nash DR, Yen SH, Mignault AA, Boomsma JJ, Pierce NE (2004) The evolution of alternative parasitic life histories in large blue butterflies. Nature, DOI 10.1038/nature03020
Elmes GW, Wardlaw JC (1981) The quantity and quality of overwintered larvae in five species of Myrmica (Hymenoptera: Formicidae). J Zool 193:429–446
Elmes GW, Wardlaw JC, Thomas JA (1991) Larvae of Maculinea rebeli, a large blue butterfly, and their Myrmica host ants: wild adoption and behaviour in ant-nests. J Zool 223:447–460
Elmes GW, Thomas JA, Wardlaw JC, Hochberg ME, Clarke RT, Simcox DJ (1998) The ecology of Myrmica ants in relation to the conservation of Maculinea butterflies. J Insect Conserv 2:67–78
Elmes GW, Barr B, Thomas JA, Clark RT (1999) Extreme host specificity by Microdon mutabilis (Diptera: Syrphidae), a social parasite of ants. Proc R Soc Lond B 266:447–453
Fiedler K (1990) New information on the biology Maculinea nausithous and M. teleius (Lepidoptera: Lycaenidae). Nota Lepidopt 12:246–256
Fiedler K (1991) Systematic, evolutionary, and ecological implication of myrmecophily within the Lycaenidae (Insecta: Lepidoptera: Papillionidea). Bonn Zool Monogr 31:5–210
Figurny E, Woyciechowski M (1998) Flowerhead selection for oviposition by females of the sympatric butterfly species Maculinea teleius and M. nausithous (Lepidoptera: Lycaenidae). Entomol Gener 23:215–222
Gross MR (1985) Disruptive selection for alternative life histories in salmon. Nature 313:47–48
Hovestadt T, Mitesser O, Elmes G, Thomas J, Hochberg M (2005) Ass ESS model for evolution of growth polymorphism in the social parasite Maculinea rebeli. In: Study in the ecology and conservation of butterflies in Europe, vol 2. Species ecology along a European Gradient: Maculinea butterflies as a model. Pensoft, Sofia, pp 126–127
Macdonald PDM (1980) A Fortran programme for analyzing distribution mixtures. Statistical technical report 80-ST-1. McMaster University, Ontario
Nowicki P, Witek M, Skórka P, Settele J, Woyciechowski M (2005) Population ecology of the endangered butterflies Maculinea teleius and M. nausithous, and its implications for conservation. Popul Ecol, DOI 10.1007/s10144–005–0222–3
Schönrogge K, Wardlaw JC, Thomas JA, Elmes GW (2000) Polymorphic growth rates in myrmecophilous insects. Proc R Soc Lond B 267:771–777
Skórka P, Witek M, Woyciechowski M (2006) A simple and nondestructive methods for estimation of worker population size in Myrmica ant nests. Insects Soc 53:97–100
Sliwinska EB, Nowicki P, Nash DR, Witek M, Settele J, Woyciechowski M (2006) Morphology of caterpillars and pupae of European Maculinea species (Lepidoptera: Lycaenidae) with identification table. Entomol Fenn (in press)
StatSoft (2003) Statistica electronic manual, version 6. StatSoft, Tulsa, Okla.
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Thomas JA, Wardlaw JC (1992) The capacity of a Myrmica ant nest to support a predacious species of Maculinea rebeli inhabitants in ant nests. Oecologia 91:101–109
Thomas JA, Elmes GW (1998) Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol Entomol 23:457–464
Thomas JA, Elmes GW, Wardlaw JC (1998a) Polymorphic growth in larvae of the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc Lond B 265:1895–1901
Thomas JA, Clark RT, Elmes GW, Hochberg ME (1998b) Population dynamics in the genus Maculinea (Lepidoptera: Lycaenidae). In: Dempster JP, McLean IFG (eds) Insect population dynamics: in theory and pratice. Chapman & Hall, London, pp 261–290
Wardlaw JC (1991) Techniques for rearing Myrmica ants (Hym.) and Maculinea rebeli Hir. caterpillars. Entomol Mon Mag 127:233–241
Wardlaw JC, Elmes GW (1996) Exceptional colony size in Myrmica species (Hymenoptera: Formicidae). Entomologist 115:191–196
Wardlaw JC, Elmes GW, Thomas JA (1998) Techniques for studying Maculinea butterflies. I. Rearing Maculinea caterpillars with Myrmica ants in the laboratory. J Insect Conserv 2:79–84
Acknowledgements
We would like to thank Karsten Schönrogge for his help with Macdonald’s optimisation routine analysis. Anna Amirowicz, Marta Wantuch and Kajetan Woyciechowski kindly assisted us with the fieldwork. Two anonymous reviewers provided helpful comments on an earlier version of the manuscript. Specimens were collected with permission of the Polish Ministry of Environment. This study was financed by the EC within its RTD project EVK2-CT-2001-00126 as well as by the Polish Committee of Scientific Research by its grant SPUB-3024.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Konrad Fiedler
Rights and permissions
About this article
Cite this article
Witek, M., Sliwinska, E.B., Skórka, P. et al. Polymorphic growth in larvae of Maculinea butterflies, as an example of biennialism in myrmecophilous insects. Oecologia 148, 729–733 (2006). https://doi.org/10.1007/s00442-006-0404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0404-5