Abstract
Stable isotopes are widely used as time-integrating tracers of trophic interactions, but turnover rates of isotopes in animal tissues remain poorly understood. Here, we report nitrogen (N) isotope turnover rates in tissues of four primary consumer species: Ancistrus triradiatus armored catfish (muscle, fins, and whole blood), Tarebia granifera snails (muscle), and Rana palmipes tadpoles (muscle) from a Venezuelan river, and Lavigeria grandis snails (muscle) from Lake Tanganyika, East Africa. Turnover was estimated from the dilution of a 15N label introduced into consumer tissues by feeding on 15N-enriched periphyton. Muscle turnover rates were rapid (0.5–3.8% per day), and were attributable to metabolic replacement of N as well as growth in catfish and snails. N turnover in catfish muscle decreased with size, and fin tissue turned over more rapidly than whole blood or muscle, though the difference was not significant. Our results indicate that stable isotope signatures of these tropical species could change markedly within weeks following a shift in diet. However, generalization across taxa or latitudes is complicated by the strong size-dependence of isotope turnover rates. The enrichment-dilution approach outlined here may facilitate measurement of isotopic turnover in a wide variety of consumers under field conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stable isotopes are widely used as tracers of energy and nutrient flow in food webs (Peterson and Fry 1987). The interpretation of stable isotope data depends on a variety of assumptions about the relationship between isotope ratios of consumers and their food resources (Gannes et al. 1997; Post 2002). Some of these issues have received intense scrutiny (e.g. trophic fractionation; Vander Zanden and Rasmussen 2001; Post 2002; McCutchan et al. 2003; Vanderklift and Ponsard 2003), but others such as temporal turnover rates are less well understood.
A key feature of stable isotopes is that they reflect animal dietary patterns over long periods of time compared to direct analysis of stomach contents (Peterson and Fry 1987; Vander Zanden et al. 1997). However, the actual period across which diet is integrated depends on the turnover rate of the sampled tissue (Hobson and Clark 1992; Hesslein et al. 1993; MacAvoy et al. 2001). Disparities in turnover rates among taxa and tissues complicate the interpretation of isotopic differences. In comparisons among consumer taxa, isotopic differences could arise from either actual trophic segregation or unequal turnover rates among consumers overlaid upon temporal shifts in resource isotope ratios (e.g. O’Reilly et al. 2002). Similarly, inferences about the relative importance of alternative foods to a consumer depend on the stability of resource isotope ratios during the time period represented by consumer tissues (Cabana and Rasmussen 1996; Post 2002).
The tissues of long-lived animals vary less in isotopic composition than those of their prey (Cabana and Rasmussen 1996; Vander Zanden and Rasmussen 1999; Post 2002). This is due in part to the larger size of consumers than their prey (Cohen et al. 1993), which is associated with lower mass-specific growth and metabolic rates (Peters 1983; Gillooly et al. 2001). At one extreme, algae and microbes exhibit frequent isotopic shifts due to their high biomass-specific growth rates, high nutrient uptake and storage capacity, and short lifespan (Cabana and Rasmussen 1996; McCutchan and Lewis 2001; Post 2002). At the other extreme, muscle tissue from long-lived animals such as mollusks and fishes integrates dietary isotope ratios over periods of months or years (Hesslein et al. 1993; Cabana and Rasmussen 1996; Vander Zanden and Rasmussen 1999; Post 2002). Isotopic turnover rates also can vary markedly among different tissues of a single animal (Tieszen et al. 1983; Hobson and Clark 1992; MacAvoy et al. 2001).
Given the increasing use of stable isotope analysis to study trophic ecology, it is critical to determine the extent and causes of variation in isotopic turnover rates in animal tissues. Our survey of the literature on N isotope turnover rates in aquatic invertebrates and fishes indicates substantial differences among taxa and even conspecific life stages (Table 1). Much of this variation appears to be associated with body size. For instance, the half-life of tissue N in larval fishes is less than a week (e.g. Herzka and Holt 2000; Vander Zanden et al. 1998; Bosley et al. 2002; Witting et al. 2005), compared to months in large fishes (e.g. Hesslein et al. 1993; MacAvoy et al. 2001; Harvey et al. 2002). There is also evidence that N turnover rates are positively related to temperature (Frazer et al. 1997; Bosley et al. 2002; Witting et al. 2005). These patterns accord with general relationships between metabolic rates and both body size and temperature (Peters 1983; Clarke and Johnston 1999; Gillooly et al. 2001).
The literature on N isotope turnover rates has expanded considerably in recent years (Table 1), but remains limited in several ways. First, most previous studies have been conducted under laboratory conditions. Second, no single study has evaluated N turnover in multiple species under the same conditions, thus taxonomic comparisons are confounded with experimental conditions. Third, experiments have relied primarily upon switching consumers between alternative diets that differ naturally in stable isotope ratios. This approach requires long periods of time in order for animals to achieve isotopic equilibrium with the new diet, and is difficult to adapt to field conditions. Finally, there has been little work on N turnover rates in tropical species, which experience warm temperatures throughout the year. These animals are expected to have higher average growth and metabolic rates than their temperate counterparts (Benke 1998; Clarke and Johnston 1999), and may show higher N turnover rates as a consequence.
This paper reports N isotope turnover rates from four species of primary consumers at two tropical sites. Rather than switching animals between alternative diets with natural differences in isotopic composition, our strategy was to measure the loss of a 15N label. We allowed focal animals to feed on 15N-enriched periphyton, then returned them to a diet of periphyton with the natural abundance of 15N. The dilution of 15N through time was used to quantify N turnover rates in multiple tissue types. This enrichment-dilution approach offers both challenges and benefits compared to traditional diet-switching methods. Our results support the dependence of turnover rates on body size and temperature, and suggest that tissue N turns over rapidly in tropical catfish and snails.
Materials and methods
Experiment 1
The first experiment compared three species of primary consumers from Rio Las Marias, a fourth-order river in the Andean piedmont of Venezuela (9°10′N, 69°44′W). We selected armored catfish (Ancistrus triradiatus), snails (Tarebia granifera), and tadpoles (Rana palmipes) because they were abundant during the dry-season study period (January–February 2002) and consume a combination of algae and detritus. A broad size range of individuals from each species was collected from the river for use in the experiment (Table 2).
To introduce the 15N label into animal tissues, we allowed them to feed on periphyton grown under 15N-enriched conditions. Enrichments were conducted in plastic pools containing 70 l of river water and periphyton-covered cobbles from the river. We added 250 mg 15NH4Cl (99 atom% 15N) to each pool, thereby increasing total dissolved N by ~850%. Periphyton grew without consumers for 2 days, then we introduced catfish into one pool, snails into another, and tadpoles into a third. After feeding on the 15N-enriched periphyton for 6 days, all animals were transferred into a non-enriched pool for a day in order to flush non-assimilated 15N from their bodies. Following the enrichment and flushing periods, animals were transferred to six replicate, non-enriched pools (70 l) for the remainder of the experiment. To ensure even size distributions, each species was divided into size classes from which individuals were haphazardly assigned to pools to yield a density of 5–6 catfish, 6–7 snails, and 7 tadpoles per pool. Fresh cobbles were transferred from the river every 3–5 days to ensure continual access to non-enriched periphyton. Pools were covered with mosquito netting to prevent entry of animals or debris. Daily fluctuations in water temperature (20–27°C) were similar to those in the river.
Six individuals of each consumer taxon were sampled before enrichment to determine their natural abundance of 15N. Following the enrichment, one individual per taxon was sampled from each non-enriched pool on six dates (days 0, 5, 10, 16, 21, and 26). Snail mortality reduced sample sizes to three individuals per date during the middle of the experiment, and one catfish blood sample was lost. The 15N of non-enriched and enriched periphyton was measured from scrapings collected before enrichment and after the enrichment period, respectively.
Three tissues were sampled from catfish: whole blood (via cardiac puncture), fin (soft rays, webbing, and associated mucus of left pectoral fin), and muscle (dorsal musculature). Tadpole tail muscle was sampled by dissection from skin and skeletal elements. The foot muscle of snails was sampled by dissection from other soft parts, shell, and operculum. The wet mass of catfish and tadpoles and the shell length of snails were measured before sampling, and snail shell length was converted into wet mass of soft tissue using a regression equation for this species (r 2=0.99, n=10). All samples were placed in glass vials and desiccated (60°C, 48 h).
Muscle samples from each individual were analyzed separately. To achieve sufficient sample mass, blood samples from six catfish were pooled to yield one datum per sampling date, and equal masses of fin material from three fish were combined to yield two data per sampling date. Dried samples were ground, subsampled (1 mg), and analyzed using a Finnigan MAT Delta Plus in the Cornell Isotope Laboratory. Measurements were calibrated against reference standards (trout tissue, methionine, N2 gas) of known 15N content. For enrichments exceeding 3,000‰ δ15N (1.45 atom% 15N), samples were diluted with non-enriched tissue from the same species to comply with instrument and laboratory guidelines while maintaining their elemental and molecular composition, and the actual enrichment was back-calculated using a mixing model. Analytical precision for non-enriched samples was 0.2‰ δ15N (SD), and duplicate analyses of enriched (δ15N=258–2857‰; n=13) samples showed SDs of 1.7–55.5‰ δ15N. Unless otherwise noted, for the remainder of the paper isotope data are expressed in units of atom% 15N, which is the percentage of N atoms as 15N {i.e. [15N/(14N+15N)]×100}.
Experiment 2
The second experiment used snails from Lake Tanganyika, the largest of the East African rift lakes. We selected Lavigeria grandis for the study because it is abundant, relatively large, and dwells on boulders and bedrock where marked individuals are easily recaptured (West et al. 2003). Individuals representing a range of adult sizes were collected from Jakobsen’s Beach (4°55′S, 29°36′W) near Kigoma, Tanzania.
The experiment was conducted during June–August 2002 using an enrichment protocol similar to that in Experiment 1. A plastic box was filled with 42 l of lake water and aerated continuously. The bottom was covered with cobbles from the lake, and we added 90 mg 15NH4Cl (99 atom% 15N) to increase the concentration of total dissolved N by ~500%. One day later, snails were collected from the lake, marked on the shell with nail polish, and allowed to feed on enriched periphyton for 4 days. They were subsequently released onto a large boulder (2 m diameter, 3 m height) in the lake where they could feed on periphyton with the natural abundance of 15N. The lake water temperature was 25–27°C.
Foot muscle samples were collected from three snails before the enrichment and three individuals on each of nine dates afterward (days 0, 8, 14, 22, 29, 35, 42, 49, and 55). Snails were processed and analyzed as described earlier, and the wet mass of soft tissues was estimated from shell size using a regression equation for this species (r 2=0.99, n=29). 15N of non-enriched and enriched periphyton was measured from scrapings collected before enrichment and at the conclusion of the enrichment period, respectively.
Turnover modeling
Daily turnover rates were estimated from time series of tissue 15N by fitting two exponential models. The first was a decay model adapted from Tieszen et al. (1983) and Hesslein et al. (1993):
where t is the number of days the consumer had access to non-enriched food sources following enrichment, 15N t is the tissue atom% 15N at time t, 15Npre is the tissue atom% 15N prior to enrichment, and 15Npost is the equilibrium atom% 15N toward which the tissue is returning following enrichment. We assumed that 15Npost=15Npre in our experiments because the potential isotopic difference between the natural-abundance diets before and after enrichment was small relative to the enrichment; the implications of this assumption are discussed later. Two parameters were fitted to the data: k and 15Npeak′. k is the exponent describing the proportion of 15N lost daily from the tissue due to both growth and metabolic replacement. 15Npeak is the 15N immediately following enrichment (t=0), though it is not critical that the actual peak value be identified as long as the turnover rate is stable throughout the dilution curve. It was fitted as a constant because destructive sampling prevented measuring both 15Npeak and 15N t (t>0) from the same individual.
To account for potential negative size-scaling of turnover rates within species due to allometry of growth and metabolism, our second model elaborated on the basic model by including the effect of body size on the initial enrichment. 15Npeak was replaced with a power function of individual mass:
where 15Npeak′ is the 15N of a consumer at t=0, M is its wet mass (g) measured or estimated at the time of sampling, and c and a are constants to be fitted along with k. A power function was chosen because growth and metabolic rates scale as a power of mass (Peters 1983). This second model was applied only to muscle data because catfish blood and fin data represented multiple individuals, hence an individual M was not applicable. Intraspecific variation in mass ranged from 2.4- to 11-fold among species (Table 2), offering sufficient power to test the effects of size.
We compared the two- (15Npeak, k) and three-parameter (c, a, k) models using AICc, a small-sample version of the Akaike Information Criterion recommended by Burnham and Anderson (2002). AICc is an index of how well alternative statistical models balance descriptive power and parsimonious parameterization. The model with lower AICc was selected for interpretation.
In addition to the potential effects of mass on the initial enrichment, which are evaluated using the three-parameter model, the subsequent rate of loss of the 15N label could be size-dependent. To assess the latter, we tested for a correlation between mass and estimated turnover of muscle N within each study species. An approximate muscle N turnover rate was calculated by rearranging Eq. 1 to solve for k for each individual (i):
where 15N t was measured (t>0) and 15Npeak (or 15Npeak′) was fitted using the turnover model with the lowest AICc for the species. If all observed 15N t were predicted perfectly by the fitted model, all k i would equal k. Given an imperfect match, a correlation among conspecifics between mass and k i indicates a systematic difference in turnover rate as a function of body mass. We tested the correlation between k i and individual size ranking using Kendall’s τ. When a significant relationship was detected, we used a contour plot to visualize the joint effects of mass and sampling date on muscle 15N.
Curve fitting was performed using the Marquardt method in the NLIN procedure of SAS, and Kendall’s τ was calculated using the CORR procedure of SAS. Contour plots were created in SigmaPlot using Loess smoothing.
Results
Experiment 1
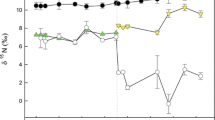
Periphyton in the enrichment pools quickly incorporated 15N, reaching ~19.2 atom% 15N. This label was passed on to all three consumer species (Table 2). Following their return to a diet with the natural abundance of 15N (0.3662 atom% 15N), the tissue 15N of consumers decreased according to the expected exponential pattern (Fig. 1). However, the 15N of catfish blood and snail muscle rose between the first (t=0) and second (t=5) dates, so we modeled data from t≥5 in those cases.
Exponential dilution of the 15N label in a catfish muscle, b catfish fin, c catfish blood, d tadpole muscle, e Tarebia snail muscle, and f Lavigeria snail muscle following a return to a diet with the natural-abundance of 15N (~0.366 atom%) on experiment day 0. The solid line illustrates the preferred turnover model; see Table 3 for equations. Including body mass in the turnover models significantly improved descriptive power in a, d, and f, though this effect is not included in the scatterplots. Dashed lines represent the 15N toward which each curve is moving
Daily turnover of N in catfish fins and blood were estimated as 5.7 and 4.1%, respectively, and the two-parameter model explained the majority of the variance in enrichment (Tables 2, 3). The three-parameter model described the muscle data better than the two-parameter model, indicating turnover of 3.8% of N per day. Overlapping confidence intervals indicated a lack of statistically significant differences in turnover rates among catfish tissues. Small catfish assimilated more 15N during the enrichment period than larger conspecifics, as indicated by the negative overall scaling of muscle 15Npeak′ with mass (a=−0.5284±0.0827 SE; Table 3), and the inverse relationship between mass and observed 15N at t=0 (Fig. 2).
Muscle N of Tarebia snails also turned over quickly (3.4% per day), and the two-parameter turnover model was sufficient to describe the data (Tables 2, 3). Tadpole muscle turned over more slowly (0.5% per day), and the estimated turnover rate was not different from zero. The three-parameter model fit tadpole data better than the two-parameter model, but explained only 24% of the variance (Table 3).
The analysis of intraspecific variation in muscle turnover estimates (k i ) suggested significant effects of body mass on muscle N turnover rates in catfish (Kendall’s τ=−0.26, P=0.044) but not tadpoles (P=0.630) or snails (P=0.205). The negative correlation coefficient indicates that dilution of the 15N label occurred more rapidly in small catfish than in larger individuals. This pattern is also evident in Fig. 2; small catfish exhibited a broader range of muscle 15N during the course of the experiment than large catfish, reflecting faster turnover.
Experiment 2
Periphyton in the enrichment pools became highly enriched (~2.4 atom% 15N), and the label was transferred to Lavigeria snails (Table 2). After snails were released into the lake to feed on periphyton with the natural abundance of 15N (0.3660 atom% 15N), their muscle 15N decreased exponentially (Fig. 1f). The N turnover rate was estimated as 1.4% per day (Table 2), and the three-parameter model offered significantly greater descriptive power than the two-parameter model (Table 3). Individual estimates of turnover (k i ) were not correlated with snail tissue wet mass (P=0.180).
Discussion
Most species in our experiments experienced rapid turnover of tissue N, as expected based on the dependence of growth and metabolic rates upon body size and temperature (Peters 1983; Gillooly et al. 2001). The half-life of muscle N in catfish and snails ranged from 18 to 50 days (Table 2), indicating that N isotope ratios in these animals integrate diet over timescales of weeks to months. Tissue N half-lives appear to be considerable longer among species of comparable size from the temperate zone (Table 1). Nevertheless, the N turnover rates that we observed in tropical primary consumers are much lower than those inferred from primary producers (Cabana and Rasmussen 1996; McCutchan and Lewis 2001; Post 2002), supporting the use of these animals to establish isotopic baselines in food web studies.
Turnover rates of N in muscle, fin, and whole blood of armored catfish were rapid and statistically equivalent (Table 2). Unfortunately, our small sample size for fins and blood resulted in low statistical power despite reasonably good descriptive power (Table 3). We had expected that fins would change more rapidly than muscle because they include a layer of mucus that is probably regenerated continuously (e.g. Schmidt et al. 1999). Although the differences among tissues were not significant, estimated daily turnover of N in fins was >1.6% higher than in blood or muscle. This potential difference merits further investigation because the combination of rapid N turnover and non-lethal sampling would make fins useful for studies of diet shifts and movement patterns. Sampling regrown fins also could allow a simple test of how the isotopic composition of new tissues compare to that of recent diet. However, these analyses would need to account for potentially complex routing of materials to fins, which are a composite of several tissue types that probably differ in derivation and turnover rate.
Based on previous results from birds (Hobson and Clark 1992) and fish (MacAvoy et al. 2001), we had also expected that catfish blood would turn over more quickly than muscle. Though the plasma fraction of blood might have changed more quickly (e.g. Pearson et al. 2003), our analyses indicate comparable turnover rates in whole blood and muscle of these small fish (Fig. 1). The increase in blood 15N in the 5 days following the enrichment period also suggests complex routing of dietary N into whole blood (see also Bearhop et al. 2002).
Muscle N of snails at both study sites turned over rapidly, but turnover rates in Venezuelan Tarebia were more than double those in Lavigeria from Lake Tanganyika (3.4 vs 1.4% per day; Fig. 1e–f). This disparity probably arises from the 10-fold difference between these species in tissue wet mass (Table 2), which is expected to result in 75% faster turnover in Tarebia based solely on the scaling of mass-specific metabolism (~M−0.25; Peters 1983; Gillooly et al. 2001). It was also notable that Tarebia muscle became more enriched than that of catfish or tadpoles (Fig. 1), perhaps due to their small size or dietary preferences.
These are the first measurements of N turnover rates in tropical snails, and we are aware of only two relevant studies involving temperate species. Monthly measurements of N turnover in the tissue of salt-marsh Littorina in Georgia (Kemp et al. 1990) suggest maximum daily turnover of ~0.7% of tissue N (Table 1). Using data from a whole-stream 15N-enrichment in Tennessee (Mulholland et al. 2000), we calculate daily dilution of the 15N label in the tissue of small Elimia snails of ~1% (Table 1). Muscle N turnover rates were much higher in the tropical snails that we studied, which are at least as large as Littorina and Elimia. Further studies of isotopic turnover rates in mollusk tissues are needed because snails and clams are widely used to set isotopic baselines in aquatic food web studies (Cabana and Rasmussen 1996; Vander Zanden and Rasmussen 1999; Post 2002).
The tadpole data suggest slower turnover of muscle N than in catfish or snails (Table 3). This is surprising because the tadpole stage in frog development is tuned for rapid growth (Werner 1986), therefore we expected rapid dilution of the 15N label. However, the low explanatory power of the three-parameter model (r 2=0.24; Table 3) and large standard error of the turnover estimate prevent any strong conclusions (Table 2). We have previously observed variable growth rates in tadpoles of this species even within a single experimental pool (McIntyre et al. 2004), and such differences probably contributed to inconsistent 15N enrichment.
Factors affecting N turnover rates
A growing number of studies on aquatic animals implicate body size and temperature as key influences on isotopic turnover rates. In accordance with general patterns of metabolic scaling (Peters 1983; Gillooly et al. 2001), there is an inverse relationship between body size and N turnover rates reported in the literature, particularly among fish (Table 1). Fish >5 g have N turnover rates of ≤1.0% per day, small fishes (0.1–0.9 g) show intermediate rates (0.7–2.1% per day), and larval fishes (<0.1 g) have rapid N turnover (>5% per day). Our study offers further evidence of the negative scaling of turnover with body size. Interspecific differences between snails roughly conformed to expectations based on metabolic scaling. Similarly, intraspecific patterns of initial enrichment (15Npeak′) and subsequent loss of the 15N label from catfish muscle were both inversely related to individual mass (Fig. 2).
Temperature may also help to explain why isotopic turnover rates in our tropical study species were more similar to those of birds (Hobson and Clark 1992) and mammals (Tieszen et al. 1983) than like-sized aquatic poikilotherms from the temperate zone (Table 1). Respiration of aquatic invertebrates and fishes almost doubles over a temperature range of 15–25°C (Clarke and Johnston 1999), and isotopic turnover rates increase with temperature in laboratory experiments (Frazer et al. 1997; Bosley et al. 2002; Witting et al. 2005; but see Herzka and Holt 2000). Under field conditions, seasonal patterns of N turnover in saltmarsh snails suggest temperature effects (Kemp et al. 1990). Further field data come from whole-system 15N enrichments, which offer an ecosystem-scale analog to our enrichment-dilution approach once the isotopic composition of food resources returns to natural-abundance levels. Results from whole-stream enrichments in Puerto Rico (22°C water; Merriam et al. 2002) and Tennessee (12.4°C water; Mulholland et al. 2000) suggest more rapid N turnover in tropical invertebrates, though the available data are insufficient to make quantitative comparisons.
Another emphasis of previous research has been the relative influence of metabolic replacement of existing biomass and growth of new biomass on changes in tissue isotopic composition. Most studies have concluded that growth explains a majority of turnover (Hesslein et al. 1993; Vander Zanden et al. 1998; MacAvoy et al. 2001; Harvey et al. 2002), though metabolic replacement of N is also important in some cases (Vander Zanden et al. 1998; Herzka et al. 2001; Maruyama et al. 2001). Our models did not differentiate between growth and metabolic replacement because we did not measure growth directly. However, the same species and sizes of catfish increase in mass by 0.9–1.1% per day in enclosures at our field site (Solomon et al. 2004). These growth rates might account for most N turnover in our largest catfish, but could explain only a minor portion in small catfish (Fig. 2). Tadpole growth rates in field enclosures and experimental pools at our study site average 0.6–1.4% per day (McIntyre et al. 2004; Solomon et al. 2004), potentially accounting for all of the N turnover observed in this study. Though no data are available for the snail species, our observations under experimental and field conditions suggest that their growth rates are much lower than the observed turnover rates of muscle N (see also Kemp et al. 1990). To our knowledge, only very small snails are capable of growing 3% per day (e.g. Hall et al. 2003). Thus, we infer that metabolic replacement of muscle N was important in catfish and snails, but not in the tadpoles that we studied.
Though it has received less attention, dietary N content could also have important effects on N turnover rates. Consumers with low dietary N are more reliant upon reusing amino acids than conspecifics with higher dietary N (Webb et al. 1998; Carter and Bransden 2001), and animal prey contain more N than algae or plants (Sterner and Elser 2002). Thus, carnivores at our study sites could have higher net N turnover than the primary consumers that we studied due to lower internal N recycling and higher growth rates.
15N enrichment-dilution approach
Our study and others demonstrate the ease of labeling primary consumers using enriched primary producers (e.g. Beviss-Challinor and Field 1982; Kemp et al. 1990), and enriched primary consumers can be used subsequently to label carnivores (Steffan et al. 2001). The greatest challenge to using this approach to measure isotopic turnover rates is achieving uniform enrichment of experimental animals. For instance, body size significantly affected muscle enrichment (15Npeak) in three of four species in our experiments (Table 3), producing much of the scatter in Fig. 1. Our study design featured a continuous range of body sizes. However, use of replicate individuals within discrete size groupings would facilitate quantification of size effects in future studies.
Even after controlling for body size, differences in ingestion or assimilation rates among conspecifics could be amplified by short-term enrichments. Similarly, variation in enrichment of alternative food resources (e.g. algal taxa, microbes) within enrichment pools could increase variance in consumer enrichment. These problems might be alleviated by offering a standardized ration of enriched foods to each individual, or extending the enrichment period in order to reduce the influence of random, short-term feeding differences.
The problem of heterogeneity in enrichment could be circumvented using non-lethal sampling of consumer tissues. Serial samples from the same individual could be used to construct an individual-level dilution curve describing turnover, and parallel analyses of many individuals would allow generalization about turnover rates at the species level. Fins and blood of large fishes would be suitable for such repeated sampling, and muscle might also be sampled non-lethally using biopsies (e.g. Baker et al. 2004). However, serial sampling of muscle is less practical for snails, tadpoles, or small fish like those used in this study.
The turnover rates estimated from the enrichment-dilution approach also represent a subtly different quantity than that measured in traditional diet-switches. Both approaches rely on exponential turnover equations that assume that all body tissues are in isotopic equilibrium before switching diets (Fry and Arnold 1982; Tieszen et al. 1983; Hesslein et al. 1993). This assumption is valid in traditional diet-switching studies, which yield estimates of isotopic turnover that reflect incorporation of material from the new diet both directly and through indirect pathways via rapid-turnover internal pools (see Ayliffe et al. 2004). However, internal mobilization of material from slow-turnover pools would be largely invisible because it reflects the isotopic composition of the old diet for a long period of time. In contrast, turnover rates measured in enrichment-dilution experiments include the contribution of slow-turnover pools because these pools reflect the isotopic signature of the pre-enrichment diet, which is similar to that of the post-enrichment diet. Thus, the enrichment-dilution approach could indicate higher tissue turnover rates than traditional diet-switching methods if use of slow-turnover internal pools is substantial (e.g. Ayliffe et al. 2004). The general importance of slow-turnover pools is not yet clear, but is likely to differ among tissues and depend on feeding rates. Thus, at present it is reasonable to consider isotopic turnover rates estimated by both approaches as comparable.
Despite these challenges, our enrichment-dilution approach offers several advantages over natural-abundance diet switches. Most importantly, natural periphyton was used as the food source throughout our experiments, and animals were allowed to regulate their own consumption rates. Both the quantity and quality of food can affect tissue turnover rates (Carter and Bransden 2001; Tominaga et al. 2003), hence turnover estimates based on natural food sources and feeding rates are likely to be more useful for interpreting field data than rates based on switches between artificial diets under laboratory conditions.
Rapid labeling of consumer tissues through short-term enrichments also eliminates the requirement that experiments proceed until isotopic equilibrium is reached. Natural-abundance diet switches require either determination of the equilibrium isotope ratio using long-lasting experiments (e.g. Hesslein et al. 1993) or use of assumed fractionation factors to estimate the equilibrium isotope ratio of consumers relative to the new diet (e.g. Frazer et al. 1997). In the enrichment-dilution approach, the isotopic equilibrium following a return to natural-abundance food sources (15Npost) should approximate the pre-enrichment condition (15Npre) as long as food resources are refreshed regularly to ensure that recycled 15N is not incorporated. To explore the implications of violating this assumption, we tested the sensitivity of turnover rates in Lavigeria snails from Experiment 2 to the value of 15Npost while holding all other parameters constant. We varied 15Npost by 0.0077 atom%, representing the total range of 15N observed within the food web at each study site. Increasing and decreasing 15Npost by that amount yielded negative and positive changes, respectively, of ~10% in the fitted turnover coefficient (k). After halving the magnitude of the enrichment, the same increase in 15Npost led to a 19% decrease in k. These analyses indicate that the enrichment-dilution method is not very sensitive to plausible inequalities between 15Npre and 15Npost, and that sensitivity is inversely related to the magnitude of the enrichment. Thus, the large enrichments in our experiments would have alleviated the influence of any differences between 15Npre and 15Npost.
Measuring isotopic dilution following a short-term enrichment also reduces the time period required to quantify turnover rates. In only a few days, enrichments drastically increase the differences between endmembers in isotopic turnover models (15Npeak and 15Npost). This allows measurement of relatively large absolute changes in isotope ratios over short periods of time, which would be particularly useful for slow-turnover species. In contrast, natural-abundance diet switches require long periods between samples in order to quantify isotopic changes, due in part to low analytical precision (SD=0.1–0.3‰ δ15N) relative to the isotopic differences between diets (2–15‰ δ15N). In our experiments, analytical precision was reduced for enriched samples (SD=1.7–55.5‰ δ15N), however the decrease was generally small in proportion to the enrichments (262–2818‰ δ15N).
Conclusions
The interpretation of stable isotope data from field studies requires an understanding of isotopic turnover rates, and this study offers the first measurements of N turnover in tropical freshwater animals. Our results suggest that tissue N turnover occurs rapidly in these primary consumers, though body size had strong effects both within and across species. It remains to be seen whether turnover is generally faster in the tropics than the temperate zone, and whether tropical carnivores exhibit even more rapid N turnover than the species we studied.
Our enrichment-dilution approach offers a promising alternative to traditional natural-abundance diet switches. Reducing the time period required for experiments will ease a major logistical barrier to quantifying turnover rates. The method is also well-suited to field-based studies, which may be more useful for interpreting stable isotope data from wild animals than laboratory results. Though important challenges remain, we are optimistic that refinements of the enrichment-dilution approach will facilitate systematic comparisons of turnover rates across taxa, tissues, and environmental conditions.
References
Ayliffe LK, Cerling TE, Robinson T, West AG, Sponheimer M, Passey BH, Hammer J, Roeder B, Dearing MD, Ehleringer JR (2004) Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia 139:11–22
Baker RF, Blanchfield PF, Paterson MJ, Flett RJ, Wesson L (2004) Evaluation of nonlethal methods for the analysis of mercury in fish tissue. Trans Am Fish Soc 133:568–576
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458
Benke AC (1998) Production dynamics of riverine chironomids: extremely high biomass turnover rates of primary consumers. Ecology 79:899–910
Beviss-Challinor MH, Field JG (1982) Analysis of a benthic community food web using isotopically labeled potential food. Mar Ecol Prog Ser 9:223–230
Bosley KL, Witting DA, Chambers RC, Wainright SC (2002) Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Mar Ecol Prog Ser 236:233–240
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach, 2nd edn. Springer, Berlin Heidelberg New York
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using nitrogen isotopes. Proc Natl Acad Sci USA 93:10844–10847
Carter CG, Bransden MP (2001) Relationships between protein-nitrogen flux and feeding in greenback flounder, Rhombosolea tapirina. Comp Biochem Physiol A 130:799–807
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Cohen JE, Pimm SL, Yodzis P, Saldana J (1993) Body sizes of animal predators and animal prey in food webs. J Anim Ecol 62:67–78
Frazer TK, Ross RM, Quetin LB, Montoya JP (1997) Turnover of carbon and nitrogen during growth of larval krill, Euphausia superba Dana: a stable isotope approach. J Exp Mar Biol Ecol 212:259–275
Fry B, Arnold C (1982) Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 54:200–205
Gannes LZ, O’Brian DM, Martinez del Rio C (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78:1271–1276
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Hall RO, Tank JL, Dybdahl MF (2003) Exotic snails dominate nitrogen and carbon cycling in a highly productive stream. Front Ecol Environ 1:407–411
Harvey CJ, Hanson PC, Essington T, Brown P, Kitchell JF (2002) Using bioenergetics models to predict stable isotope ratios in fishes. Can J Fish Aquat Sci 59:115–124
Herzka SZ, Holt GJ (2000) Changes in isotopic composition of red drum (Sciaenops ocellatus) larvae in response to dietary shifts: potential applications to settlement studies. Can J Fish Aquat Sci 57:137–147
Herzka SZ, Holt SA, Holt GJ (2001) Documenting the settlement history of individual fish larvae using stable isotope ratios: model development and validation. J Exp Mar Biol Ecol 265:49–74
Hesslein RH, Hallard KA, Ramlal P (1993) Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to change in diet traced by δ34S, δ13C, and δ15N. Can J Fish Aquat Sci 50:2071–2076
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of δ13C in tissues. Condor 94:181–188
Kemp PF, Newell SY, Hopkinson CS (1990) Importance of grazing on the salt-marsh grass Spartina alterniflora to nitrogen turnover in a macrofaunal consumer, Littorina irrorata, and to decomposition of standing-dead Spartina. Mar Biol 104:311–319
MacAvoy SE, Macko SA, Garman GC (2001) Isotopic turnover in aquatic predators: quantifying the exploitation of migratory prey. Can J Fish Aquat Sci 58:923–932
Maruyama A, Yamada Y, Rusuwa B, Yuma M (2001) Change in stable nitrogen isotope ratio in the muscle tissue of a migratory goby, Rhinogobius sp., in a natural setting. Can J Fish Aquat Sci 58:2125–2128
McCutchan JH, Lewis WM Jr (2001) Seasonal variation in stable isotope ratios of stream algae. Verh Int Verein Limnol 27:3304–3307
McCutchan JH, Lewis WM Jr, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
McIntyre PB, Baldwin S, Flecker AS (2004) Effects of behavioral and morphological plasticity on risk of predation in a Neotropical tadpole. Oecologia 141:130–138
Merriam JL, McDowell WH, Tank JL, Wollheim WM, Crenshaw CL, Johnson SL (2002) Characterizing nitrogen dynamics, retention, and transport in a tropical rainforest stream using an in situ 15N addition. Freshw Biol 47:143–160
Mulholland PJ, Tank JL, Sanzone DM, Wollheim WM, Peterson BJ, Webster JR, Meyer JL (2000) Nitrogen cycling in a forest stream determined by a 15N tracer addition. Ecol Monogr 70:471–493
O’Reilly CM, Hecky RE, Cohen AS, Plisnier PD (2002) Interpreting stable isotopes in food webs: recognizing the role of time averaging at different trophic levels. Limnol Oceanogr 47:306–309
Pearson SF, Levey DJ, Greenberg CH, Martinez del Rio C (2003) Effects of elemental composition on the incorporation of dietary nitrogen and carbon signatures in an omnivorous songbird. Oecologia 135:516–523
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Schmidt O, Scrimgeouer CM, Curry JP (1999) Carbon and nitrogen stable isotope ratios in body tissue and mucus of feeding and fasting earthworms. Oecologia 118:9–15
Solomon C, Flecker AS, Taylor B (2004) Testing the role of sediment-mediated interactions between tadpoles and armored catfish in a Neotropical stream. Copeia 2004:610–616
Steffan S, Daane K, Mahr D (2001) 15N-enrichment of plant tissue to mark phytophagous insects, parasitoids, and flower-visiting entemophaga. Entomol Exp Appl 98:173–180
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, N.J.
Tieszen LL, Boutton T, Tesdahl K, Slade N (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37
Tominaga O, Uno N, Seikai T (2003) Influence of diet shift from formulated feed to live mysids on the carbon and nitrogen stable isotope ratio (δ13C and δ15N) in dorsal muscles of juvenile Japanese flounders, Paralichthys olivaceus. Aquaculture 218:265–276
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Vander Zanden MJ, Cabana G, Rasmussen JB (1997) Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ15N) and literature dietary data. Can J Fish Aquat Sci 54:1142–1158
Vander Zanden MJ, Hulshof M, Ridgway MS, Rasmussen JB (1998) Application of stable isotope techniques to trophic studies of age-0 smallmouth bass. Trans Am Fish Soc 127:729–739
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80:1395–1404
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ13C and δ15N trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Webb SC, Hedges REM, Simpson SJ (1998) Diet quality influences the δ13C and δ15N of locusts and their biochemical components. J Exp Biol 201:2903–2911
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341
West K, Michel E, Todd J, Brown D, Clabaugh J (2003) The gastropods of Lake Tanganyika: diagnostic key, classification, and notes on the fauna. Soc Int Limnol Spec Publ 2:1–132
Witting DA, Chambers RC, Bosley KL, Wainright SC (2005) Experimental evaluation of ontogenetic diet transitions in summer flounder (Paralichthys dentatus), using stable isotopes as diet tracers. Can J Fish Aquat Sci 61:2069–2084
Acknowledgments
Our study design was greatly improved by suggestions from Beth Boyer, Francis Chan, and Bob Howarth. We thank Martin Genner, Bob Hall, Ellinor Michel, Steve Thomas, two anonymous reviewers, and especially David Post for comments on the manuscript. Bryon Daley, Bob Hall, Corbin Hodges, George Kazumbe, Ellinor Michel, Michelle Olsgard, and Brad Taylor provided advice and assistance in the field. Chris Solomon and Ellinor Michel provided access to unpublished data, and Art Kasson analyzed the samples at the Cornell Isotope Laboratory. We thank Steve Ellner and Francoise Vermeylen for assistance with curve fitting and model selection, and Frank Wesselingh for identifying Tarebia. Logistical support in Venezuela was provided by the Perez family, Donald Taphorn, and the Figueredo family. The staff of the Tanzanian Fisheries Research Institute and Nyanza Project kindly provided logistical support at Lake Tanganyika, and the Tanzanian Committee for Science and Technology granted permission to conduct the research. This work was funded by the NSF through grants to PBM and ASF (INT-0321443 and DEB-0321471), the Nyanza Project (ATM-0223920), and the IGERT in Biogeochemistry and Environmental Biocomplexity at Cornell University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Post
Rights and permissions
About this article
Cite this article
McIntyre, P.B., Flecker, A.S. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia 148, 12–21 (2006). https://doi.org/10.1007/s00442-005-0354-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0354-3